Abstract
Background—Infection of pancreatic necrosis has a major impact on clinical course, management, and outcome in acute pancreatitis. Currently, guided fine needle aspiration is the only means for an early and accurate diagnosis of infected necrosis. Procalcitonin (PCT), a 116 amino acid propeptide of calcitonin, and interleukin 8 (IL-8), a strong neutrophil activating cytokine, are markers of severe inflammation and sepsis. Aims—To analyse the clinical value of PCT and IL-8 as biochemical parameters for predicting infected necrosis in acute pancreatitis. Patients and methods—Fifty patients with acute pancreatitis entered this prospective study and were stratified into three groups according to morphological and bacteriological findings: 18 patients with oedematous pancreatitis (group I), 14 patients with sterile necrosis (group II), and 18 patients who developed infected necrosis a median of 13.5 days after the onset of symptoms (group III). After admission serum samples were drawn daily for two weeks. Concentrations of PCT and IL-8 were measured by chemoluminescent immunoassays (upper reference range 0.5 ng/ml for PCT and 70 pg/ml for IL-8). The routine parameter C-reactive protein was determined by laser nephelometry (upper reference range 10 mg/l). Results—Median concentrations of PCT and IL-8 were significantly higher in patients with infected necrosis than in those with sterile necrosis during the observation period, whereas there was no difference in C-reactive protein. In oedematous pancreatitis overall median concentrations of all three parameters were low. By receiver operating characteristics best cut off levels for predicting infected necrosis or persisting pancreatic sepsis were 1.8 ng/ml for PCT and 112 pg/ml for IL-8. If these cut off levels were reached on at least two days, sensitivity, specificity, and accuracy for the prediction of infected necrosis were 94%, 91%, and 92% for PCT and 72%, 75%, and 74% for IL-8, respectively. After surgical treatment of infected necrosis median PCT and IL-8 values continued to be significantly higher in patients with persisting pancreatic sepsis (n=11) compared with those having an uneventful postoperative course (n=7). For the preoperative differentiation between infected necrosis and sterile necrosis guided fine needle aspiration was performed in 24 patients with necrotising pancreatitis and reached a diagnostic accuracy of 84% compared with 87% for PCT, and 68% for IL-8. There was no correlation between the aetiology of acute pancreatitis or the extent of necrosis and PCT or IL-8. Conclusion—PCT and IL-8 are found in high concentrations in infected necrosis and associated systemic complications in patients with acute pancreatitis. The course of PCT shows the closest correlation with the presence of infected necrosis. Monitoring of serum PCT is a potential new marker for the non-invasive and accurate prediction of infected necrosis as well as for the selection of patients with persisting septic complications after surgical debridement.
Keywords: procalcitonin; interleukin 8; infected necrosis; acute pancreatitis
Full Text
The Full Text of this article is available as a PDF (154.0 KB).
Figure 1 .
: Peak concentrations of PCT reached on at least two days during the observation period in patients with infected necrosis, sterile necrosis, and oedematous pancreatitis (p<0.0001, infected versus sterile necrosis and infected necrosis versus oedematous pancreatitis).
Figure 2 .
: Peak concentrations of IL-8 reached on at least two days during the observation period in patients with infected necrosis, sterile necrosis, and oedematous pancreatitis (p<0.0001, infected versus sterile necrosis; p<0.05, infected necrosis versus oedematous pancreatitis).
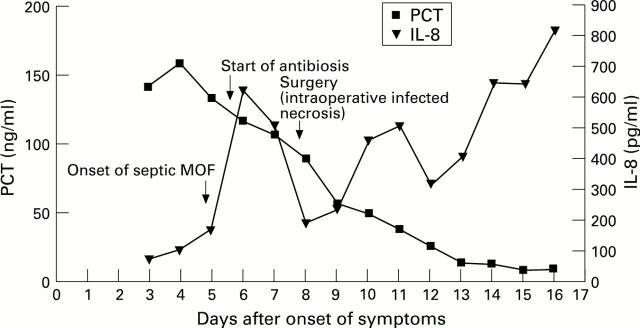
Figure 3 .
: Typical course of PCT and IL-8 in a patient with infected necrosis and severe septic multiple organ failure.
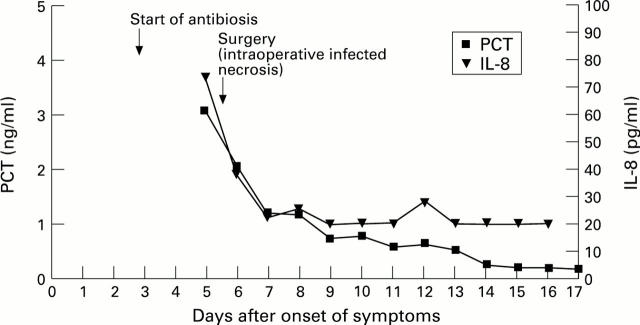
Figure 4 .
: Typical course of PCT and IL-8 in a patient with infected necrosis and an uncomplicated course.
Figure 5 .
: ROC for PCT, IL-8, and C-reactive protein (CRP) in predicting infected necrosis. Analysis was based on at least two maximum levels of each parameter (p<0.05, PCT versus CRP; p<0.0001, PCT versus IL-8; p<0.012, CRP versus IL-8).
Figure 6 .
: ROC for PCT, IL-8, and C-reactive protein (CRP) in predicting septic multiple organ failure. Analysis was based on at least two maximum levels of each parameter (p<0.001, PCT versus IL-8; p<0.02, PCT versus CRP; NS, IL-8 versus CRP).
Figure 7 .
: ROC for PCT, IL-8, and C-reactive protein (CRP) in predicting necrotising pancreatitis. Analysis was based on the two highest levels of each parameter (p<0.008, CRP versus PCT; p<0.0001, CRP versus IL-8; p<0.0001, PCT versus IL-8).
Figure 8 .
: Peak concentrations of PCT did not correlate with the intrapancreatic extent of necrosis.
Figure 9 .
: Peak concentrations of IL-8 did not correlate with the intrapancreatic extent of necrosis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardyce D. B. Incidence of necrotizing pancreatitis and factors related to mortality. Am J Surg. 1987 Sep;154(3):295–299. doi: 10.1016/0002-9610(89)90614-4. [DOI] [PubMed] [Google Scholar]
- Assicot M., Gendrel D., Carsin H., Raymond J., Guilbaud J., Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993 Feb 27;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks P. A., Gerzof S. G., Chong F. K., Worthington M. G., Doos W. G., Sullivan J. G., Johnson W. C. Bacteriologic status of necrotic tissue in necrotizing pancreatitis. Pancreas. 1990 May;5(3):330–333. doi: 10.1097/00006676-199005000-00013. [DOI] [PubMed] [Google Scholar]
- Beck J. R., Shultz E. K. The use of relative operating characteristic (ROC) curves in test performance evaluation. Arch Pathol Lab Med. 1986 Jan;110(1):13–20. [PubMed] [Google Scholar]
- Becker K. L., Monaghan K. G., Silva O. L. Immunocytochemical localization of calcitonin in Kulchitsky cells of human lung. Arch Pathol Lab Med. 1980 Apr;104(4):196–198. [PubMed] [Google Scholar]
- Beger H. G., Bittner R., Block S., Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986 Aug;91(2):433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Bradley E. L., 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg. 1991 Jan;161(1):19–25. doi: 10.1016/0002-9610(91)90355-h. [DOI] [PubMed] [Google Scholar]
- D'Egidio A., Schein M. Surgical strategies in the treatment of pancreatic necrosis and infection. Br J Surg. 1991 Feb;78(2):133–137. doi: 10.1002/bjs.1800780204. [DOI] [PubMed] [Google Scholar]
- Dandona P., Nix D., Wilson M. F., Aljada A., Love J., Assicot M., Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994 Dec;79(6):1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- Donnelly S. C., Strieter R. M., Kunkel S. L., Walz A., Robertson C. R., Carter D. C., Grant I. S., Pollok A. J., Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993 Mar 13;341(8846):643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- Elebute E. A., Stoner H. B. The grading of sepsis. Br J Surg. 1983 Jan;70(1):29–31. doi: 10.1002/bjs.1800700111. [DOI] [PubMed] [Google Scholar]
- Formela L. J., Galloway S. W., Kingsnorth A. N. Inflammatory mediators in acute pancreatitis. Br J Surg. 1995 Jan;82(1):6–13. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- Gerzof S. G., Banks P. A., Robbins A. H., Johnson W. C., Spechler S. J., Wetzner S. M., Snider J. M., Langevin R. E., Jay M. E. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987 Dec;93(6):1315–1320. doi: 10.1016/0016-5085(87)90261-7. [DOI] [PubMed] [Google Scholar]
- Goris R. J., te Boekhorst T. P., Nuytinck J. K., Gimbrère J. S. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985 Oct;120(10):1109–1115. doi: 10.1001/archsurg.1985.01390340007001. [DOI] [PubMed] [Google Scholar]
- Gross V., Andreesen R., Leser H. G., Ceska M., Liehl E., Lausen M., Farthmann E. H., Schölmerich J. Interleukin-8 and neutrophil activation in acute pancreatitis. Eur J Clin Invest. 1992 Mar;22(3):200–203. doi: 10.1111/j.1365-2362.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Gross V., Leser H. G., Heinisch A., Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993 Dec;40(6):522–530. [PubMed] [Google Scholar]
- Joyce C. D., Fiscus R. R., Wang X., Dries D. J., Morris R. C., Prinz R. A. Calcitonin gene-related peptide levels are elevated in patients with sepsis. Surgery. 1990 Dec;108(6):1097–1101. [PubMed] [Google Scholar]
- Kusske A. M., Rongione A. J., Reber H. A. Cytokines and acute pancreatitis. Gastroenterology. 1996 Feb;110(2):639–642. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- Nylen E. S., O'Neill W., Jordan M. H., Snider R. H., Moore C. F., Lewis M., Silva O. L., Becker K. L. Serum procalcitonin as an index of inhalation injury in burns. Horm Metab Res. 1992 Sep;24(9):439–443. doi: 10.1055/s-2007-1003354. [DOI] [PubMed] [Google Scholar]
- Pezzilli R., Billi P., Miniero R., Fiocchi M., Cappelletti O., Morselli-Labate A. M., Barakat B., Sprovieri G., Miglioli M. Serum interleukin-6, interleukin-8, and beta 2-microglobulin in early assessment of severity of acute pancreatitis. Comparison with serum C-reactive protein. Dig Dis Sci. 1995 Nov;40(11):2341–2348. doi: 10.1007/BF02063235. [DOI] [PubMed] [Google Scholar]
- Rau B., Pralle U., Uhl W., Schoenberg M. H., Beger H. G. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg. 1995 Oct;181(4):279–288. [PubMed] [Google Scholar]
- Rau B., Uhl W., Buchler M. W., Beger H. G. Surgical treatment of infected necrosis. World J Surg. 1997 Feb;21(2):155–161. doi: 10.1007/s002689900208. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988 Mar;3(2-3):105–112. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- Schoonjans F., Zalata A., Depuydt C. E., Comhaire F. H. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995 Dec;48(3):257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- Vesentini S., Bassi C., Talamini G., Cavallini G., Campedelli A., Pederzoli P. Prospective comparison of C-reactive protein level, Ranson score and contrast-enhanced computed tomography in the prediction of septic complications of acute pancreatitis. Br J Surg. 1993 Jun;80(6):755–757. doi: 10.1002/bjs.1800800633. [DOI] [PubMed] [Google Scholar]
- Widdison A. L., Karanjia N. D. Pancreatic infection complicating acute pancreatitis. Br J Surg. 1993 Feb;80(2):148–154. doi: 10.1002/bjs.1800800208. [DOI] [PubMed] [Google Scholar]
- Wilson C., Heath D. I., Imrie C. W. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990 Nov;77(11):1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]