MYELOPROLIFERATIVE DISORDERS
The myeloproliferative disorders (MPD) are a group of haematological conditions where there is a primary disorder at the level of the multi-potent haematopoietic stem cell leading to increased production in one or more blood cell types. The three main disorders in the group are polycythaemia vera (PV), essential thrombocythaemia (ET) and idiopathic myelofibrosis (IMF). PV is characterised by an increase in red cells, white cells and platelets and clinically a plethoric appearance, itch and splenomegaly. The disease can be complicated by thromboembolic phenomena and haemorrhage and in the end stages can progress to myelofibrosis and acute leukaemia. ET is characterised by an increased platelet count. Clinically it is frequently asymptomatic but the thromboembolic events may lead to disease detection. There is a small propensity to progress to myelofibrosis and acute leukaemia which may be influenced by the treatment modalities used. IMF is defined by a leukoerythroblastic blood picture, splenomegaly and bone marrow fibrosis. The blood picture includes anaemia, thrombocythaemia or thrombocytopenia and variable white cell counts. The disease frequently progresses inexorably to transfusion dependent anaemia, symptomatic splenomegaly and transformation to acute leukaemia.
A number of different biological phenomena have been described in haematopoietic cells from PV patients and other MPDs, the majority of which involve dysregulation of key signalling mediators. The key molecular events in the pathogenesis of these disorders have been poorly defined to date, except in the case of Chronic Myeloid Leukaemia (CML) with the associated characteristic chromosomal translocation ‘the Philadelphia chromosome’ and associated rearranged gene BCR – ABL.
PV progenitor cells have been shown to grow in the absence of added erythropoietin, so called endogenous erythroid colony (EEC) formation 1 and to be hypersensitive to a variety of other cytokines including insulin-like growth factor-1.2 EEC formation however is not specific to PV and is also identified in other MPDs. Other properties include increased expression of the inhibitor of apoptosis Bcl-xL in the absence of Epo in PV erythroid cells suggesting that deregulated expression of Bcl-xL may contribute to the erythropoietin dependent survival of erythroid lineage cells in PV.3 Expression of the thrombopoietin receptor, Mpl, by platelets and megakaryocytes from patients with PV has been shown to be reduced compared to normal controls.4 This again is not specific to PV however and can occur in other MPDs. RNA synthesis from the polycythaemia rubra vera 1 (PRV-1) gene has been found to be over expressed in PV granulocytes.5 Erythroid colonies from PV patients have been shown to contain a hyperactive membrane-associated tyrosine phosphatase PTP-MEG2, although the exact role in erythrogenesis requires further investigation.6
Under normal conditions binding of the Epo receptor by its ligand induces rapid phosphorylation of Akt and subsequent stimulation of survival pathways for erythroid colonies. Erythroid cells derived from individuals with PV have been shown to demonstrate increased phosphorylation of Akt/PKb and also Glycogen synthase Kinase 3.7 This contributes to inherent survival properties of the erythroid cells. Microarray analysis has identified candidate genes involved in the pathophysiology of PV including the transcription factor NF-E2 (Nuclear Factor (Erythroid-derived 2)). This has been shown to be over expressed in the bone marrow megakaryocytic, erythroid and myeloid precursors of PV subjects.8
Over 50 years ago Dameshek 9 linked together the recognised disorders PV, chronic myeloid leukaemia and IMF and speculated on the common myelostimulatory factors. In the early 1970s chronic myeloid leukaemia was separated as a distinct clonal disorder defined by a single chromosomal and latterly gene rearrangement (BCR/ABL).10 Until very recently the other MPDs continue to be separated and diagnosed on the basis of their clinical and laboratory findings (Table I). However, recent molecular findings in the JAK2 gene are common to all these disorders.
Table I.
Diagnostic criteria for common myeloproliferative disorders
| Polycythaemia Vera | Essential Thrombocythaemia | Idiopathic Myelofibrosis | |
|---|---|---|---|
| A1 Raised Red Cell mass (>25% above mean predicted value) or Hct ≥ 0.6 males; ≥ 0.56 females | A2 Absence of cause for secondary erythrocytosis | 1. Platelet Count > 600 × 109/l* | NECESSARY CRITERIA |
| A) Diffuse Bone Marrow Fibrosis | |||
| B) Absence of Philadelphia Chromosome or BCR-ABL transcript in peripheral blood cells. | |||
| A3 Palpable splenomegaly | A4 Clonality marker i.e acquired abnormal marrow karyotype | 2. No evidence of overt polycythaemia/polycythaemia masked by co-existing Iron deficiency | OPTIONAL CRITERIA |
| (i) Splenomegaly of any grade | |||
| (ii) Anisopoikilocytosis with tear drop erythrocytes | |||
| B1 Thrombocytosis (platelet count > 400 × 109/l) | B2 Neutrophil Leucocytosis (neutrophil count >10 × 109 in non smokers; >12.5 × 109 in smokers) | 3. Absence of a Philadelphia chromosome | (iii) Presence of circulating immature myeloid cells |
| 4. Absence of peripheral blood and/or marrow appearances of myelodysplasia or myelofibrosis | (iv) Presence of circulating erythroblasts | ||
| B3 Splenomegaly (demonstrated on isotope/ultrasound scanning) | B4 Characteristic BFUE growth or reduced serum erythropoietin | 5. No known cause of reactive thrombocytosis. Care should be taken to exclude iron deficiency in premenopausal women | (v) Presence of clusters of megakaryoblasts and anomalous megakaryocytes in bone marrow sections. |
| (vi) Myeloid metaplasia | |||
| Diagnosis | * In asymptomatic patients the platelet count should be observed for a period. | Diagnosis of IMF if: | |
| A1+A2+A3 or A4 establishes PV A1+A2+any 2 B Criteria establishes PV | Diagnosis is made if all above 5 criteria are met. | The two necessary criteria (designated A and B) are present with optional criteria as follows | |
| (1) any two other features if splenomegaly present | |||
| Adapted from British Committee for Standards in Haematology Guidelines on Polycythaemia36 | Adapted from diagnostic criteria in PT-1 trial coordinated by UK MPD group38. | (2) any four optional criteria when splenomegaly absent. | |
| Adapted from Italian Consensus on Diagnostic Criteria for Myelofibrosis39. | |||
JANUS KINASE 2
The JAK2 gene was first cloned in 1989 11 and is a member of a family of four Janus kinases 1, 2 and 3 and tyrosine kinase 2.
It was originally named ‘just another kinase’ but the protein group was renamed Janus kinases after the Roman God of gates and passages. These non receptor kinases have two similar ‘active’ and ‘inactive’ domains and this is reminiscent of the God Janus who had the ability to look simultaneously in two directions.
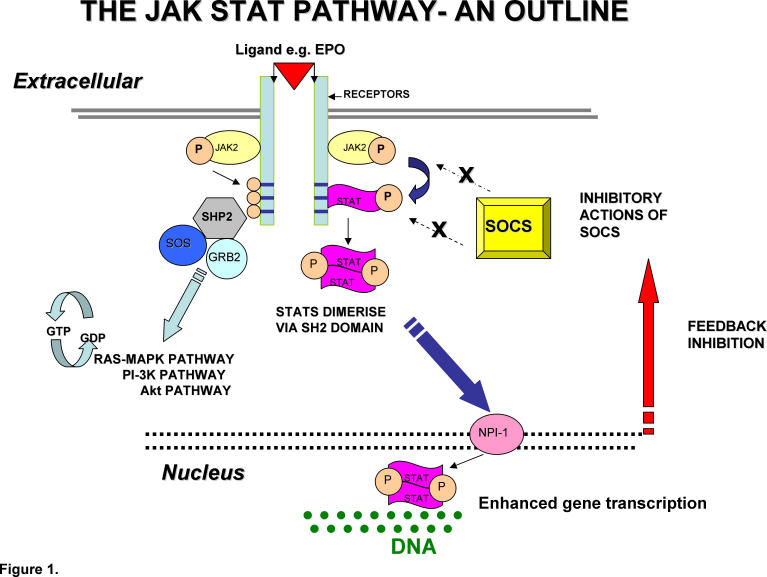
Each JAK has an active tyrosine kinase domain, JAK homology 1(JH1), a catalytically inactive pseudokinase domain, JAK homology 2 (JH2), a SRC homology 2 domain (SH2), and an amino terminal FERM (4-point-1, Erzin, Radixin, Moesin) homology domain where binding to type 1 cytokine receptors takes place. The interactions of the JAK2 FERM domain also comprises a role in trafficking of the EPO receptor (EPOR) cytoplasmic domain to the cell surface.12 Under normal physiological circumstances when a ligand (for example erythropoietin) binds with a receptor a conformational change occurs (see fig 1). The JAK2 protein then makes contact with the cytoplasmic domain of the receptor where it catalyses tyrosine phosphorylation. This primarily leads to the recruitment of STAT (signal transducer and activator of transcription) molecules which are then phosphorylated, homodimerise and translocate to the nucleus where they act as transcription factors. Tyrosine phosphorylation also modifies other key regulatory events involved in cytokine signalling pathways.
Fig 1.
Diagram illustrating Functional JAK STAT Pathway
This “JAK STAT” pathway appears to be ubiquitous amongst vertebrates. Following ligand binding the activated JAK2 protein catalyses tyrosine phosphorylation in the cytoplasmic domain of the receptor and also leads to phospharylation of the Signal Transducers and Activators of Transcription (STATS).
Phosphorylation of STAT leads to dimerisation via conserved Src homology 2 (SH2) domains. Translocation of these dimers to the nucleus then occurs facilitated via Nucleoprotein Interactor 1 (NPI-1). Subsequent regulation of gene expression following interaction with DNA response elements occurs. This leads to a transcriptional response. There is also interaction with the RAS/MAPK, Pl-3 K and Akt downstream pathways.
Under normal conditions the enhanced gene expression is under complex negative feedback mechanisms including amongst others the production of the negative regulator Suppressors of Cytokine Signalling (SOCS).
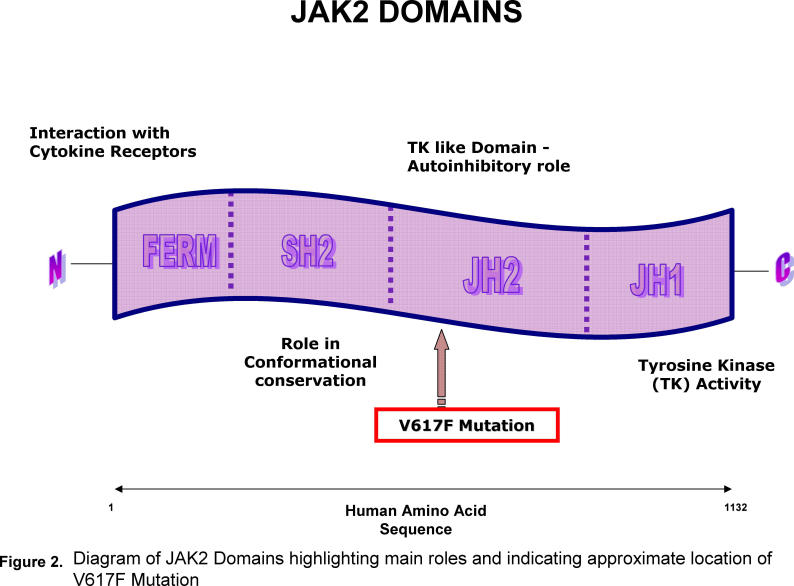
The JH2 domain is a non catalytic pseudokinase and has several crucial regulatory functions.13 It appears that in the absence of ligand binding it has autoinhibitory properties, most likely manifest by a JH2/JH1 interaction, and if an alteration in this area occurred dysregulation of this autoinhibition would result. It also appears that maximal JAK2 activity in response to cytokines requires an intact JH2 region.
In a short period in early 2005 four different groups described an identical mutation in JAK2 V617F in large numbers of patients with MPDs.14–17 Although all groups arrived at the same result they approached it by different methods. The Vainchenker group, who are acknowledged to have made the discovery first, approached it from the point of view of the underlying biology of the disease.14 They had previously observed that inhibitors of JAK2 and other kinases interfered with the erythropoietin independent differentiation in PV.18 Therefore they looked at potential mechanisms leading to the formation of EECs.
Identifying JAK2 as a potential candidate gene, and aware of its role as an upstream signalling molecule directly linked to the erythropoietin receptor, they focussed attention on this tyrosine kinase. They discovered that down regulating JAK2 expression via the introduction of short interfering RNA led to a marked inhibition of EEC formation in individuals with PV. This obviously alerted the investigators to the key role of JAK2 in the formation of EEC and prompted sequencing of the coding exons and intron – exon junctions of the gene in three patients with PV and in two normal controls. Two of the individuals with PV demonstrated the presence of the JAK2 V617F mutation. (fig 2) In a further, larger group of patients with PV the mutation was present in 88% of cases. In all of the controls, in addition to all 35 samples of patients diagnosed with a secondary erythrocytosis, only wild type JAK2 was detected.
Fig 2.
Diagram of JAK2 Domains highlighting main roles and indicating approximate location of V617F Mutation.
The mutation was shown to be acquired, as it was present in the myeloid lineage but absent in T cells. This group also identified the ability of the mutated JAK2 to spontaneously activate downstream STAT mediated transcription in the absence of the ligand erythropoietin. This is in contrast to the inability of wildtype JAK2 to mediate such events. There was also activation of the ERK/MAP kinase and P13K/AKT pathways in the absence of alternative cytokine stimulation. In conclusion, the auto-inhibitory activity of JAK2 was disrupted by the presence of this V617F mutation.
Skoda's 15 group followed on from previous work where they had identified loss of heterozygosity (LOH) on the short arm of chromosome 9 in a proportion of MPD patients via genome wide microsatellite screening. This suggested that 9p may harbour a pathogenic mutation. They initally utilised 10 microsatellite markers covering chromosme 9p and found 9p LOH in granulocytes derived from patients with MPDs in 21% (51/244) of cases and in no control cases, including CML. All 51 patients with 9p LOH possessed the JAK2 V617F mutation. Further investigation found the mutation in 65% of patients with PV of whom 27% had an acquired homozygous mutation and 38% a heterozygous mutation.
Dysregulation of key tyrosine kinases is paramount to the pathogenesis of many cancers, including CML. This prompted further in depth searches for mutations in tyrosine kinases in the conventional myeloproliferative disorders. Gilliland's group16 undertook a search for mutations of tyrosine kinases using high-throughput sequence analysis and found theJAK2 V61F mutation. As part of a large study looking at protein kinase genes in MPDs, Green's group17 found the mutation in 57% of individuals with ET, 50% of individuals with IMF and 97% of those with PV. It was detected in both granulocyte-macrophage and erythroid colonies and intriguingly was present in all EECs, demonstrating a link with growth factor hypersensitivity.
The JAK2 V61F mutation accounts for some of the abnormalities described in PV although the molecular events linking the mutation with the biological parameters require further delineation. The JH2 domain is a pseudokinase and possesses autoinhibitory properties which prevent receptor phosphorylation. From modelling studies, the highly conserved valine at position 617 is predicted to lie on the upper surface of the N-terminal lobe of the JH2 domain. Substituting the valine for a large phenlyalanine destabilises the fold of the domain.16 Therefore the presence of the mutation would lead to a JAK2 which is constitutively active. Interestingly, in heterozygotes there appears to be competition between the wild-type and mutant genes.
Haematopoietic stem cells from MPD patients are hypersensitive to a range of growth factors and use JAK2 for signalling. Observations suggest a disruption of signal transduction downstream of JAK2 including constitutive activation of STAT3,20 up-regulation of the anti-apoptotic protein Bcl-xL3 and increased AKT activity.7 Death receptor stimulated apoptotic pathways also appear disrupted in JAK2 V617F PV derived erythroblasts with deregulated expression of the short isoform of c-FLIP 21 which fundamentally plays an essential role in the normal homeostatic apoptotic cascade.
DETECTION METHODS
The JAK2 V617F mutation in MPDs can be detected by a variety of methods. The simplest method is to isolate DNA from whole blood leukocytes and use PCR-direct sequencing. However, since the mutation is acquired and restricted to the myeloid lineage this method has a sensitivity of between 20 to 30%. By implication the JAK2 V617F mutant clone would have to constitute a significant proportion of the total leukocyte population to be detectable by this method.
Ficoll gradient centrifugation can be used to isolate mononuclear cells, with subsequent separation into granulocyte/macrophage lineages and lymphocytes. Methods to ensure an absence of gross contamination of the fractions should be utilised, for example magnetic sorting/flow cytometry. Isolation of DNA from the fractions can then occur and direct sequencing methods instituted. This allows detection of an acquired JAK2 V617F mutation in cells of myeloid lineage.
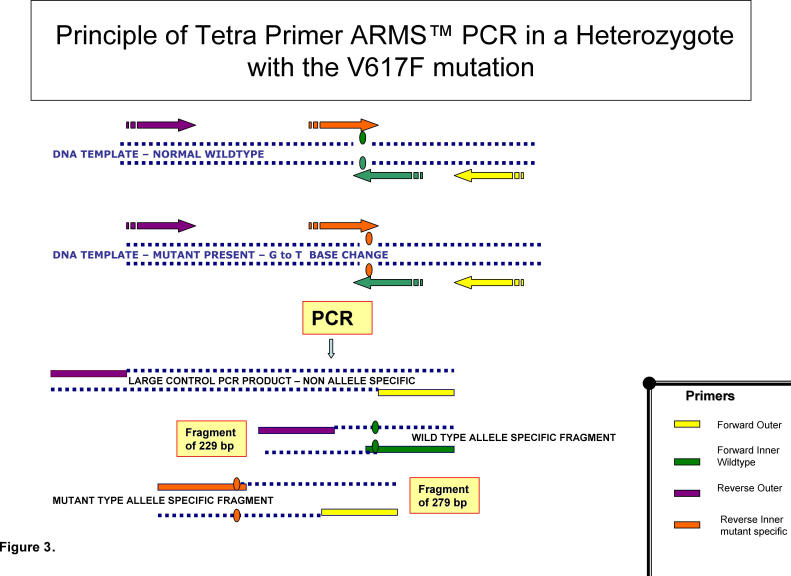
Amplification Refractory Mutation Screening (ARMS) PCR permits a single base change to be detected under ideal PCR conditions. This is ideal for detection of the single base G → T transversion associated with the JAK2 mutation in question. The ARMS-PCR technique (fig 3) uses 4 primers as follows; a forward outer primer, a reverse outer primer, a forward inner wild type specific primer and a reverse inner mutant specific primer. The forward primer from one set and the reverse from the other are able to amplify a positive control band. The other two primers span the site of the JAK2 V617F mutation. Therefore in the presence of the JAK2 mutation the reverse inner mutant specific primer and the forward outer primer bind to give a fragment of 279 bp. In the presence of wild-type JAK2 the reverse outer primer and the forward inner wild-type specific primer produce a fragment of 229bp. Performing a dilution series indicates the level of sensitivity of ARMS-PCR to be 1-2 %.22 The assay allows discrimination between homozygous and heterozygous individuals with the JAK2 V617F mutation and has a key role in acting as a reliable screening test for the presence or absence of the mutation in individuals with MPDs.
Fig 3.
- Tetra Primer Amplification Refractory Mutation Screening Polymerase Chain Reaction – ARMS-PCR-is an extremely efficient detection method of single nucleotide polymorphisms (SNPS).
- It consists of two pairs of primers – to amplify wildtype and mutant respectively.
- Two amplification allele-specific reactions occur in opposite directions, simultaneously.
- The mismatch is in the middle of the inner primers in contrast to conventional ARMS PCR.
- There is the outer forward primer, the forward inner wildtype primer, the reverse outer primer and the reverse inner mutant specific primer.
- It requires two temperature programs during the PCR reaction.
- Due to the positioning of the outer primers at varying distance from the site of the mutation there is the generation of three fragments in this example in a heterozygote: two small allele specific fragments and a large control PCR product.
- DNA Fragments can be distinguished via electrophoresis on agarose gel.
Although ARMS-PCR can indicate whether an individual is homozygous or heterozygous for the V617F JAK2 mutation it does not quantify the ratios of the wild type and mutant alleles. This also applies to PCR-direct sequencing. Pyrosequencing which uses a quantitative technique originally designed for the detection of single nucleotide polymorphisms (SNP) has been developed for the detection of the G1849T JAK2 mutation, providing accurate allele ratios.23,24 This technique is unique in that it provides a quantitative result for each allele. Pyrosequencing entails amplification by PCR of exon 12 of JAK2, with a set of primers where one is labelled with a biotin tag. Single-stranded PCR products are prepared post PCR. Any biotin tagged single stranded DNA molecules are then specifically isolated by capture with streptavidin beads and genotypes are determined using the SNP software, which allows allele frequency quantification and scoring of individuals as homozygous when the mutant allele is greater than 50%.
CLINICAL CORRELATES
Other groups have proceeded to look at their series of patients and identified similar rates of the presence of the mutation in the various disease groups (Table II).14–17,22–25 Of interest is that the mutation is found extremely rarely in those with no identified cause of erythrocytosis and screening is therefore of benefit for the detection of clonal disease.26 The presence of the JAK2 V617F mutation has been correlated with other described biological phenomena such as PRV1 expression and EEC formation.27 Patients who are JAK2 V617F mutation heterozygous or homozygous have been shown to express higher levels of NF-E2 compared to mutation negative individuals.28
Table 2.
Some reported incidences of JAK2 V61F mutation in myeloproliferative disorders
| Investigators | ||||||||
|---|---|---|---|---|---|---|---|---|
| Disorder | Green17 | Vainchenker14 | Gilliland16 | Skoda15 | Tefferi24 | Cross22 | Zhao25 | Jelinek23 |
| PV | 97% | 88% | 74% | 65% | 81% | 83% | 86% | |
| ET | 57% | 43% | 32% | 23% | 41% | 30% | ||
| IMF | 50% | 43% | 35% | 57% | 43% | 95% | ||
| SM | 25% | 0% | ||||||
| CNL | 17% | 33% | ||||||
| HES | 0% | 2% | ||||||
| UN | 20% | |||||||
| MDS | 5% | 1.5% | ||||||
| CMML | 3% | 13% |
KEY TO ABBREVIATIONS
PV: polycythemia vera, ET: essential thrombocythemia, IMF: idiopathic myelofibrosis, SM: systmemic mastocytosis, CNL: chronic neutrophilic leukemia, HES: hypereosinophilic syndrome, UN: unclassified MPD, MDS: myelodysplastic syndrome, CMML: chronic myelomonocytic leukemia.
Those who are homozygous for the mutation may have different or more advanced disease compared to those who are heterozygous. Kravolics 15 showed that those who were homozygous had a longer duration of disease and were more likely to develop secondary myelofibrosis. Tefferi 29 demonstrated that in PV those possessing the homozygous JAK2 mutation tended to have higher haemoglobin levels, an increased incidence of pruritis and higher rates of fibrotic complications.
The significance of considering cases with a very small percentage of JAK2 mutant positive clones as being “truly” JAK2 V617F positive remains unclear. Obviously one needs to consider what ‘cut off’ value we use to distinguish those possessing the mutation from those classified as mutation negative. This is of importance if the presence of the JAK2 V61F mutation is to become paramount within the classification system of MPDs. Familial MPDs, including polycythaemia,30 have been well documented and it appears that even in these rare cases, the JAK2 mutation appears to be somatic rather than germ-line in nature.
The impact of the mutation in IMF, from a clinical stance, appears somewhat less well defined. In a large study of myelofibrosis, Campbellet al 31 showed that patients with the JAK2 V617F mutation had higher neutrophil and white cell counts compared to patients without the mutation and overall tended to have a poorer prognosis. Tefferi et al 32 looked for the mutation in a variety of patients with myelofibrosis and found that the mutation was more likely to be present in patients with a previous history of PV compared to de novo MF. The presence of the JAK2 V617F mutation in this fairly large cohort of 157 patients did not appear to have prognostic significance.
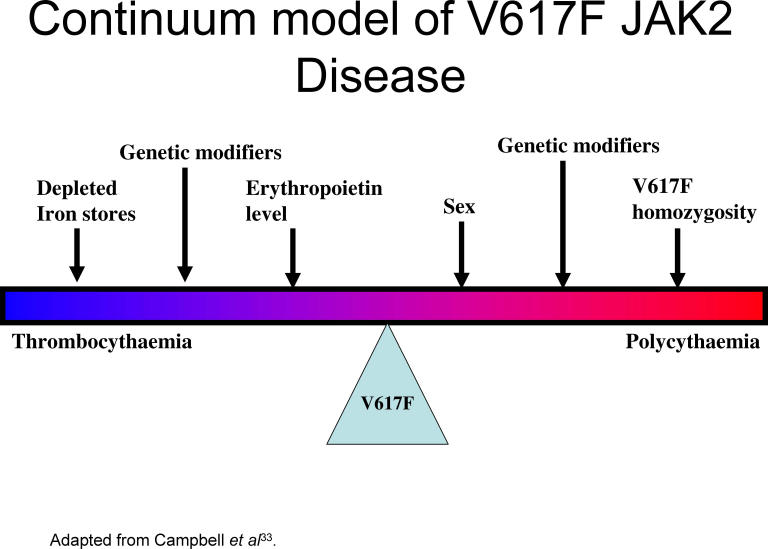
The MRC-PT-1 prospective study of ET allowed comparison of mutation positive and negative patients.33 Those with the mutation had features resembling PV, higher haemoglobin, higher neutrophil counts, more venous thrombosis and a higher rate of transformation to PV but they had lower serum erythropoietin levels and ferritin levels. JAK2 V617F positive patients appeared to more sensitive to treatment with hydroxycarbamide but not anagrelide. Another series of ET patients showed that those with the mutation were more likely to transform to PV.34 These findings call into question the separation of the diseases PV and ET. There may be a continuum of disease with the effects of the JAK2 V617F mutation on clinical presentation influenced by other modifiers including iron supply, erythropoietin suppression and other genetic modifiers (see fig 4).
Fig 4.
Continuum model of JAK2 V61F Disease.
OTHER DISEASES
The presence of the mutation has been investigated in atypical MPDs including systemic mastocytosis, hypereosinophilic syndrome, chronic neutrophilic leukaemia, unclassified myeloproliferative disorders.24 Of interest, McLornan et al 35 detected the mutation in one chronic neutrophilic leukaemia patient with an unusual protracted course where the mutation may in some way influence the course of the disease. The mutation has been found in varying proportions of patients as summarised in Table II. It has been detected rarely in patients with myelodysplastic disorders.22 The prevalence of the mutation is higher in patients with acute myeloid leukaemia (AML) with antecedent PV or IMF than in the overall cohort.36 It has been reported rarely in other series of patients with AML and no patients with lymphoid leukaemia.23,36
FURTHER QUESTIONS
The fascinating discovery of a single mutation in a wide spectrum of MPDs has lead to rapid progress in the investigation of MPDs but leads to a number of further questions. The hierarchical position of this mutation requires further study. It is still not clear whether the JAK2 V61F mutation is the primary initiating event or a secondary event with an as yet unknown primary event. While the presence of the mutation was sufficient to induce erythrocytosis in mice this is a manipulated experimental situation and it may not be sufficient to initiate disease in the human.
Classification of MPD needs revision. The presence of the mutation demonstrates a clonal disorder but splitting of diseases on the basis of clinical characteristics needs reconsideration. Questions remain about the underlying pathogenesis in those with mutation negative myeloproliferative disorders. In summary, the discovery of a single mutation JAK2 V61F in a large number of MPD patients has lead to great progress in the understanding of MPDs but leads to many more exciting biological questions.
The authors have no conflict of interest.
REFERENCES
- 1.Prchal JF, Axelrad AA. Bone marrow responses in polycythemia vera. N Engl J Med. 1974;290(24):1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 2.Correa PN, Eskinazi D, Axelradd AA. Circulating erythroid progenitors in polycythemia vera are hypersensitive to insulin-like growth factor-1 in vitro: studies in an improved serum-free medium. Blood. 1994;83(1):99–112. [PubMed] [Google Scholar]
- 3.Silva M, Richard C, Benito A, Sanz C, Olalla I, Fernandez-Luna JL. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338(9):564–71. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 4.Moliterno AR, Hankins WD, Spivak JL. Impaired expression of the thrombopoietin receptor by platelets from patients with polycythemia vera. N Engl J Med. 1998;338(9):572–80. doi: 10.1056/NEJM199802263380903. [DOI] [PubMed] [Google Scholar]
- 5.Temerinac S, Klippel S, Strunck E, Roder S, Lubbert M, Lange M, et al. Cloning of PRV-1, a novel member of the uPAR receptor superfamily, which is overexpressed in polycythemia rubra vera. Blood. 2000;95(8):2569–76. [PubMed] [Google Scholar]
- 6.Xu MJ, Sui X, Zhao R, Dai C, Krantz SB, Zhao ZJ. PTP-MEG2 is activated in polycythemia vera erythroid progenitor cells and is required for growth and expansion of erythroid cells. Blood. 2003;102(13):4354–60. doi: 10.1182/blood-2003-04-1308. [DOI] [PubMed] [Google Scholar]
- 7.Dai C, Chung IJ, Krantz SB. Increased erythropoiesis in polycythemia vera is associated with increased erythroid proliferation and increased phosphorylation of Akt/PKB. Exp Hematol. 2005;33(2):152–8. doi: 10.1016/j.exphem.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Goerttler PS, Kreutz C, Donauer J, Faller D, Maiwald T, Marz E, et al. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. Br J Haematol. 2005;129(1):138–50. doi: 10.1111/j.1365-2141.2005.05416.x. [DOI] [PubMed] [Google Scholar]
- 9.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–5. [PubMed] [Google Scholar]
- 10.Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–3. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 11.Wilks AF. Two putative protein-tyrosine kinases identified by the application of the polymerase chain reaction. Proc Natl Acad Sci. 1989;86(5):1603–7. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell. 2001;8(6):1327–38. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- 13.Saharinen P, Vihinin M, Silvennoinen O. Autoinhibition of Jak2 tyrosine kinase is dependent on specific regions in its pseudokinase domain. Mol Biol Cell. 2003;14(4):1448–59. doi: 10.1091/mbc.E02-06-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 15.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 16.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. doi: 10.1016/S0140-6736(05)71142-9. Erratum in: Lancet 2005; 366(9480)122. [DOI] [PubMed] [Google Scholar]
- 18.Ugo V, Marzac C, Teyssandier I, Larbet F, Lecluse Y, Debili N, et al. Multiple signalling pathways are involved in erythropoietin independent differentiation of erythroid progenitors in polycythemia vera. Exp Hematol. 2004;32(2):179–87. doi: 10.1016/j.exphem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp Hematol. 2002;30(3):229–36. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 20.Roder S, Steimle C, Meinhardt G, Pahl HL. STAT3 is constitutively activated in some patient with Polycythemia rubra vera. Exp Hematol. 2001;29(6):694–702. doi: 10.1016/s0301-472x(01)00637-3. [DOI] [PubMed] [Google Scholar]
- 21.Zeuner A, Pedini F, Signore M, Ruscio G, Messina C, Tafuri A, et al. Increased death receptor resistance and FLIP short expression in polycythemia vera erythroid precursor cells. Blood. 2005 Dec 29; doi: 10.1182/blood-2005-07-3037. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 23.Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, et al. JAK2 mutation 1849 G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106(10):3370–3. doi: 10.1182/blood-2005-05-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both ‘atypical’ myeloproliferative disorders and myelodysplastic syndrome. Blood. 2005;106(4):1207–9. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280(24):22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Percy MJ, Jones FG, Green AR, Reilly JT, McMullin MF. The V617F JAK2 mutation is rare in patients with idiopathic erythrocytosis. Haematologica. 2006;91(3):413–4. [PubMed] [Google Scholar]
- 27.Goerttler PS, Steimle C, Marz E, Johansson PL, Andreasson B, Greisshammer M, et al. The Jak2V617F mutation, PRV-1 overexpression and EEC formation define a similar cohort of MPD patients. Blood. 2005;106(8):2862–4. doi: 10.1182/blood-2005-04-1515. [DOI] [PubMed] [Google Scholar]
- 28.Kravolics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, et al. Altered gene expression in myeloproliferative disorders correlates with activation of signalling by the V617F mutation of JAK2. Blood. 2005;106(10):3374–6. doi: 10.1182/blood-2005-05-1889. [DOI] [PubMed] [Google Scholar]
- 29.Tefferi A, Lasho TL, Schwager SM, Strand JS, Elliott M, Mesa R, et al. The clinical phenotype of wild-type, heterozygous, and homozygous JAK2(V617F) in polycythemia vera. Cancer. 2005;106(3):631–5. doi: 10.1002/cncr.21645. [DOI] [PubMed] [Google Scholar]
- 30.Cario H, Goerttler PS, Steimle C, Levine RL, Pahl HL. The JAK2 V617F mutation is acquired secondary to the predisposing alteration in familial polycythaemia vera. Br J Haematol. 2005;130(5):800–1. doi: 10.1111/j.1365-2141.2005.05683.x. [DOI] [PubMed] [Google Scholar]
- 31.Campbell PJ, Griesshammer M, Dohner K, Dohner H, Kusec R, Hasselbalch HC, et al. The V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood. 2006;107(5):2098–100. doi: 10.1182/blood-2005-08-3395. [DOI] [PubMed] [Google Scholar]
- 32.Tefferi A, Lasho TL, Schwager SM, Steensma DP, Mesa RA, Li CY, et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br J Haematol. 2005;131(3):320–8. doi: 10.1111/j.1365-2141.2005.05776.x. [DOI] [PubMed] [Google Scholar]
- 33.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. United Kingdom Myeloproliferative Disorders Study Group; Medical Research Council Adult Leukaemia Working Party; Australasian Leukaemia and Lymphoma Group Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945–53. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 34.Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, Lee SJ, et al. JAK2V617F mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br J Haematol. 2005;131(2):208–13. doi: 10.1111/j.1365-2141.2005.05764.x. [DOI] [PubMed] [Google Scholar]
- 35.McLornan DP, Percy MJ, Jones AV, Cross NC. McMullin MF Chronic neutrophilic leukemia with an associated V617F JAK2 tyrosine kinase mutation. Haematologica. 2005;90(12):1696–7. [PubMed] [Google Scholar]
- 36.Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukaemia and acute myeloid leukaemia, but not in acute lymphoblastic leukemia or chronic leukemia. Blood. 2005;106(10):3377–9. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMullin MF, Bareford D, Campbell P, Green AR, Harrison C, Hunt B, et al. Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br J Haematol. 2005;130(2):174–95. doi: 10.1111/j.1365-2141.2005.05535.x. [DOI] [PubMed] [Google Scholar]
- 38.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythaemia. N Engl J Med. 2005;353(1):33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 39.Barosi G, Ambrosetti A, Finelli C, Grossi A, Leoni P, Liberato NL, et al. The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol. 1999;104(4):730–7. doi: 10.1046/j.1365-2141.1999.01262.x. [DOI] [PubMed] [Google Scholar]