Abstract
Background—Mast cells have been shown to regulate intestinal ion transport in animal models and normal human colon but their physiological role in human intestinal inflammatory disorders is unknown. Aims—To examine mast cell regulation of ion transport in inflammatory bowel disease (IBD). Subjects and methods—Small and large intestine was obtained from patients with and without IBD undergoing surgical resection. Short circuit current (Isc) responses to rabbit antihuman IgE, histamine, and electrical stimulation were measured in Ussing chambers. Specimens were also examined for mast cell numbers and degree of inflammation. Results—Isc responses to anti-IgE and histamine were smaller in magnitude in IBD compared with non-IBD tissues. In all tissues, anti-IgE Isc responses were reduced by about 80% in chloride free buffer. The histamine H1 receptor antagonist, pyrilamine, decreased anti-IgE responses in non-IBD tissues. Greater inhibition with pyrilamine was seen in IBD small intestine but its effect was less in IBD colon. Histamine pretreatment of non-IBD control tissues reduced anti-IgE responses to levels seen in IBD colon but had no effect in small intestine. Mast cell numbers were greater in IBD compared with non-IBD small intestine while no differences were observed between the colonic groups. Isc responses to anti-IgE were not correlated with the degree of mucosal inflammation. Conclusions—This study provides further evidence that mast cells are capable of mediating alterations of ion transport in human gut but that this regulatory role may be altered in IBD. The data suggest that prior activation of mast cells with release of histamine may account for the reduced secretory response to anti-IgE observed in IBD colonic tissues.
Keywords: mast cells; intestine; ion transport; histamine; ulcerative colitis; Crohn's disease
Full Text
The Full Text of this article is available as a PDF (139.3 KB).
Figure 1 .
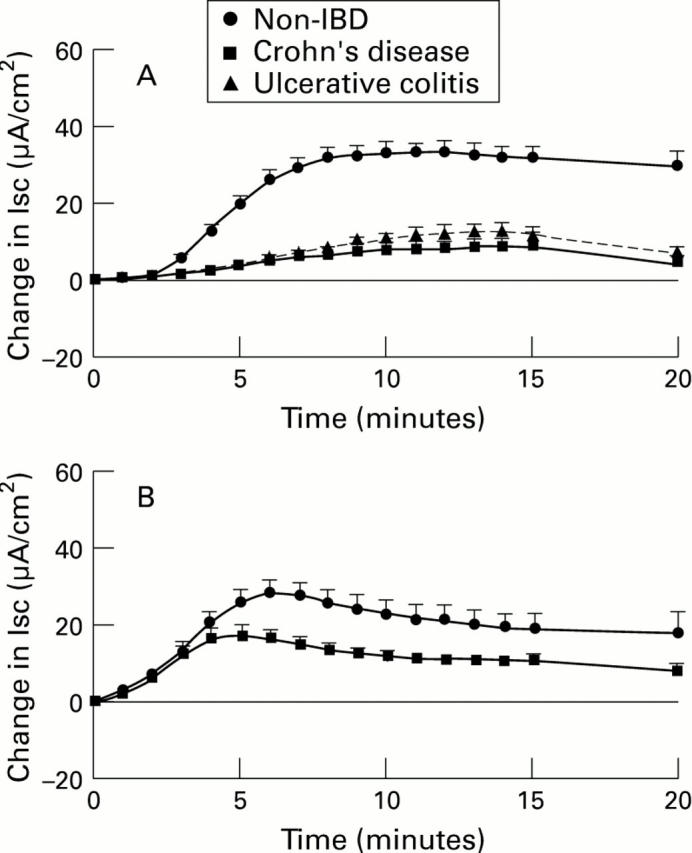
: Kinetics of Isc responses to rabbit anti-human IgE serum (10 µg/ml) added to large (A) or small bowel (B) from subjects without IBD, with Crohn's disease (CD), or with ulcerative colitis (UC). For comparison, results (in µA/cm2, mean (SEM)) are shown as if each stimulus was added at time 0. For large bowel (A), n=100 tissues from 48 subjects (non-IBD), 23 tissues from 12 subjects (CD), and 21 tissues from 9 subjects (UC). For small bowel (B), n=20 tissues from 9 subjects (non-IBD), and 68 tissues from 30 subjects (CD).
Figure 2 .
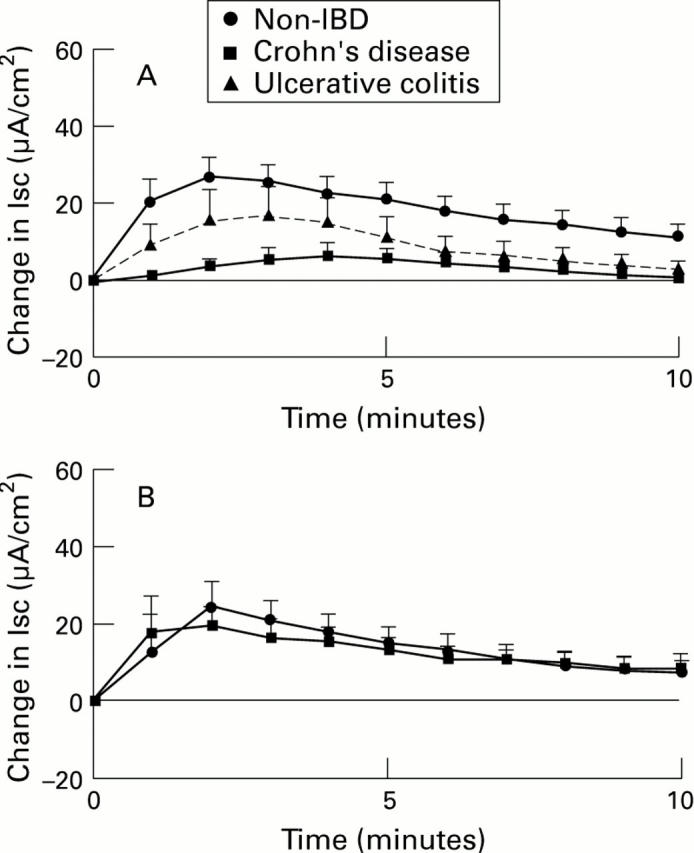
: Kinetics of Isc responses to histamine (10-4 M) added to the serosal surface of muscle stripped large (A) or small bowel (B) from subjects without IBD, with Crohn's disease (CD), or with ulcerative colitis (UC). For comparison, results (in µA/cm2, mean (SEM)) are shown as if each stimulus was added at time 0. For large bowel (A), n=33 tissues from 15 subjects (non-IBD), 4 tissues from 2 subjects (CD), and 9 tissues from 3 subjects (UC). For small bowel, n=12 tissues from 6 subjects (non-IBD), and 16 tissues from 8 subjects (CD).
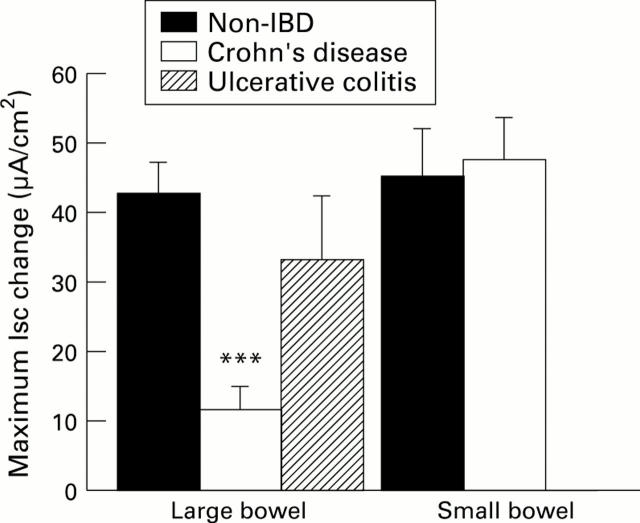
Figure 3 .
: Peak increase in Isc (µA/cm2, as mean (SEM)) to forskolin (10-5 M) compared in large and small intestine from subjects without IBD, with Crohn's disease (CD), or with ulcerative colitis (UC). The number of tissues tested for each large bowel group was 75 from 34 non-IBD subjects, 14 from 9 CD subjects, and 12 from 6 UC subjects. In small intestine, 30 tissues from 15 non-IBD subjects and 51 tissues from 24 CD subjects were used. ***p<0.001 compared with non-IBD large bowel.
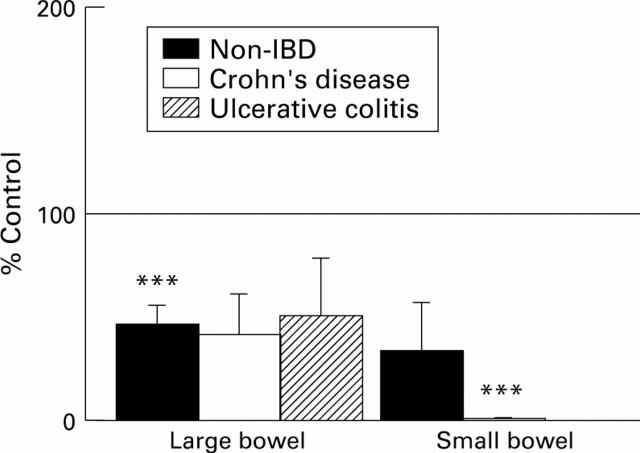
Figure 4 .
: Effect of pyrilamine (10-4 M) on the maximum Isc response to anti-IgE in large and small bowel from subjects without IBD, with Crohn's disease (CD), or with ulcerative colitis (UC). The mean Isc responses to anti-IgE (calculated on a per subject basis) in drug treated tissues are depicted as a percentage of the mean response to anti-IgE in untreated control tissues of the corresponding category. The SEM are also shown as a percentage of control results. The horizontal line indicates the control values (100%). Treatment and control groups included 6-26 tissues from 4-14 subjects per category. ***p<0.001 compared with control.
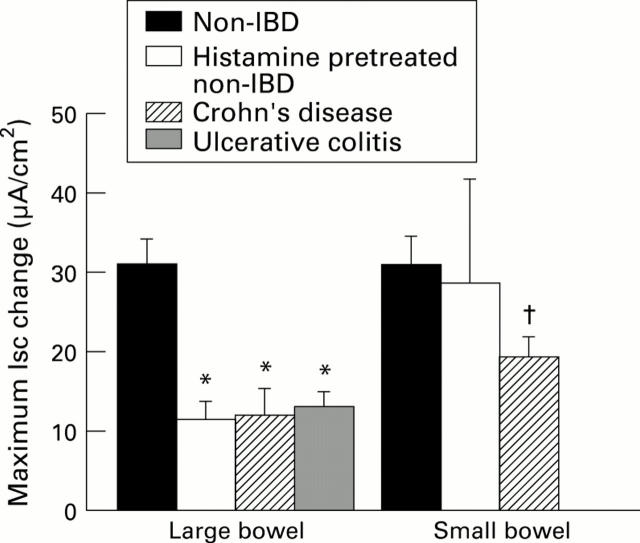
Figure 5 .
: Peak average increases in Isc (in µA/cm2, as mean (SEM)) to rabbit anti-human IgE serum (10 µg/ml) are compared in non-IBD large and small bowel pretreated with histamine 10-4 M × 2 and untreated control tissues from the same subjects as well as tissues from subjects with Crohn's disease (CD), or ulcerative colitis (UC). For large bowel, n=5 non-IBD, 15 CD, 9 UC subjects; and for small bowel, n=3 non-IBD, 30 CD subjects. *p<0.05 compared with non-IBD large bowel; †p<0.05 compared with non-IBD small bowel.
Figure 6 .
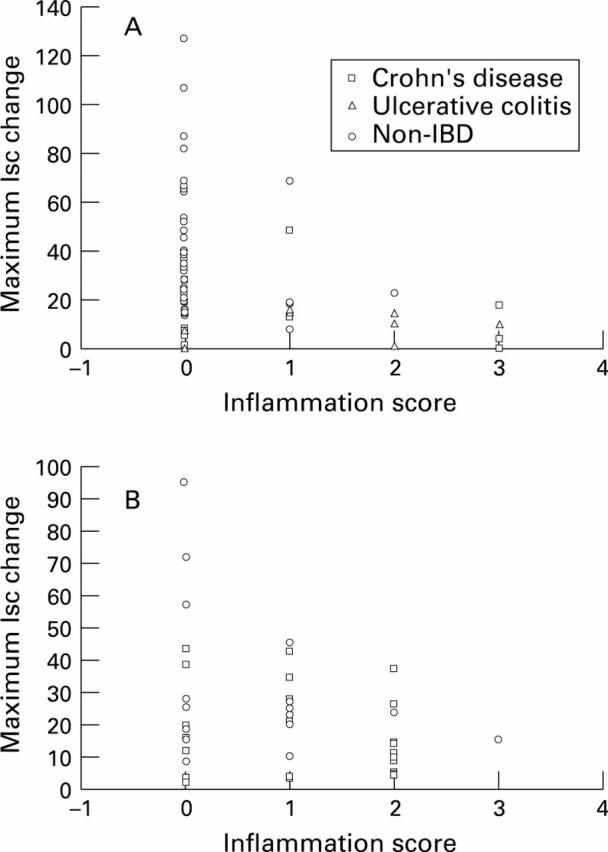
: Comparison of individual mucosal inflammation scores and the corresponding mean Isc response to anti-IgE in large (A) and small (B) bowel categorised according to tissue group. Mean Isc values (in µA/cm2) are plotted against the inflammation score for each patient. No significant differences were detected by Pearson's correlational analysis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archampong E. Q., Harris J., Clark C. G. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut. 1972 Nov;13(11):880–886. doi: 10.1136/gut.13.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs M., Illyés G., Vadász G. Mast cells in ulcerative colitis. Quantitative and ultrastructural studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(6):353–360. doi: 10.1007/BF02899101. [DOI] [PubMed] [Google Scholar]
- Barrett K. E. Immune-related intestinal chloride secretion. III. Acute and chronic effects of mast cell mediators on chloride secretion by a human colonic epithelial cell line. J Immunol. 1991 Aug 1;147(3):959–964. [PubMed] [Google Scholar]
- Befus A. D., Dyck N., Goodacre R., Bienenstock J. Mast cells from the human intestinal lamina propria. Isolation, histochemical subtypes, and functional characterization. J Immunol. 1987 Apr 15;138(8):2604–2610. [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S. C., Schwengberg S., Wordelmann K., Weimann A., Raab R., Manns M. P. Effect of c-kit ligand, stem cell factor, on mediator release by human intestinal mast cells isolated from patients with inflammatory bowel disease and controls. Gut. 1996 Jan;38(1):104–114. doi: 10.1136/gut.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff S. C., Wedemeyer J., Herrmann A., Meier P. N., Trautwein C., Cetin Y., Maschek H., Stolte M., Gebel M., Manns M. P. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996 Jan;28(1):1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Parkos C. A., Matthews J. B., D'Andrea L., Awtrey C. S., Lichtman A. H., Delp-Archer C., Madara J. L. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994 Aug;267(2 Pt 1):C402–C410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Resnick M. B., Parkos C. A., Delp-Archer C., McGuirk D., Bacarra A. E., Weller P. F., Madara J. L. IL-4 directly modulates function of a model human intestinal epithelium. J Immunol. 1994 Sep 1;153(5):2122–2129. [PubMed] [Google Scholar]
- Crowe S. E., Perdue M. H. Anti-immunoglobulin E-stimulated ion transport in human large and small intestine. Gastroenterology. 1993 Sep;105(3):764–772. doi: 10.1016/0016-5085(93)90894-i. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Perdue M. H. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology. 1992 Sep;103(3):1075–1095. doi: 10.1016/0016-5085(92)90047-3. [DOI] [PubMed] [Google Scholar]
- Crowe S. E., Sestini P., Perdue M. H. Allergic reactions of rat jejunal mucosa. Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology. 1990 Jul;99(1):74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Monahan R. A., Osage J. E., Dickersin G. R. Mast-cell degranulation in Crohn's disease. Lancet. 1978 Mar 4;1(8062):498–498. doi: 10.1016/s0140-6736(78)90155-1. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Lazenby A. J., Moore W. C., Yardley J. H., Bayless T. M., Lichtenstein L. M. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology. 1990 Jul;99(1):119–124. doi: 10.1016/0016-5085(90)91238-2. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Lichtenstein L. M., Roche J. K. Intestinal mast cell responses in idiopathic inflammatory bowel disease. Histamine release from human intestinal mast cells in response to gut epithelial proteins. Dig Dis Sci. 1993 Jun;38(6):1105–1112. doi: 10.1007/BF01295728. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Hawker P. C., McKay J. S., Turnberg L. A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980 Sep;79(3):508–511. [PubMed] [Google Scholar]
- Jenkins H. R., Milla P. J. The effect of colitis on large-intestinal electrolyte transport in early childhood. J Pediatr Gastroenterol Nutr. 1993 May;16(4):402–405. doi: 10.1097/00005176-199305000-00010. [DOI] [PubMed] [Google Scholar]
- Keren D. F., Appelman H. D., Dobbins W. O., 3rd, Wells J. J., Whisenant B., Foley J., Dieterle R., Geisinger K. Correlation of histopathologic evidence of disease activity with the presence of immunoglobulin-containing cells in the colons of patients with inflammatory bowel disease. Hum Pathol. 1984 Aug;15(8):757–763. doi: 10.1016/s0046-8177(84)80167-7. [DOI] [PubMed] [Google Scholar]
- King T., Biddle W., Bhatia P., Moore J., Miner P. B., Jr Colonic mucosal mast cell distribution at line of demarcation of active ulcerative colitis. Dig Dis Sci. 1992 Apr;37(4):490–495. doi: 10.1007/BF01307568. [DOI] [PubMed] [Google Scholar]
- Knutson L., Ahrenstedt O., Odlind B., Hällgren R. The jejunal secretion of histamine is increased in active Crohn's disease. Gastroenterology. 1990 Apr;98(4):849–854. doi: 10.1016/0016-5085(90)90006-m. [DOI] [PubMed] [Google Scholar]
- Nolte H., Spjeldnaes N., Kruse A., Windelborg B. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut. 1990 Jul;31(7):791–794. doi: 10.1136/gut.31.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. H., Masson S., Wershil B. K., Galli S. J. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991 Feb;87(2):687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampton D. S., Murdoch R. D., Sladen G. E. Rectal mucosal histamine release in ulcerative colitis. Clin Sci (Lond) 1980 Nov;59(5):389–391. doi: 10.1042/cs0590389. [DOI] [PubMed] [Google Scholar]
- Ranlöv P., Nielsen M. H., Wanstrup J. Ultrastructure of the ileum in Crohn's disease. Immune lesions and mastocytosis. Scand J Gastroenterol. 1972;7(5):471–476. doi: 10.3109/00365527209180772. [DOI] [PubMed] [Google Scholar]
- Rao S. N. Mast cells as a component of the granuloma in Crohn's disease. J Pathol. 1973 Jan;109(1):79–82. doi: 10.1002/path.1711090109. [DOI] [PubMed] [Google Scholar]
- Sanderson I. R., Leung K. B., Pearce F. L., Walker-Smith J. A. Lamina propria mast cells in biopsies from children with Crohn's disease. J Clin Pathol. 1986 Mar;39(3):279–283. doi: 10.1136/jcp.39.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle G. I., Higgs N., Crowe P., Marsh M. N., Venkatesan S., Peters T. J. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990 Jul;99(1):97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- Serafin W. E., Austen K. F. Mediators of immediate hypersensitivity reactions. N Engl J Med. 1987 Jul 2;317(1):30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- Sommers S. C. Mast cells and paneth cells in ulcerative colitis. Gastroenterology. 1966 Nov;51(5):841–850. [PubMed] [Google Scholar]
- Stack W. A., Keely S. J., O'Donoghue D. P., Baird A. W. Immune regulation of human colonic electrolyte transport in vitro. Gut. 1995 Mar;36(3):395–400. doi: 10.1136/gut.36.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeze H. J., Sinaasappel M., Bijman J., Bouquet J., de Jonge H. R. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology. 1991 Aug;101(2):398–403. doi: 10.1016/0016-5085(91)90017-f. [DOI] [PubMed] [Google Scholar]