Abstract
Background—Recent studies indicate that gastric emptying may be influenced by patterns of previous nutrient intake. Endogenous cholecystokinin (CCK), whose synthesis and release can be affected by dietary intake, has a major role in the regulation of gastric emptying. Aims—To evaluate the influence of diets with differing protein content on gastric emptying of differing liquid test meals and plasma CCK levels in the rat and to check whether the inhibitory effect of exogenous CCK on gastric emptying is modified after long term intake of diets with differing protein content. Methods—Rats were fed for three weeks with high protein, medium protein (regular), or low protein diet. On day 22 gastric emptying of a peptone meal was studied. In addition, basal and postprandial CCK levels after the different dietary regimens were measured by bioassay. The time course of dietary adaptation was studied and its specificity assessed through the use of different (peptone, glucose, and methylcellulose) test meals. The effect of exogenous CCK-8 on gastric emptying was studied at the end of the adaptation period (three weeks). Results—Feeding the animals with a high protein diet for three weeks resulted in a significant (p<0.05) acceleration (by 21.2(8.2)%) of gastric emptying while feeding with a low protein diet was followed by a significant (p<0.05) delay (by 24.0 (6.2)%) in the emptying rate. When the time course of the effect of dietary adaptation on gastric emptying was studied, it appeared that at least two weeks are required for dietary protein to be effective. The regulatory effect of dietary protein on gastric emptying proved to be dependent on meal composition. Only the emptying rate of a protein containing meal (40% peptone) was significantly modified by previous dietary intake. No significant (p>0.05) changes were observed with glucose and methylcellulose meals whose emptying rates were similar in rats receiving a high protein or low protein diet. A peptone meal strongly and significantly (p<0.05) increased plasma CCK levels in rats fed a medium protein (regular) diet. Results were similar in rats receiving a low protein diet (p<0.05) but not in rats on a high protein diet (p>0.05). As a consequence, postprandial plasma levels of CCK in rats fed with a medium or low protein diet were significantly (p<0.05) higher than those in rats receiving a high protein diet. In rats on high and low protein diets, dose response curves to CCK-8 were virtually identical, suggesting that dietary protein intake has no influence on the effect of exogenous CCK. Conclusions—These results clearly show that gastric emptying of a protein containing meal can be modified by previous dietary protein intake. This effect, which is time dependent and meal specific, may be related to changes in endogenous CCK release which will affect emptying rate. While the exact mechanisms underlying this adaptive response need to be studied and clarified further, these results emphasise the importance of dietary history in the evaluation and interpretation of gastric emptying data.
Keywords: diet; protein content; gastric emptying; cholecystokinin
Full Text
The Full Text of this article is available as a PDF (129.4 KB).
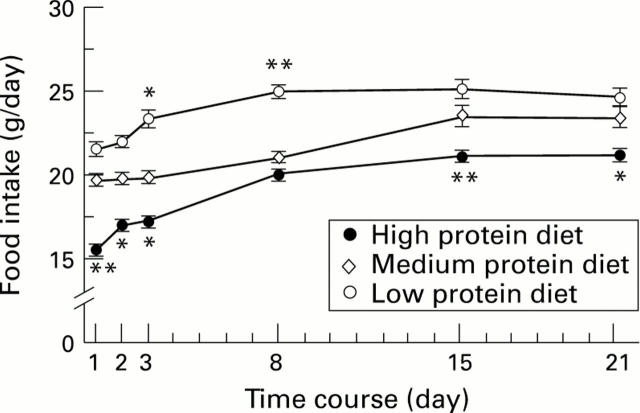
Figure 1 .
: Food intake of rats fed with a high, medium, or low protein diet. Points represent the means. Vertical bars are standard errors. *p<0.05 versus medium protein diet, **p<0.01 versus medium protein diet.
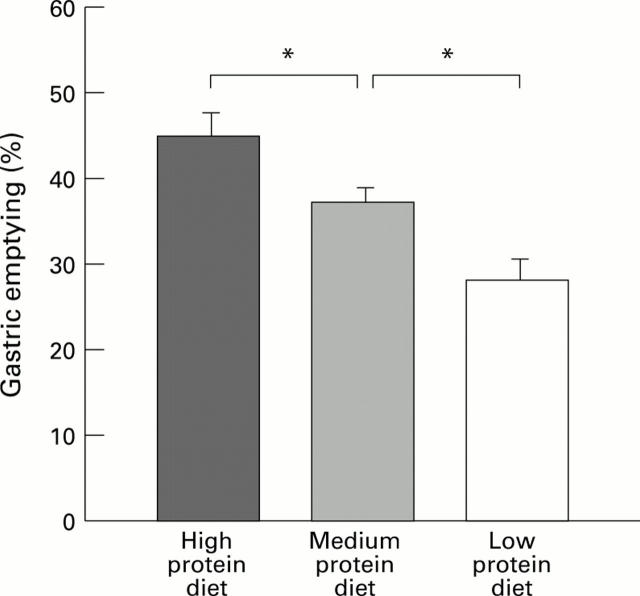
Figure 2 .
: Gastric emptying of a peptone meal (over 40 minutes) in rats fed for 21 days with a high, medium, or low protein diet. Each column represents the mean of the values obtained from six to eight animals. Vertical bars are standard errors. *p<0.05.
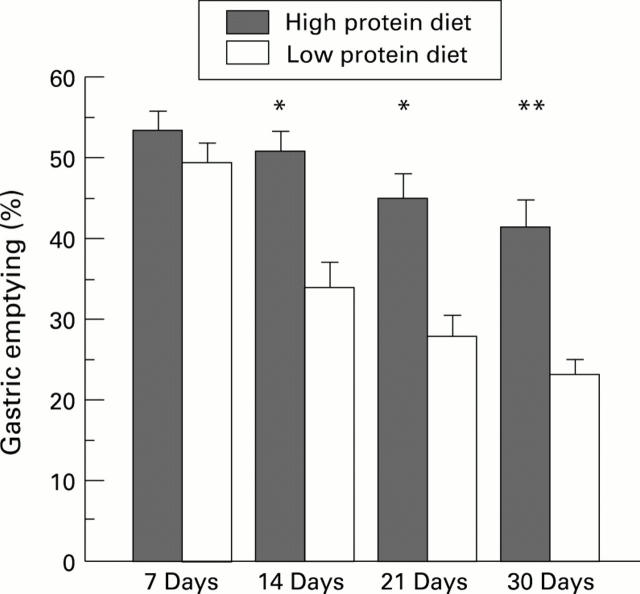
Figure 3 .
: Gastric emptying of a peptone meal (over 40 minutes) in rats fed with a high or low protein diet for 7, 14, 21, or 30 days. Each column represents the mean of the values obtained from six to eight animals. Vertical bars are standard errors. *p<0.01 versus low protein diet, **p<0.001 versus low protein diet.
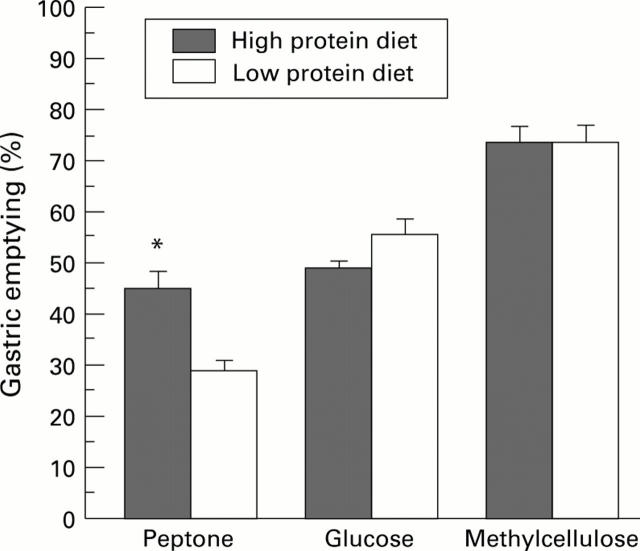
Figure 4 .
: Gastric emptying of a peptone (over 40 minutes), glucose (over 20 minutes), or methylcellulose (over 15 minutes) meal in rats fed for 21 days with a high or low protein diet. Each column represents the mean of the values obtained from six to eight animals. Vertical bars are standard errors. *p<0.01 versus low protein diet value.
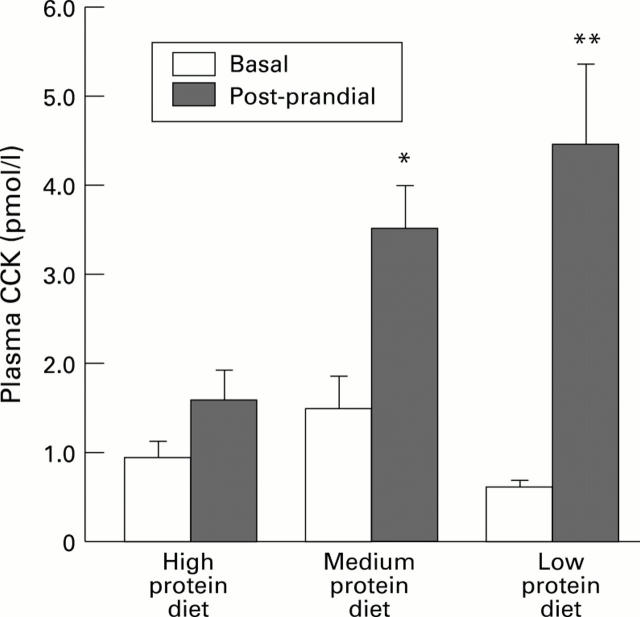
Figure 5 .
: Basal and postprandial plasma CCK levels in rats fed for 21 days with a high, medium, or low protein diet. Peptone induced CCK release was measured eight minutes after the test meal. Each column represents the mean of the values obtained from six to nine animals. Vertical bars are standard errors. *p<0.05 versus basal value, **p<0.01 versus basal value.
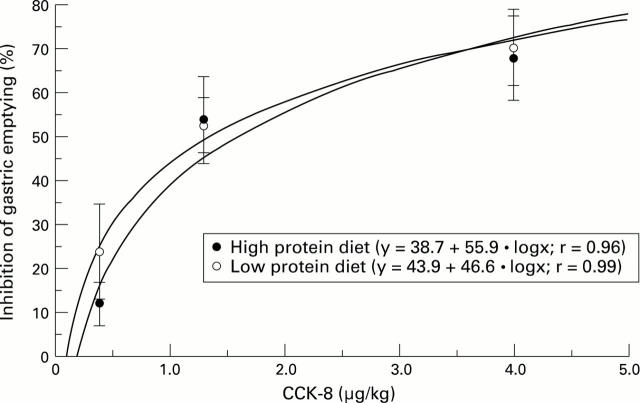
Figure 6 .
: Dose response curves of the effect of CCK-8 on gastric emptying of a methylcellulose meal in rats fed for 21 days with a high or low protein diet. Each point represents the mean of the values obtained from six to eight animals. Vertical bars are standard errors. The graph displays a semilogarithmic plot of percentage inhibition versus dose.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baile C. A., McLaughlin C. L., Della-Fera M. A. Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev. 1986 Jan;66(1):172–234. doi: 10.1152/physrev.1986.66.1.172. [DOI] [PubMed] [Google Scholar]
- Berthélemy P., Bouisson M., Vellas B., Moreau J., Nicole-Vaysse, Albarede J. L., Ribet A. Postprandial cholecystokinin secretion in elderly with protein-energy undernutrition. J Am Geriatr Soc. 1992 Apr;40(4):365–369. doi: 10.1111/j.1532-5415.1992.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corvilain B., Abramowicz M., Féry F., Schoutens A., Verlinden M., Balasse E., Horowitz M. Effect of short-term starvation on gastric emptying in humans: relationship to oral glucose tolerance. Am J Physiol. 1995 Oct;269(4 Pt 1):G512–G517. doi: 10.1152/ajpgi.1995.269.4.G512. [DOI] [PubMed] [Google Scholar]
- Cunningham K. M., Daly J., Horowitz M., Read N. W. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991 May;32(5):483–486. doi: 10.1136/gut.32.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K. M., Horowitz M., Read N. W. The effect of short-term dietary supplementation with glucose on gastric emptying in humans. Br J Nutr. 1991 Jan;65(1):15–19. doi: 10.1079/bjn19910061. [DOI] [PubMed] [Google Scholar]
- Edelbroek M., Horowitz M., Fraser R., Wishart J., Morris H., Dent J., Akkermans L. Adaptive changes in the pyloric motor response to intraduodenal dextrose in normal subjects. Gastroenterology. 1992 Dec;103(6):1754–1761. doi: 10.1016/0016-5085(92)91431-3. [DOI] [PubMed] [Google Scholar]
- Forster E. R., Dockray G. J. The role of cholecystokinin in inhibition of gastric emptying by peptone in the rat. Exp Physiol. 1992 Sep;77(5):693–699. doi: 10.1113/expphysiol.1992.sp003635. [DOI] [PubMed] [Google Scholar]
- Gregory P. C., McFadyen M., Rayner D. V. Control of gastric emptying in the pig: influence of cholecystokinin, somatostatin and prokinetic agents. Exp Physiol. 1995 Jan;80(1):159–165. doi: 10.1113/expphysiol.1995.sp003831. [DOI] [PubMed] [Google Scholar]
- Harty R. F., Pearson P. H., Solomon T. E., McGuigan J. E. Cholecystokinin, vasoactive intestinal peptide and peptide histidine methionine responses to feeding in anorexia nervosa. Regul Pept. 1991 Oct 1;36(1):141–150. doi: 10.1016/0167-0115(91)90202-r. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Cunningham K. M., Wishart J. M., Jones K. L., Read N. W. The effect of short-term dietary supplementation with glucose on gastric emptying of glucose and fructose and oral glucose tolerance in normal subjects. Diabetologia. 1996 Apr;39(4):481–486. doi: 10.1007/BF00400681. [DOI] [PubMed] [Google Scholar]
- Jansen J. B., Fried M., Hopman W. P., Lamers C. B., Meyer J. H. Relation between gastric emptying of albumin-dextrose meals and cholecystokinin release in man. Dig Dis Sci. 1994 Mar;39(3):571–576. doi: 10.1007/BF02088345. [DOI] [PubMed] [Google Scholar]
- Kanayama S., Liddle R. A. Influence of food deprivation on intestinal cholecystokinin and somatostatin. Gastroenterology. 1991 Apr;100(4):909–915. doi: 10.1016/0016-5085(91)90263-k. [DOI] [PubMed] [Google Scholar]
- Konturek J. W., Thor P., Maczka M., Stoll R., Domschke W., Konturek S. J. Role of cholecystokinin in the control of gastric emptying and secretory response to a fatty meal in normal subjects and duodenal ulcer patients. Scand J Gastroenterol. 1994 Jul;29(7):583–590. doi: 10.3109/00365529409092476. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Kwiecien N., Obtulowicz W., Kopp B., Oleksy J., Rovati L. Cholecystokinin in the inhibition of gastric secretion and gastric emptying in humans. Digestion. 1990;45(1):1–8. doi: 10.1159/000200218. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Carter J. D., McDonald A. R. Dietary regulation of rat intestinal cholecystokinin gene expression. J Clin Invest. 1988 Jun;81(6):2015–2019. doi: 10.1172/JCI113552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A. Decreased sensitivity of Zucker obese rats to the putative satiety agent cholecystokinin. Physiol Behav. 1980 Oct;25(4):543–548. doi: 10.1016/0031-9384(80)90119-5. [DOI] [PubMed] [Google Scholar]
- Moran T. H., Kornbluh R., Moore K., Schwartz G. J. Cholecystokinin inhibits gastric emptying and contracts the pyloric sphincter in rats by interacting with low affinity CCK receptor sites. Regul Pept. 1994 Aug 4;52(3):165–172. doi: 10.1016/0167-0115(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Zittel T. T. Inhibition of gastric motility induced by intestinal glucose in awake rats: role of Na(+)-glucose co-transporter. Neurogastroenterol Motil. 1995 Mar;7(1):9–14. doi: 10.1111/j.1365-2982.1995.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Robinson P. H., Stephenson J. S. Dietary restriction delays gastric emptying in rats. Appetite. 1990 Jun;14(3):193–201. doi: 10.1016/0195-6663(90)90087-o. [DOI] [PubMed] [Google Scholar]
- Scarpignato C., Capovilla T., Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980 Aug;246(2):286–294. [PubMed] [Google Scholar]
- Scarpignato C., Kisfalvi I., D'Amato M., Varga G. Effect of dexloxiglumide and spiroglumide, two new CCK-receptor antagonists, on gastric emptying and secretion in the rat: evaluation of their receptor selectivity in vivo. Aliment Pharmacol Ther. 1996 Jun;10(3):411–419. doi: 10.1111/j.0953-0673.1996.00411.x. [DOI] [PubMed] [Google Scholar]
- Scarpignato C., Varga G., Corradi C. Effect of CCK and its antagonists on gastric emptying. J Physiol Paris. 1993;87(5):291–300. doi: 10.1016/0928-4257(93)90035-r. [DOI] [PubMed] [Google Scholar]
- Wisén O., Björvell H., Cantor P., Johansson C., Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid test meal. Regul Pept. 1992 Apr 29;39(1):43–54. doi: 10.1016/0167-0115(92)90007-h. [DOI] [PubMed] [Google Scholar]