Abstract
Some tumor cells can be stimulated to differentiate and undergo terminal cell division and loss of tumorigenicity. The in vitro differentiation of murine erythroleukemia (MEL) cells is a dramatic example of tumor-cell reprogramming. We found that reentry of MEL cells into terminal differentiation is accompanied by an early transient decline in the activity of cyclin-dependant kinase (CDK) 2, followed by a decline of CDK6. Later, as cells undergo terminal arrest, CDK2 and CDK4 activities decline. By analyzing stable MEL-cell transfectants containing vectors directing inducible expression of specific CDK inhibitors, we show that only inhibitors that block the combination of CDK2 and CDK6 trigger differentiation. Inhibiting CDK2 and CDK4 does not cause differentiation. Importantly, we also show that reprogramming through inhibition of CDKs is restricted to G1 phase of the cell cycle. The results imply that abrogation of normal cell-cycle controls in tumor cells contributes to their inability to differentiate fully and that restoration of such controls in G1 can lead to resumption of differentiation and terminal cell division. The results also indicate that CDK4 and CDK6 are functionally distinct and support our hypothesis that the two CDKs regulate cell division at different stages of erythroid maturation.
Leukemias, like many other cancers, exhibit both loss of normal proliferation controls and features of immature cells because of a block in completing differentiation (1, 2). Identifying ways to reestablish normal cell-division control and differentiation in such malignant cells may lead to new cancer therapies. Certain leukemic cell lines can be reprogrammed in vitro to undergo terminal differentiation (3). Resumption of differentiation by these cells is often accompanied by a switch from a state of uncontrolled proliferation typical of malignant cells to a state in which the cells begin to differentiate and undergo a limited number of cell divisions, like normal hematopoietic progenitors, and then withdraw from the cell cycle (4). The mechanisms controlling this switch from unlimited proliferative capacity to a fixed number of cell divisions is not fully understood.
Cell-cycle progression in mammalian cells is controlled by a family of cyclin-dependent kinases (CDKs): CDK2, CDK4, and CDK6. The enzymatic activities of the CDKs are controlled at several levels: cyclin binding, CDK phosphorylation and dephosphorylation, and by binding of CDK inhibitors (CDKIs; ref. 5). To date, two families of CDKIs have been identified that differ in their specificity and mechanism of inhibition. INK4 family members p16INK4A, p15INK4B, p18INK4C, and p19INK4D inhibit CDK4 and CDK6 by interfering with cyclin D binding (6, 7). The kinase inhibitor protein (KIP) family of inhibitors, p21CIP, p27KIP1, and p57KIP2, is thought to inhibit primarily CDK2 in vivo (8). Small-molecule inhibitors of CDKs have also been identified, some of which may represent promising anticancer agents (9). Effective use of CDKIs in cancer therapy requires knowing which CDKs control proliferation in specific tumor-cell types.
We have studied the changes in specific CDK activities during in vitro differentiation of murine erythroleukemia (MEL) cells (4, 10). MEL cells are transformed erythroid precursors that are blocked at about the proerythroblast stage of differentiation. Treatment of the cells with a variety of agents causes them to reinitiate erythroid differentiation culminating in accumulation of erythrocyte-specific markers, cell-cycle arrest, and loss of tumorigenicity. We found that reentry of the cells into differentiation is accompanied by a temporal sequence of changes in specific CDKs and CDKIs. By stable transfection experiments, we show that these changes are coupled to the decision to reinitiate differentiation. Inhibiting specific CDKs induces the cells to undergo differentiation and terminal cell division. Interestingly, reprogramming MEL cells to differentiate by inhibiting CDKs can occur only in G1 and seems to require a specific order of inhibition. The results also indicate that the two highly related D cyclin kinases, CDK4 and CDK6, participate in controlling cell division at different stages of MEL cell differentiation.
Materials and Methods
Cell Culture, Differentiation, and Transfections.
Clone DS19 MEL cells were grown and differentiation was initiated as described (11, 12). Stable MEL cell transfectants expressing doxycycline-inducible cell-cycle regulators were generated by transfecting MEL cell clone B1 that stably expresses the reverse tetracycline-controlled transactivator (rtTA; ref. 13). Clone B1 was prepared by cotransfecting DS19 MEL cells with pPGK-neo and pUHD 172-1. p15, p16, p21, and p27 MEL cell transfectants were generated by cotransfecting clone B1 with pPGK-puro and pUHD 10-3 p15, pUHD 10-3 p16, pUHD 10-3 p21, or pUHD 10-3 p27. Transfectant clones were selected and maintained in medium containing 1 mg/ml G418 and 5 μg/ml puromycin. Antibiotic-resistant clones were expanded and treated with 1 μg/ml doxycycline, and cell extracts were analyzed by immunoblotting. The degree of differentiation was measured at various times by staining for the presence of hemoglobin with the benzidine reagent as described (11, 12). A minimum of 100 cells was scored for each determination of cell differentiation.
Plasmids.
cDNAs encoding human p15, p16, and p21 were the generous gifts of R. Pestell (Albert Einstein College of Medicine) with permission from J. Koh. pUHD 10-3 p27 was the generous gift of R. Pestell.
Plasma Clot Assay.
Cells were grown in either 5 mM hexamethylenebisacetamide (HMBA) or 1 μg/ml doxycycline for the indicated times and then washed in DMEM and plated in plasma clots (without HMBA or doxycycline) at ≈100 cells per well in 96-well microtiter plates as described (11, 12). The clots were incubated at 37°C for 4 days, transferred to slides, fixed with glutaraldehyde, and stained with 1% benzidine in methanol containing 2.5% H2O2, and counterstained with hematoxylin. The percentage of cells committed to differentiation was determined by calculating the ratio of colonies that stained positive with benzidine as compared with the total number of colonies scored. At least 100 colonies were scored for each experiment.
Immunoblot and Immunoprecipitation Assays.
Immunoblot assays were performed on 100 μg of total protein extract by published procedures (14). Immunoblotting for hemoglobin was performed similarly, except sonication extracts were used and PAGE gels were run under nondenaturing conditions. Immunoprecipitation assays were performed on 500 μg of total protein extract from cells lysed by sonication by published procedures. Nuclear and cytoplasmic extracts were isolated as described (15), except that protein extract from nuclear isolates was prepared by sonication by published procedures (14).
Antibodies.
Immunoblot analysis was performed with polyclonal human: α-CDK2, α-CDK4, α-CDK6, α-p15, α-p18, and α-p27 (all gifts of Y. Xiong, University of North Carolina, Chapel Hill); α-p16 (M-156; Santa Cruz Biotechnology); monoclonal α-human p21 (65951A; PharMingen); and polyclonal α-mouse hemoglobin (ICN-Koeppell). Horseradish peroxidase-conjugated anti-mouse IgG, anti-rabbit IgG, and enhanced chemiluminescence were obtained from Amersham Pharmacia. Blocking peptides (bp) used included CDK2 bp, CDK4bp, and CDK6bp (gifts of Y. Xiong). Immunoprecipitation-kinase assays used included polyclonal α-human CDK2, α-human CDK4, α-human CDK6 (gifts of Y. Xiong), α-human CDK4, and α-human CDK6 (gifts of M. Paguano, New York University).
Immunoprecipitation-Kinase Assays.
Either 100 μg (CDK2) or 500 μg (CDK4, CDK6) of total cellular protein extract was immunoprecipitated with a mouse-specific anti-CDK antibody for the corresponding CDK complexes. Kinase assays were performed by published procedures (14) with either histone H1 or a glutathione S-transferase-tagged, carboxy-terminal fragment of Rb (generous gift of R. Pestell) as substrate.
Fractionation by Centrifugal Elutriation.
MEL-transfected cells (clone p21.12) were maintained in logarithmic growth and then treated with 1 μg/ml doxycycline for 36 h. Cells were washed twice with DMEM, and then cells at various stages of the cell cycle were prepared by size fractionation by centrifugal elutriation as described (16). Briefly, 5 × 108 cells were resuspended in 20 ml of DMEM with 5% (vol/vol) FCS. The cells were passed through an 18-gauge needle and a 70-μm needle-top filter (Fisher). Three liters of additional DMEM with 5% (vol/vol) FCS were prepared for use as circulating fluid. The JE-5.0 Beckman–Spinco Elutriator was set at 2,000 rpm and 22°C for a 3-h run. The cells were loaded at a pump speed of 50 (arbitrary units). The speed was gradually raised to 62, and the first 100-ml fraction was collected. Fractions were collected at pump speeds of 66, 70, 74, 78, 82, 86, 88, 92, 96, 100, and 104. Aliquots of 1 × 106 cells were resuspended in a propidium iodide/sodium citrate buffer and subjected to fluorescence-activated cell sorter scan analysis. Elutriated cell fractions representative of G1, S, and G2 were incubated in growth medium and also plated directly in plasma clots. Benzidine staining for hemoglobinized cells and plasma clot assays were performed as described (11, 12).
Results
Resumption of Terminal Differentiation Is Accompanied by Changes in Specific Cell-Cycle Regulators.
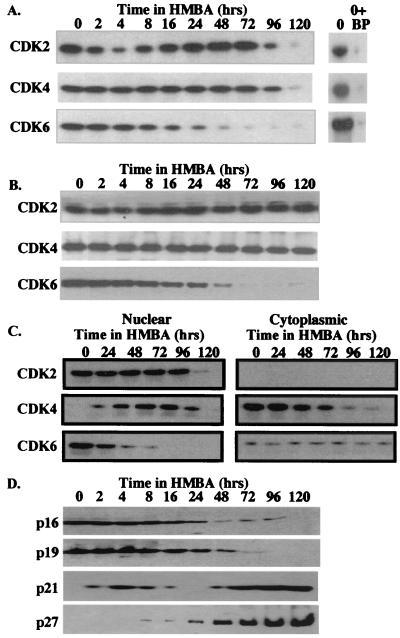
Treatment of MEL cells with chemical inducers of differentiation such as HMBA leads initially to cells that are not overtly differentiated but that are irreversibly committed to differentiate (17). These committed cells, which no longer require the presence of the inducer to execute the terminal-differentiation program, first appear 12–24 h after adding the inducer depending on conditions. By 48 h of HMBA treatment, most cells have become committed. After committing to differentiation, MEL cells continue to proliferate for several division cycles while undergoing phenotypic differentiation, and they then arrest between 96 and 120 h. To investigate possible changes in cell-cycle regulators during these transitions, we assayed the levels and activities of CDK2, CDK4, and CDK6 (Fig. 1 A and B). A very rapid but transient reduction in CDK2 activity was observed soon after initiating HMBA treatment, with the lowest level seen after 4 h. CDK2 activity is restored to pretreatment levels by 24 h. The transient loss of CDK2 activity is not accompanied by reduced CDK2 protein levels, suggesting possible inhibition by CDKIs. CDK6 activity also declines during HMBA treatment, but the decline is gradual and continuous, such that CDK6 activity is reduced to very low levels by 48 h. Loss of CDK6 kinase activity is paralleled by a reduction in CDK6 protein levels. In contrast, throughout this early period, no change was observed in CDK4 activity or protein levels. Subsequently, as cells undergo proliferation arrest between 96–120 h, there is a marked decline in CDK2 and CDK4 activities. A previous study (18) found that CDK4 protein levels decline soon after HMBA treatment. However, in agreement with the data in Fig. 1A, this study also showed that CDK4 kinase activity declined much later as cells complete differentiation and undergo cell-cycle arrest. How CDK4 activity could be maintained at early times as CDK4 protein levels decline was not explained in this earlier work. CDK6 was not investigated.
Figure 1.
Changes in cell-cycle regulatory proteins during erythroleukemia cell differentiation. MEL cells were treated with 5 mM HMBA for the indicated times, and total cellular-protein extracts were prepared and analyzed for specific CDK activities by immunoprecipitation-kinase assays (A) or CDK protein levels were analyzed by immunoblotting (B) as described in Materials and Methods. BP = Blocking peptides. (C) MEL cells were treated with 5 mM HMBA for the indicated times, and nuclear and cytoplasmic protein extracts were prepared and analyzed for specific CDK activities as described in Materials and Methods. (D) CDKI protein levels were analyzed by immunoblotting as described in Materials and Methods.
The differential regulation of CDK4 and CDK6 might suggest that these two cyclin D-dependent kinases participate in controlling proliferation at different stages of MEL cell differentiation. Consistent with this suggestion, we found that >90% of CDK6 activity is localized in the nucleus in untreated MEL cells, whereas nearly all of CDK4 activity is found in the cytoplasm (Fig. 1C). As cells commit to differentiation and CDK6 declines, increasing amounts of CDK4 appear in the nucleus. At all times, nearly all CDK2 protein and activity is found in the nucleus. The distributions of the three kinase activities are paralleled by the distributions of the proteins themselves as determined by immunoblotting (data not shown). These observations suggest that CDK6 (along with CDK2) regulates proliferation in untreated MEL tumor cells, and that CDK4 (along with CDK2) controls cell division in differentiating cells. Data shown in Fig. 2 and Table 1 and other data (see Discussion) support this conclusion.
Figure 2.
Characterization of MEL cell transfectants. (A) MEL cell transfectant clones expressing the indicated CDKI under control of the tetracycline-inducible promoter in pUHD 10-3 were cultured either in the absence (−) or in the presence (+) of 1 μg/ml doxycycline (dox) for 36 h. p16 MEL cell transfectants were first cultured in HMBA for 84 h, at which point endogenous p16 is present at very low levels (Fig. 1). Total cellular-protein extracts were prepared, and the levels of the CDKIs were determined by immunoblotting. The parental MEL cells (clone B1) containing only the rtTA regulator were cultured in the absence (0) or presence (120) of 5 mM HMBA for 120 h to indicate the levels of endogenous p16 in undifferentiated cells or that of p15, p21, and p27 present in fully differentiated MEL cells. For further details see Materials and Methods. (B) The indicated MEL cell transfectants were cultured in the presence (+) or absence (−) of doxycycline for 36 h as described in Materials and Methods. MEL and MEL rtTA cells were cultured in the presence (+) or absence (−) of roscovitine for 24 h as described in Materials and Methods. Extracts were prepared and immunoprecipitated-Kinase assays were performed as described in Materials and Methods. (C) p21 MEL cell transfectants (clone p21.12 and p21.35) were cultured in the presence (+) or absence (−) of doxycycline for 36 h. Total cellular protein extracts were prepared and immunoprecipitated (IP) with antibodies specific for the indicated CDKI as described in Materials and Methods. The immunoprecipitates were subjected to SDS/PAGE and immunoblotted for the indicated CDK.
Table 1.
Characterization of MEL cell transfectants
| Clone | Doxycycline | T(g)* | G1, %† | B+, %‡ | C, %§ |
|---|---|---|---|---|---|
| p15.25 | − | 12.2 | 31.6 | 2 | 1 |
| + | 12.4 | 31.7 | 1 | 2 | |
| p15.47 | − | 11.8 | 31.7 | 2 | 2 |
| + | 11.9 | 32.4 | 2 | 2 | |
| p16.8 | − | 11.9 | 33.4 | 1 | 2 |
| + | 24.3 | 50.6 | 3 | 2 | |
| p16.11 | − | 11.9 | 30.7 | 2 | 3 |
| + | 25.3 | 52.3 | 1 | 3 | |
| p21.12 | − | 11.8 | 29.6 | 2 | 2 |
| + | 55.5 | 33.3 | 34 | 36 | |
| p21.35 | − | 11.9 | 30.7 | 1 | 3 |
| + | 52.3 | 33.4 | 31 | 33 | |
| p27.16 | − | 12.1 | 28.3 | 2 | 2 |
| + | 42.1 | 84.9 | 1 | 3 | |
| p27.77 | − | 11.9 | 27.6 | 1 | 3 |
| + | 40.2 | 87.8 | 1 | 1 | |
| MELrtTA | − | 11.9 | 29.1 | 1 | 2 |
| + | 11.7 | 28.5 | 2 | 3 |
Doubling time [T(g)] was determined by counting cell numbers with a Coulter counter at intervals of 24 h in samples of the indicated transfectants incubated in the presence (+) or absence (−) of doxycycline for 14 days at 37°C.
Percentage of cells in (G1, %) was determined by fluorescence-activated cell sorter scan analysis as described in Materials and Methods on aliquots of cells incubated the presence (+) or absence (−) of doxycycline for 36 h at 37°C.
Percentage of benzidine-positive (B+) cells was determined as described in Materials and Methods on aliquots of cells incubated for 5 days.
Percentage of cells committed to terminal differentiation (C, %) was determined by plasma clot assays as described in Materials and Methods on aliquots of cells incubated for 4 days.
To investigate the basis for the early transient drop in CDK2 activity, we assayed the levels of CDKIs p21 and p27 and the INK4 family throughout differentiation. During the first 24 h of HMBA treatment, p21 levels were observed to undergo a rapid, transient increase and decline (Fig. 1D). This early, transient increase of p21 has been reported to be caused by an increase in p21mRNA level and posttranscriptional regulation (19). These changes are a mirror image of the early transient decline and subsequent restoration in CDK2 activity. Later, as cells underwent differentiation and terminal cell division, p21 levels increased again, concomitant with the final decline in CDK2 activity. On the other hand, p27 was not detected during the early, first decline in CDK2 activity, but it was induced at later times during the differentiation and terminal cell division phase. p16 and p19 were readily detectable in untreated MEL cells, but their levels were observed to decline during commitment and terminal differentiation (Fig. 1D). p15 and p18 were not detected in untreated cells nor during the early stages of HMBA treatment, but their levels rose substantially along with p21 and p27 at later times as cells underwent terminal cell division (20).
Inhibition of CDK2 and CDK6 (but Not CDK2 and CDK4) Is Required for Commitment to Terminal Differentiation.
The foregoing observations suggest that a transient decline of CDK2 activity, possibly caused by a transient induction of p21, followed by loss of CDK6 may play a role in reprogramming MEL cells to terminal differentiation. To investigate this possibility, we generated stable MEL cell transfectants containing tetracycline-controlled expression vectors (13) driving the synthesis of four different human CDKIs. For each inhibitor, many transfectants were screened, and two transfectant clones were chosen that exhibited no detectable expression of the exogenous inhibitor in the absence of doxycycline and doxycycline-induced levels equivalent to the levels of endogenous CDKIs present in fully differentiated parental MEL cells (Fig. 2A). Two p16 transfectant clones were chosen that exhibited doxycycline-induced levels equivalent to levels found in undifferentiated MEL cells before the decline in endogenous p16 levels that occurs during differentiation.
The functionality of the transfected CDKIs was ascertained by measuring their effect on endogenous CDK activities and on proliferation. Induction of p21 in transfectants by doxycycline led to nearly complete inhibition of all three CDK activities (Fig. 2B) and a 5-fold increase in cell-doubling time (Table 1). However, induction of p21 caused very little change in the distribution of cells in the cell cycle compared with p21 transfectants not treated with doxycycline or the parental MEL cells (Table 1), suggesting that it can inhibit progression at all phases of the cell cycle. On the other hand, induction of p27 in transfectants caused inhibition of CDK2 and CDK4, activities but it did not inhibit CDK6 (Fig. 2B). Induction of p27 led to a marked increase in cell-doubling time and accumulation of cells in G1. The effects of exogenous p21 and p27 on cell growth are consistent with their ability to inhibit CDK2 activity. However, only induced expression of p21 led to the inhibition of CDK6. The ability of p21 to inhibit CDK6 correlates with the ability of p21 to dissociate endogenous p16 and p19 from CDK4, causing them to associate with CDK6 (Fig. 2C).
Induction of p15 inhibited CDK4 activity but not that of CDK6, and it had no effect on cell doubling nor the cell-cycle phase-distribution pattern. On the other hand, induction of p16 led to inhibition of both CDK4 and CDK6 activities, an increase in cell-doubling time, and a moderate increase of cells in G1. The difference in the effects of p15 vs. p16 support the conclusion that CDK6, but not CDK4, is required for proliferation in undifferentiated MEL tumor cells.
To investigate the effects of the CDKIs and the specific patterns of CDK inhibition that they cause on termina1 differentiation, we measured both the production of benzidine-positive hemoglobinized cells and commitment to terminal cell division by plasma clot assay in the various CDKI transfectants treated with doxycycline. In the plasma clot assay, which was performed by plating doxycycline-treated cells in the absence of doxycycline, cells committed to differentiation exhibit limited proliferative capacity and give rise to small colonies consisting of a maximum of 32–64 cells, all of which stain positive with benzidine. In contrast, uncommitted cells proliferate extensively and produce very large colonies consisting of hundreds or thousands of cells. Treatment of p15, p16, or p27 transfectants with doxycycline did not induce differentiation or commitment to terminal cell division. On the other hand, treatment of p21 transfectants with doxycycline induced ≈35% of the cells to become benzidine-positive and about the same percentage were found to be committed to terminal cell division (Table 1).
p21 is unique among the CDKIs in its ability to induce differentiation and to inhibit both CDK2 and CDK6, suggesting that inhibition of these two CDKs is crucial for reprogramming MEL cells to terminal differentiation. Because p16 induction by doxycycline led to inhibition of CDK6 in p16 transfectants, we considered whether also inhibiting CDK2 in these transfectants with the chemical inhibitor roscovitine (ref. 21; see Fig. 2B) would lead to differentiation. Treating p16 transfectants with 50 μM roscovitine for 12 h followed by induction of p16 with doxycycline caused ≈85% of cells to undergo differentiation as measured after 5 days in cell culture (Table 2). Because p27 inhibited CDK2 and CDK4 (Fig. 2B) but did not induce differentiation (Table 1), CDK4 probably is not involved in this step. Treating p15 transfectants with roscovitine and then doxycycline did not induce differentiation (data not shown). Because p15 induction only causes inhibition of CDK4, these results also suggest that inhibiting the combination of CDK2 and CDK6 is essential for inducing differentiation.
Table 2.
Effect of roscovitine and doxycycline treatment of p16 transfectants
| Clone | Roscovitine* | Doxycycline* | B+, %† |
|---|---|---|---|
| p16.8 | − | − | 2 |
| − | + | 2 | |
| + | − | 2 | |
| + | + | 84 | |
| p16.11 | − | − | 3 |
| − | + | 1 | |
| + | − | 2 | |
| + | + | 85 | |
| MELrtTA | − | − | 2 |
| − | + | 3 | |
| + | − | 2 | |
| + | + | 2 |
The indicated cell types were first cultured in the presence (+) or absence (−) of 50 μM roscovitine for 12 h, followed by further incubation with or without roscovitine and the presence (+) or absence (−) of 1 μg/ml doxycycline, as indicated.
Percentage of benzidine-positive (B+) cells was determined as described in Materials and Methods on aliquots of cells incubated for 5 days.
Because the early decline in CDK2 activity precedes that of CDK6, we also investigated whether the order of inhibiting the two kinases is important in reprogramming MEL cells. Two different protocols were used to inhibit the two kinases in p16 transfectants. The first protocol described above mimicked the changes that occur during HMBA-induced differentiation—inhibiting CDK2 first with roscovitine and then inhibiting CDK6 by p16 induction with doxycycline caused most cells to differentiate. On the other hand, when p16 transfectants were treated first with doxycycline and then with roscovitine, they did not differentiate; instead, most cells died. Cell death also occurred when p16 transfectants were treated simultaneously with doxycycline and roscovitine (data not shown). At present we do not understand the reasons for the different outcomes with the different protocols, but the results indicate that a specific order of inhibition of the CDKs is important for triggering differentiation.
Reprogramming by CDK Inhibition Is Restricted to G1.
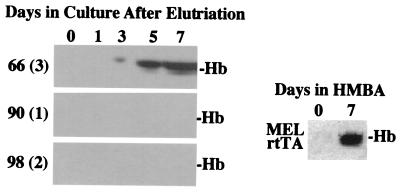
We noted that the percentage of differentiation induced by doxycycline in p21 transfectants was very similar to the percentage of cells blocked in G1. Numerous studies have suggested that decisions to differentiate may occur in G1 (22). To investigate whether cells undergoing differentiation following p21 induction arise specifically from cells arrested in G1, we treated p21 transfectants with doxycycline for 36 h, separated the cells by size by centrifugal elutriation, and then incubated the cells in medium lacking doxycycline and measured commitment to differentiation and hemoglobin production. Nearly all cells in elutriated fractions highly enriched in cells in G1 were committed to terminal differentiation (plasma clot assay) and developed into benzidine-positive cells by 5 days in cell culture (Table 3). These cells developed hemoglobin levels equivalent to those of HMBA-treated parental MEL cells (Fig. 3). In contrast, very few of the cells arrested in S or G2/M phases differentiated (Table 3 and Fig. 3). We conclude that reprogramming of MEL cells through inhibition of CDK2 and CDK6 is restricted to the G1 phase of the cell cycle.
Table 3.
Characterization of p21 elutriated fractions
| Cell fraction* | G1, %† | S, %† | G2, %† | B+, %‡ | C, %§ |
|---|---|---|---|---|---|
| 66 (1) | 93.7 | 6.3 | 0 | 96 | 97 |
| 66 (3) | 93.7 | 6.3 | 0 | 97 | 99 |
| 70 (2) | 94.8 | 4 | 1.1 | 99 | 98 |
| 78 (2) | 0 | 98.8 | 1.2 | 1 | 1 |
| 90 (1) | 0 | 99.9 | 0.1 | 1 | 2 |
| 94 (1) | 0 | 99.9 | 0.1 | 2 | 2 |
| 98 (1) | 4.2 | 38.8 | 56.7 | 2 | 3 |
| 98 (2) | 2.6 | 50.6 | 46.8 | 2 | 1 |
| 106 (1) | 2.4 | 52.7 | 45.5 | 1 | 2 |
p21 MEL cell transfectant cells (p21.12) were treated with doxycycline for 36 h, and cells were separated by size by centrifugal elutriation as described in Materials and Methods. The number indicates the pump speed at which the fractions were elutriated. Several fractions were collected at each pump speed, and the number of the fraction is indicated in parentheses. Cells were resuspended at 1 × 105 cells per ml in normal growth medium and incubated at 37°C.
Percentage of cells in the indicated phase of the cell cycle was determined by fluorescence-activated cell sorter scan analysis as described in Materials and Methods immediately after elutriation.
Percentage of benzidine-positive (B+, %) cells was determined as described in Materials and Methods on aliquots of cells incubated for 5 days after elutriation.
Percentage of cells committed to terminal differentiation (C, %) was determined by plasma clot assay as described in Materials and Methods on aliquots of cells incubated for 4 days after elutriation.
Figure 3.
Hemoglobin levels in elutriated G1, S, and G2 cell fractions of doxycycline-treated p21 MEL cell transfectant cells. p21 MEL transfectant cells (clone p21.12) were treated with doxycycline for 36 h, and cells were separated by size by centrifugal elutriation as described in Materials and Methods. Elutriated cell fractions were analyzed by fluorescence-activated cell sorter scan, and fractions representing G1 [66(3)], S [90(1)], and G2/M [98(2)] were resuspended at 1 × 105 cells per ml in normal growth medium and incubated at 37°C for the indicated number of days. Extracts were prepared and analyzed by nondenaturing PAGE and immunoblotting for hemoglobin (Hb). The levels of hemoglobin in extracts of the parental MEL rtTA cell line treated for 7 days with 5 mM HMBA are indicated (Right).
Discussion
Although differences in the regulation of CDK4 and CDK6 synthesis have been seen (23–25), functional differences between these two highly related cyclin D-kinases have not been reported to date. The results reported here suggest that CDK4 and CDK6 participate in controlling cell division at different stages of erythroid maturation. We believe that CDK6 (along with CDK2) controls proliferation in undifferentiated MEL cells and that CDK4 is not involved at this stage. The patterns of inhibition of CDKs and proliferation achieved by inducing specific CDKIs in transfectants (Fig. 2 and Table 1) and the cellular location of CDK4 and CDK6 in MEL cells (Fig. 1C) support this view. The observation that inhibiting CDK6 plus CDK2 is required for triggering MEL cell differentiation, whereas inhibiting CDK4 plus CDK2 does not induce differentiation, is also consistent with a key role for CDK6 in the block to differentiation present in MEL tumor cells. Along these lines, we also have found that overexpressing an INK4-resistant form of CDK6 in MEL cells blocks chemical induction of differentiation, whereas an INK4-resistant form of CDK4 does not have such activity (unpublished observations). Of course it is possible that the differential regulation and different effects of inhibiting CDK4 and CDK6 observed here are caused by some abnormal feature of the MEL cell differentiation program. However, we have also observed an early decline of CDK6 activity, which is followed by a later decline of CDK4 activity during erythropoietin-dependent terminal erythroid differentiation of primary erythroblasts from spleens of mice infected with the anemia-inducing strain of Friend virus (26). Thus CDK6 may be the functionally important D cyclin kinase in immature erythroid progenitors, corresponding to the stage at which MEL cells are blocked. As such, it may be subject to dysregulation during oncogenesis in erythroleukemia. If this model is also true in other leukemias, then CDK6 may prove to be a key target for inhibition by anticancer agents. As shown here, such inhibition can also lead to reentry of tumor cells into their normal differentiation program, which culminates in cell-cycle withdrawal.
The proposed change in the roles of CDK6 and CDK4 during erythroid maturation is supported further by observations made at later stages of MEL cell differentiation. By 48 h of inducer treatment, as most of the MEL cells have become irreversibly committed to differentiate and have acquired a limited proliferative capacity, there is a marked decline in CDK6 levels, and much of CDK4 is now localized into the nucleus (Fig. 1C). We believe that these events reflect a switch to CDK4-mediated (along with CDK2) control of proliferation in more mature, differentiating-erythroid cells. Consistent with this view, we found that overexpressing the INK4-resistant form of CDK4, along with CDK2, in already committed MEL cells caused significantly extended proliferation of hemoglobinized cells, whereas the INK4-resistant form of CDK6 did not have such activity (20).
The findings reported here also provide a dramatic demonstration of the crucial importance of timing within the cell cycle for decisions of cell fate in differentiation programs. We found that p21 is able to induce MEL cell differentiation only in G1 (Table 3). Many studies (22), including some with MEL cells (27), have suggested that differentiation decisions made by both normal and transformed cells occur in G1. The mechanism underlying the centrality of G1 for differentiation may lie in the signal-transduction pathways that link mitogenic signals to specific regulation of the cyclin D-dependent kinases early in G1 (28). After the brief window in G1 during which mitogens can influence cell proliferation, it is thought that the cell becomes insensitive to external stimuli. Although the exact molecular mechanisms that confer this highly specific timing on differentiation decisions are not known, the system described here may provide further insights into this important aspect of cell-fate decisions. In this regard, it is interesting to note that another highly specific aspect of reprogramming MEL cells by CDK inhibition is that it seems to require a specific order of CDK inhibition. The required order matches the order seen during HMBA-induced differentiation. Thus, inhibiting CDK2 first with roscovitine followed by inhibition of CDK6 by induction of p16 caused differentiation, whereas inverting the order of inhibitors did not induce differentiation. We believe that the first step, transient inhibition of CDK2, causes a transient lengthening of G1. A transient delay in traversal of G1 has been described during chemical induction of MEL cell differentiation with several inducers (29). Perhaps this delay is needed to allow the second step, caused by loss of CDK6 activity, to be effective. If this hypothesis is correct then a substrate of CDK6 may play a crucial role in preventing MEL cells from differentiating. Besides inhibiting CDKs (5), several different biochemical activities have been ascribed to p21, including binding to proliferating-cell nuclear antigen (PCNA) (30) and the growth arrest and DNA damage induced protein (31), affecting G2 progression (32), and inhibiting apoptosis (33). However, we think it is quite likely that p21 induces MEL cell differentiation through inhibition of CDK2, and indirectly CDK6, for several reasons. First, it can induce differentiation only in G1 where it mostly acts on the CDKs and not on PCNA. Second, we found that inhibiting the CDKs in p16 transfectants with roscovitine and then with doxycycline also causes the cells to differentiate. These changes mimic the changes in kinase activities seen during chemically induced differentiation. In fact, we have found that overexpression of CDK2 and CDK6 in MEL cells blocks chemically induced differentiation, showing that down-regulation of the kinases is needed to trigger differentiation (unpublished observations).
Our finding that MEL cells can be reprogrammed to commit to terminal differentiation through overexpression of p21 suggests that abrogation of normal cell cycle controls in these transformed cells contributes to their inability to fully differentiate. Overexpression of CDKIs might be useful for reprogramming other tumor-cell types into terminal differentiation, adding to the arsenal of differentiation therapies. Interestingly, reprogramming of MEL cells occurred only in response to p21 induction, and expression of other CDKIs including p27 did not lead to differentiation. We believe that transfected p27 is unable to induce differentiation, because it cannot cause p16 and p19 to dissociate from cytoplasmic CDK4 complexes and thereby inhibit CDK6, whereas p21 has this ability (Fig. 2C). This difference between p27 and p21 may be caused by an efficient nuclear localization signal in p27 (34), causing it to be translocated more rapidly to the nucleus. On the other hand, the inability of INK4 CDKIs to cause MEL cell differentiation may be due to their inability to inhibit CDK2 and cause the important lengthening of G1. INK4 CDKIs have been reported to accelerate differentiation but only in the presence of other differentiation-inducing agents (35, 36). The involvement of the KIPs in terminal differentiation has also been suggested from studies in several cell systems. For example, several lines of evidence suggest that p21 is involved in myogenesis (37, 38). Furthermore, overexpression of p27 (39) and p21 (40–42) has been reported to cause differentiation in several cell lines. Chemical inhibitors of CDKs have also been reported to cause differentiation of tumor-cell lines (43). However, most often treatment of tumor cells with chemical inhibitors (21, 44) or expression of CDKIs in such cells has resulted in cell-cycle arrest without differentiation. These differences have been attributed to cell-type variation. We propose that the different responses among different tumor lines may be caused by the basal levels of INK4 family members in different tumor cells. For example, in tumor cells like MEL cells, in which some INK4 family members are present at moderate to high levels, overexpression of KIPs would be expected to cause not only inhibition of CDK2 but also displacement and redistribution of the INK4 family members from one cyclin D-kinase to another, e.g., from cytoplasmically localized CDK4 to nuclear localized CDK6 (Fig. 2C). The ensuing inhibition of the cyclin D dependent kinases might then promote differentiation. The reverse rearrangement of CDKIs, i.e., INK4-mediated displacement of KIPs from CDK4 complexes to CDK2 complexes, leading to simultaneous inhibition of CDK2 and CDK4 has been reported (45–47). On the other hand, in other tumor cells in which the basal levels of INK4 family members are low or absent, overexpression of KIPs would be expected to cause only inhibition of CDK2, resulting in reduced cell-cycle progression or arrest. In these cases using a combination of inhibitors that target both CDK2 and CDK4 or CDK6 might prove effective for promoting differentiation as we showed here with roscovitine and p16.
Acknowledgments
We are extremely grateful to David Franklin and Yue Xiong for providing us with constructs, antibodies, technical support, and advice that were essential to the completion of these experiments. We also thank Richard Pestell and Liang Zhu for providing us with critical reagents and advice. A.I.S. receives support from National Cancer Institute Cancer Center Grant 2P30CA13330. F.R. was supported by National Institutes of Health Grant 5T32AG00194, and I.M. was supported by National Institutes of Health/Medical Scientist Training Program 5T32GM07288-25. This work was supported by National Institutes of Health Grant 5R37CA16368.
Abbreviations
- bp
blocking peptides
- CDK
cyclin-dependent kinase
- CDKI
CDK inhibitors
- KIP
kinase inhibitor protein
- HMBA
hexamethylenebisacetamide
- MEL
murine erythroleukemia
- rtTA
reverse tetracycline-controlled transactivator
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250488697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250488697
References
- 1.Livingston D M. Biochim Biophys Acta. 1997;1332:R25–R31. doi: 10.1016/s0304-419x(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 2.Clurman B E, Roberts J M, Groudine M. Curr Opin Hematol. 1996;3:315–320. doi: 10.1097/00062752-199603040-00011. [DOI] [PubMed] [Google Scholar]
- 3.Rifkind R A, Richon V M, Marks P A. Pharmacol Ther. 1996;69:97–102. doi: 10.1016/0163-7258(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 4.Marks P A, Rifkind R A. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- 5.Sherr C J, Roberts J M. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 7.Hunter T, Pines J. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 8.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 9.Garrett M D, Fattaey A. Curr Opin Genet Dev. 1999;9:104–111. doi: 10.1016/s0959-437x(99)80015-x. [DOI] [PubMed] [Google Scholar]
- 10.Marks P A, Sheffery M, Ramsay R, Ikeda K, Rifkind R A. Ann NY Acad Sci. 1987;511:246–255. doi: 10.1111/j.1749-6632.1987.tb36252.x. [DOI] [PubMed] [Google Scholar]
- 11.Rao G, Rekhtman N, Cheng G, Krasikov T, Skoultchi A I. Oncogene. 1997;14:123–131. doi: 10.1038/sj.onc.1200807. [DOI] [PubMed] [Google Scholar]
- 12.Rekhtman N, Radparvar F, Evans T, Skoultchi A I. Genes Dev. 1999;13:1398–1411. doi: 10.1101/gad.13.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gossen M, Bonin A L, Freundlieb S, Bujard H. Curr Opin Biotechnol. 1994;5:516–520. doi: 10.1016/0958-1669(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 14.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y, Liu D, Skoultchi A I. Mol Cell Biol. 1995;15:1889–1900. doi: 10.1128/mcb.15.4.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown E H, Schildkraut C L. J Cell Physiol. 1979;99:261–278. doi: 10.1002/jcp.1040990213. [DOI] [PubMed] [Google Scholar]
- 17.Gusella J, Geller R, Clarke B, Weeks V, Housman D. Cell. 1976;9:221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- 18.Kiyokawa H, Richon V M, Rifkind R A, Marks P A. Mol Cell Biol. 1994;14:7195–7203. doi: 10.1128/mcb.14.11.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macleod K F, Sherry N, Hannon G, Beach D, Tokino T, Kinzler K, Vogelstein B, Jacks T. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 20.Matushansky I, Radparvar F, Skoultchi A I. Blood. 2000;96:2755–2764. [PubMed] [Google Scholar]
- 21.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala L A, Scovassi A I, Meijer L, Prosperi E. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 22.Yee A S, Shih H H, Tevosian S G. Front Biosci. 1998;3:D532–D547. doi: 10.2741/a301. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Guo K, Wills K N, Walsh K. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- 24.Akagi T, Ono H, Shimotohno K. Oncogene. 1996;13:399–405. [PubMed] [Google Scholar]
- 25.Chilosi M, Doglioni C, Yan Z, Lestani M, Menestrina F, Sorio C, Benedetti A, Vinante F, Pizzolo G, Inghirami G. Am J Pathol. 1998;152:209–217. [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh F H, Barnett L A, Green W F, Freedman K, Matushansky I, Skoultchi A I, Kelley L L. Blood. 2000;96:2746–2754. [PubMed] [Google Scholar]
- 27.Geller R, Levenson R, Housman D. J Cell Physiol. 1978;95:213–222. doi: 10.1002/jcp.1040950211. [DOI] [PubMed] [Google Scholar]
- 28.Sherr C J. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 29.Friedman E A, Schildkraut C L. Proc Natl Acad Sci USA. 1978;75:3813–3817. doi: 10.1073/pnas.75.8.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Hurwitz J, Massague J. Nature (London) 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 31.Vairapandi M, Balliet A G, Fornace A J, Jr, Hoffman B, Liebermann D A. Oncogene. 1996;12:2579–2594. [PubMed] [Google Scholar]
- 32.Dulic V, Stein G H, Far D F, Reed S I. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyoshima H, Hunter T. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 35.Adachi M, Roussel M F, Havenith K, Sherr C J. Blood. 1997;90:126–137. [PubMed] [Google Scholar]
- 36.Morse L, Chen D, Franklin D, Xiong Y, Chen-Kiang S. Immunity. 1997;6:47–56. doi: 10.1016/s1074-7613(00)80241-1. [DOI] [PubMed] [Google Scholar]
- 37.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 38.Parker S B, Eichele G, Zhang P, Rawls A, Sands A T, Bradley A, Olson E N, Harper J W, Elledge S J. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 39.Kranenburg O, Scharnhorst V, Van der Eb A J, Zantema A. J Cell Biol. 1995;131:227–234. doi: 10.1083/jcb.131.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z Y, Perkins N D, Ohno T, Nabel E G, Nabel G J. Nat Med. 1995;1:1052–1056. doi: 10.1038/nm1095-1052. [DOI] [PubMed] [Google Scholar]
- 41.Kokunai T, Izawa I, Tamaki N. Int J Cancer. 1998;75:643–648. doi: 10.1002/(sici)1097-0215(19980209)75:4<643::aid-ijc24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Rots N Y, Iavarone A, Bromleigh V, Freedman L P. Blood. 1999;93:2721–2729. [PubMed] [Google Scholar]
- 43.Lee H R, Chang T H, Tebalt M J, III, Senderowicz A M, Szabo E. Int J Oncol. 1999;15:161–166. [PubMed] [Google Scholar]
- 44.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 45.Reynisdottir I, Polyak K, Iavarone A, Massague J. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 46.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parry D, Mahony D, Wills K, Lees E. Mol Cell Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]