Abstract
Background—Mice deficient in interleukin-2 (IL-2) develop inflammatory bowel disease resembling ulcerative colitis in humans. Recent studies provided evidence that αβ T cells, particularly CD4 T cells, rather than B cells, are involved in the pathogenesis of bowel inflammation of IL-2 deficient mice. Aim—To analyse the pattern of expression of cytokine mRNA in intestinal tissue of normal and IL-2 deficient mice. Methods—Expression of β-actin, IL-1α, IL-1β, IL-6, IL-10, tumour necrosis factor α (TNF-α), interferon γ (IFN-γ) and transforming growth factor β1 (TGF-β1) mRNA was analysed in colon and small intestinal tissue of both IL-2 deficient (IL-2−/−) mice and normal (wild type) litter mates (IL-2+/+) at different ages by using qualitative, as well as semiquantitative, competitive reverse transcription polymerase chain reaction (RT-PCR). Results were correlated with the phase of progression of the disease, as determined by histology. Results—IL-2−/− mice had expressed low levels of IL-1α, IL-1β, IL-6, TNF-α, and IFN-γ mRNA in the colon by 1.5 weeks of age. In advance of the development of histologically and clinically detectable bowel inflammation, expression of IL-1α, IL-1β, IL-6, TNF-α, IFN-γ, and IL-10, but not TGF-β1, mRNA increased in the colon of IL-2 deficient mice. In contrast, IL-2+/+ mice expressed TGF-β1 mRNA in colon tissue at 13 and 23 weeks of age, but not IL-1α, IL-1β, IL-6, TNF-α, IL-10, or IFN-γ mRNA. Levels of expression of cytokine mRNA in tissue from the small intestine were comparable in IL-2−/− and IL-2+/+ mice. Conclusions—Bowel inflammation in IL-2 deficient mice is preceded by an increase in IL-1α, IL-1β, TNF-α, and IFN-γ mRNA expression in colon tissue. Low levels of TGF-β1, but high levels of IL-1α, IL-1β, IL-6, TNF-α, IFN-γ, and IL-10 mRNA expression correlate with the manifestation of severe colitis, and suggest that T cells and macrophages are involved in bowel inflammation of IL-2 deficient mice.
Keywords: cytokine; mRNA expression; interleukin-2 deficient mice; bowel inflammation
Full Text
The Full Text of this article is available as a PDF (269.6 KB).
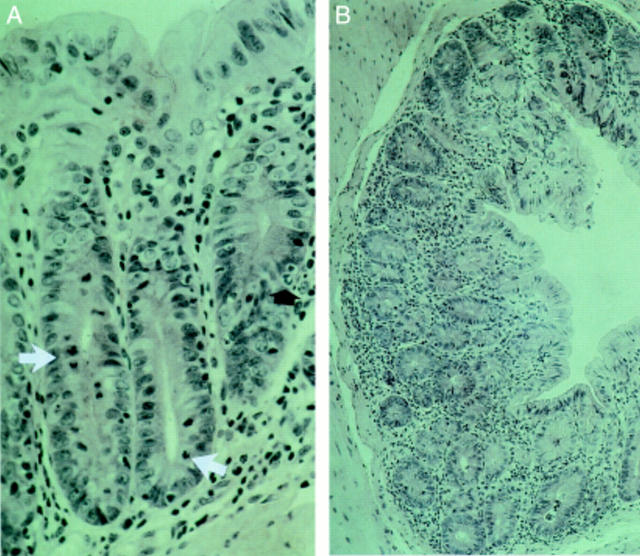
Figure 1 .
: Colon of IL-2-/- mice at (A) 8 weeks (early phase with few alterations, mild colitis), (B-D) 13 weeks of age (later phase with severe colitis). (A) Infiltration of the lamina propria with lymphocytes, plasma cells and granulocytes. A few transmigrating polymorphonuclear leucocytes are present (black arrow). The number of mitotic epithelial cells is increased (white arrows). (B) Severe inflammation of the colon (overview). (C) Crypt abscesses and an increased number of mitotic epithelial cells (arrows). (D) Transverse section, infiltration of the lamina propria, crypt abscesses (haematoxylin and eosin).
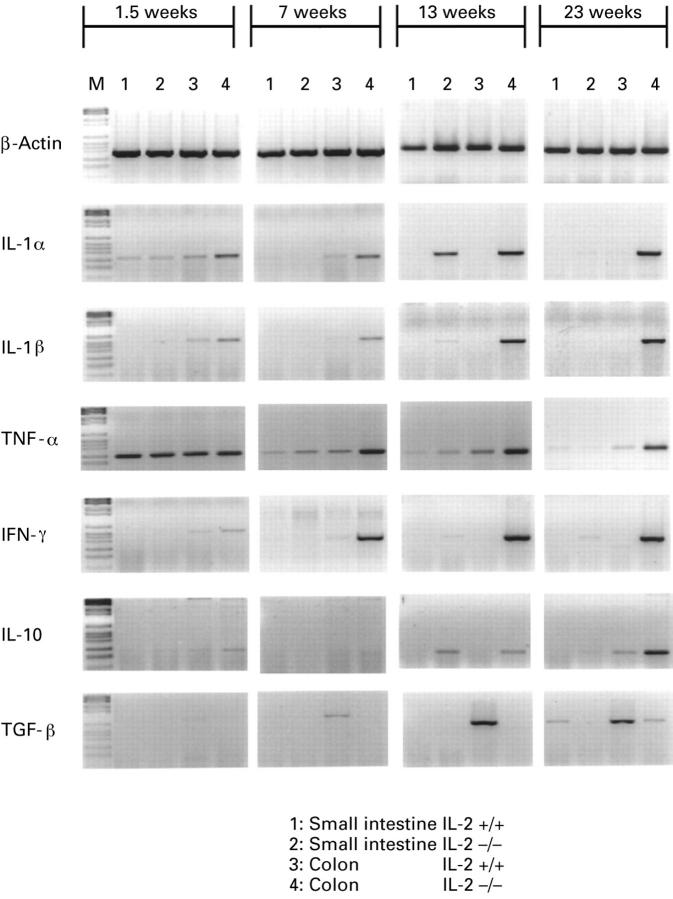
Figure 2 .
: PCR assisted amplification of β-actin, IL-1α, IL-1β, TNF-α, IFN-γ, IL-10, and TGF-β mRNA extracted from intestinal and colonic tissue of IL-2+/'+ and IL-2−/− mice at 1.5, 7, 13, and 23 weeks of age. Amplification cycles: β-actin, 21 cycles; IL-1α, IL-1β, TNF-α, and IFN-γ, 30 cycles; IL-10 and TGF-β1, 35 cycles. M, molecular weight markers. The data shown are the results from one knockout and from one wild type mouse and are representative of five mice from each group.
Figure 3 .
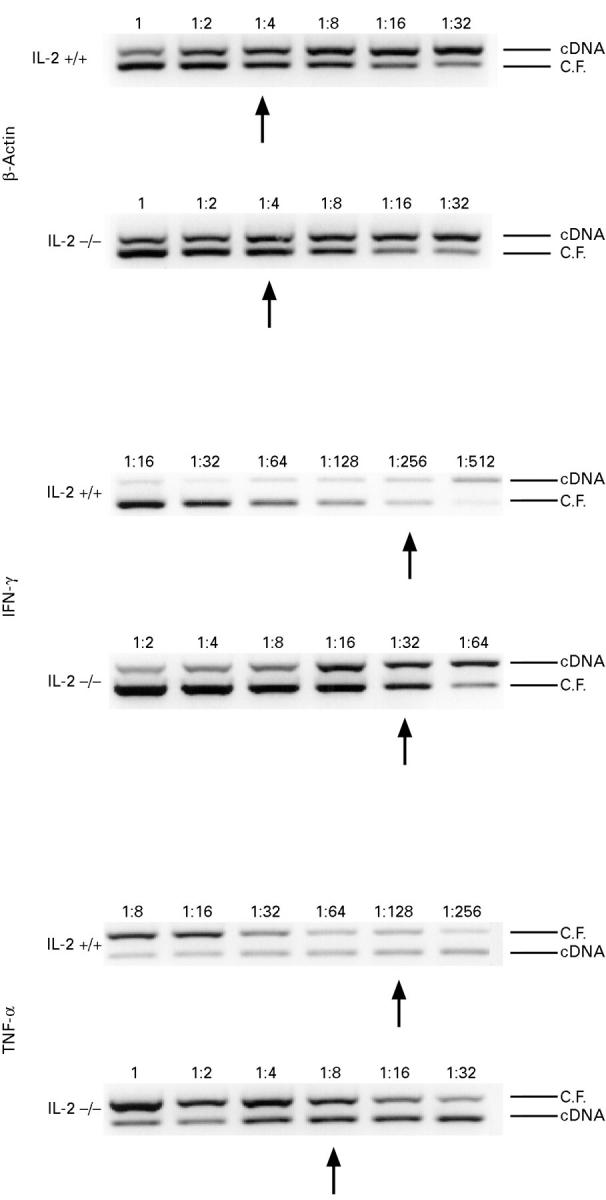
: Semiquantitative competitive PCR assisted amplification of β-actin, IFN-γ and TNF-α mRNA extracted from colonic tissue of IL-2−/− and IL-2+/+ mice at 13 weeks of age. Constant amounts of target cDNA were amplified in the presence of serially diluted competitor control DNA (1, undiluted cDNA: β-actin, pMCQ 3 ng, 28 cycles; TNF-α, pMCQ 3 pg 35 cycles; IFN-γ, pG2PCR106g4 0.125 pg 35 cycles). The dilution at which equally dense bands for control and target DNA were obtained was used for determination of cytokine mRNA expression levels. C.F. = control fragment (competitor control DNA). Arrows indicate the dilution step at which equally dense bands were obtained. The data shown are the results from one knockout and from one wild type mouse and are representative of five mice from each group.
Figure 4 .
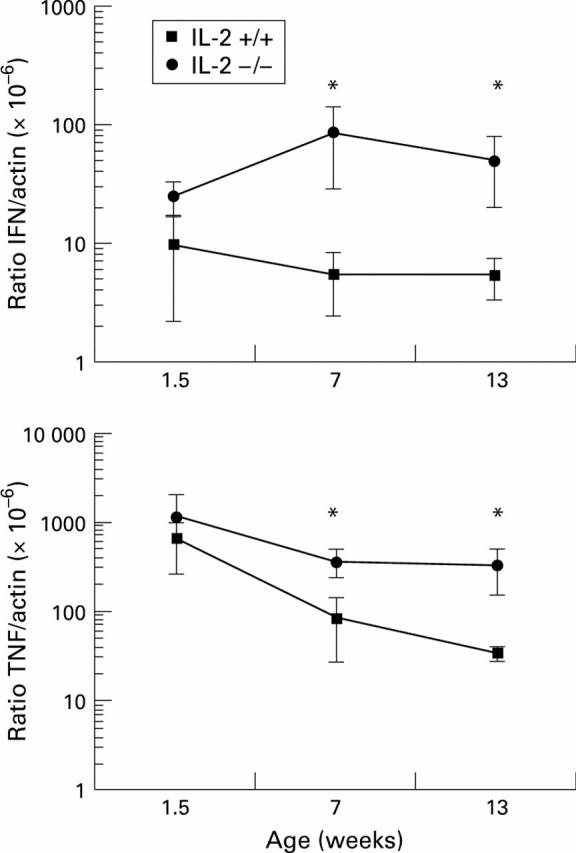
: Semiquantitative determination of IFN-γ and TNF-α mRNA expression in the colon of IL-2−/− and IL-2+/+ mice at 1.5, 7 and 13 weeks of age was performed as described in the legend to fig 3. The values represent the means of five mice per time point for each group. *p<0.05 between IL-2 −/− and IL-2+/+ mice. The ratio of IFN-γ or TNF-α to β-actin was calculated as follows: [concentration of IFN-γ control fragment×dilution factor of target DNA (IFN-γ or TNF-α)]÷[concentration of β-actin control fragment×dilution factor of target DNA β-actin]
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg J., Ebert E. C., Mayer L. T-cell activation in human intestinal mucosa: the role of superantigens. Gastroenterology. 1993 Nov;105(5):1421–1430. doi: 10.1016/0016-5085(93)90147-5. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Firsching R. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996 Apr;44(4):285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- Autenrieth I. B., Hantschmann P., Heymer B., Heesemann J. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology. 1993 Jan;187(1-2):1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- Bohn E., Autenrieth I. B. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J Immunol. 1996 Feb 15;156(4):1458–1468. [PubMed] [Google Scholar]
- Bohn E., Heesemann J., Ehlers S., Autenrieth I. B. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect Immun. 1994 Jul;62(7):3027–3032. doi: 10.1128/iai.62.7.3027-3032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese E. J., Michie C. A., Nicholls S. W., Murch S. H., Williams C. B., Domizio P., Walker-Smith J. A., MacDonald T. T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994 Jun;106(6):1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Casini-Raggi V., Kam L., Chong Y. J., Fiocchi C., Pizarro T. T., Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995 Mar 1;154(5):2434–2440. [PubMed] [Google Scholar]
- Chiodini R. J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989 Jan;2(1):90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciacci C., Mahida Y. R., Dignass A., Koizumi M., Podolsky D. K. Functional interleukin-2 receptors on intestinal epithelial cells. J Clin Invest. 1993 Jul;92(1):527–532. doi: 10.1172/JCI116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman L. A., Ridwan B. U., Tennyson G. S., Beagley K. W., Bucy R. P., Elson C. O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994 Dec;107(6):1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Mielke M. E., Blankenstein T., Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992 Nov 1;149(9):3016–3022. [PubMed] [Google Scholar]
- Ehrhardt R. O., Lúdvíksson B. R., Gray B., Neurath M., Strober W. Induction and prevention of colonic inflammation in IL-2-deficient mice. J Immunol. 1997 Jan 15;158(2):566–573. [PubMed] [Google Scholar]
- Fukushima K., West G., Fiocchi C. Adequacy of mucosal biopsies for evaluation of intestinal cytokine-specific mRNA. Comparative study of RT-PCR in biopsies and isolated cells from normal and inflamed intestine. Dig Dis Sci. 1995 Jul;40(7):1498–1505. doi: 10.1007/BF02285198. [DOI] [PubMed] [Google Scholar]
- Hanauer S. B. Inflammatory bowel disease. N Engl J Med. 1996 Mar 28;334(13):841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Holländer G. A., Simpson S. J., Mizoguchi E., Nichogiannopoulou A., She J., Gutierrez-Ramos J. C., Bhan A. K., Burakoff S. J., Wang B., Terhorst C. Severe colitis in mice with aberrant thymic selection. Immunity. 1995 Jul;3(1):27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Chen H. L., Hu X. L., Chen T. Y., Morrison D. C. Tumor necrosis factor-alpha gene expression in the tissues of normal mice. Cytokine. 1992 Sep;4(5):340–346. doi: 10.1016/1043-4666(92)90076-4. [DOI] [PubMed] [Google Scholar]
- Keshav S., Lawson L., Chung L. P., Stein M., Perry V. H., Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990 Jan 1;171(1):327–332. doi: 10.1084/jem.171.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusugami K., Matsuura T., West G. A., Youngman K. R., Rachmilewitz D., Fiocchi C. Loss of interleukin-2-producing intestinal CD4+ T cells in inflammatory bowel disease. Gastroenterology. 1991 Dec;101(6):1594–1605. doi: 10.1016/0016-5085(91)90397-4. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Kündig T. M., Schorle H., Bachmann M. F., Hengartner H., Zinkernagel R. M., Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993 Nov 12;262(5136):1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- Ma A., Datta M., Margosian E., Chen J., Horak I. T cells, but not B cells, are required for bowel inflammation in interleukin 2-deficient mice. J Exp Med. 1995 Nov 1;182(5):1567–1572. doi: 10.1084/jem.182.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Stenson W. F. Alterations of the immune system in ulcerative colitis and Crohn's disease. Adv Immunol. 1988;42:285–328. doi: 10.1016/s0065-2776(08)60848-2. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. Gastrointestinal inflammation. Inflammatory bowel disease in knockout mice. Curr Biol. 1994 Mar 1;4(3):261–263. doi: 10.1016/s0960-9822(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Mansfield J. C., Holden H., Tarlow J. K., Di Giovine F. S., McDowell T. L., Wilson A. G., Holdsworth C. D., Duff G. W. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology. 1994 Mar;106(3):637–642. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- McCabe R. P., Secrist H., Botney M., Egan M., Peters M. G. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunopathol. 1993 Jan;66(1):52–58. doi: 10.1006/clin.1993.1007. [DOI] [PubMed] [Google Scholar]
- McCall R. D., Haskill S., Zimmermann E. M., Lund P. K., Thompson R. C., Sartor R. B. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994 Apr;106(4):960–972. doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Grusby M. J., Glimcher L. H., Bhan A. K., Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Mullin G. E., Lazenby A. J., Harris M. L., Bayless T. M., James S. P. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992 May;102(5):1620–1627. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Presky D. H., Waegell W., Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996 Jun 1;183(6):2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Müller W., Blöcker H., Diamantstein T., Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (1) N Engl J Med. 1991 Sep 26;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (2) N Engl J Med. 1991 Oct 3;325(14):1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- Powrie F., Carlino J., Leach M. W., Mauze S., Coffman R. L. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996 Jun 1;183(6):2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Reinecker H. C., Loh E. Y., Ringler D. J., Mehta A., Rombeau J. L., MacDermott R. P. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995 Jan;108(1):40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Finegold M. J., Rich S. S., Harriman G. R., Srinivasan Y., Brabet P., Boulay G., Bradley A., Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995 Jun;10(2):143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Finegold M. J., Rich S. S., Harriman G. R., Srinivasan Y., Brabet P., Bradley A., Birnbaumer L. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J Clin Immunol. 1995 Nov;15(6 Suppl):101S–105S. doi: 10.1007/BF01540899. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Kühn R., Schorle H., Rajewsky K., Müller W., Horak I. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur J Immunol. 1994 Jan;24(1):281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Löhler J., Schorle H., Klebb G., Haber H., Sickel E., Noelle R. J., Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995 Nov;25(11):3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sanderson J. D., Hermon-Taylor J. Mycobacterial diseases of the gut: some impact from molecular biology. Gut. 1992 Feb;33(2):145–147. doi: 10.1136/gut.33.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Panzer U., Reinking R., Bouchard A., Stahl P. D., Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995 Jan;108(1):21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Sher M. E., D'Angelo A. J., Stein T. A., Bailey B., Burns G., Wise L. Cytokines in Crohn's colitis. Am J Surg. 1995 Jan;169(1):133–136. doi: 10.1016/s0002-9610(99)80121-4. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., Mizoguchi E., Allen D., Bhan A. K., Terhorst C. Evidence that CD4+, but not CD8+ T cells are responsible for murine interleukin-2-deficient colitis. Eur J Immunol. 1995 Sep;25(9):2618–2625. doi: 10.1002/eji.1830250932. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2. Curr Opin Immunol. 1992 Jun;4(3):271–276. doi: 10.1016/0952-7915(92)90076-q. [DOI] [PubMed] [Google Scholar]
- Strober W., Ehrhardt R. O. Chronic intestinal inflammation: an unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- Tahéri A., Lavagna C., Rampal P. Effet de l'interleukine 2 sur la prolifération de deux lignées cellulaires intestinales et sur l'expression du gène TGF beta et des oncogènes JUN/FOS. Gastroenterol Clin Biol. 1993;17(12):925–931. [PubMed] [Google Scholar]
- Taurog J. D., Richardson J. A., Croft J. T., Simmons W. A., Zhou M., Fernández-Sueiro J. L., Balish E., Hammer R. E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994 Dec 1;180(6):2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]