Abstract
HOX genes are evolutionarily highly conserved. The HOX proteins which they encode are master regulators of embryonic development and continue to be expressed throughout postnatal life. The 39 human HOX genes are located in four clusters (A-D) on different chromosomes at 7p15, 17q21.2, 12q13, and 2q31 respectively and are assumed to have arisen by duplication and divergence from a primordial homeobox gene. Disorders of limb formation, such as hand-foot-genital syndrome, have been traced to mutations in HOXA13 and HOXD13. Evolutionary conservation provides unlimited scope for experimental investigation of the functional control of the Hox gene network which is providing important insights into human disease. Chromosomal translocations involving the MLL gene, the human homologue of the Drosophila gene trithorax, create fusion genes which exhibit gain of function and are associated with aggressive leukaemias in both adults and children. To date 39 partner genes for MLL have been cloned from patients with leukaemia. Models based on specific translocations of MLL and individual HOX genes are now the subject of intense research aimed at understanding the molecular programs involved, and ultimately the design of chemotherapeutic agents for leukaemia. Investigation of the role of HOX genes in cancer has led to the concept that oncology may recapitulate ontology, a challenging postulate for experimentalists in view of the functional redundancy implicit in the HOX gene network.
INTRODUCTION
It is a fascinating thought that the single cell zygote contains all the information required for the development of the adult organism. Understanding how this information is encoded and deciphered is a major uncompleted scientific challenge. A group of genes known as homeobox genes has emerged as important master regulators of development. These genes have been highly conserved throughout evolution. They are expressed during embryonic development in a highly co-ordinated manner and continue to be expressed in virtually all tissues and organs throughout adult life.
Homeobox (Hox) genes were discovered following the observation of two striking mutations in the fruit fly, Drosophila melanogaster. In the antennapedia mutation the antennae are changed into legs, whereas in the bithorax mutation, the haltere (a balancing organ on the third thoracic segment) is transformed into part of a wing. These changes were described as homeotic transformations from the Greek word homeosis, signifying a change of a complete body structure into another. Drosophila geneticists devised the term ‘homeotic selector gene’ to encapsulate the concept that a master regulatory gene could control the development of each segment of the fly. Subsequently Drosophila was found to contain a cluster of genes consisting of the bithorax complex with three homeobox genes (Ubx, Abd-A, and Abd-B) and the antennapedia complex with five homeobox genes (Lab, Pb, Dfd, Scr and Antp). The relationship between the chromosomal arrangement of Hox genes and the localisation of their expression was established by Lewis in 1978. In effect, these genes specify positional identity of the body segments of the fly along the anterior-posterior axis.1
EVOLUTION OF HOX GENES
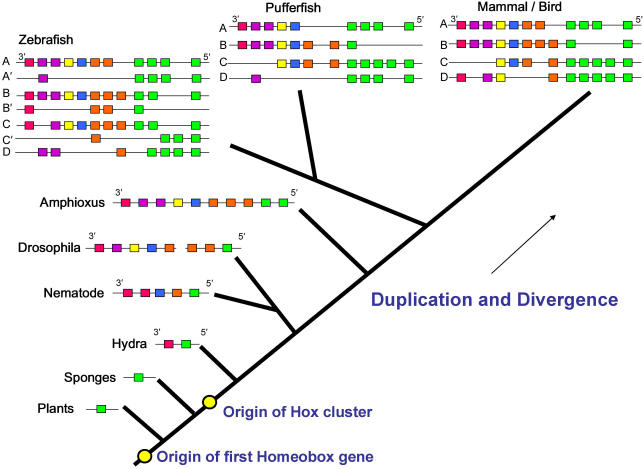
Homeobox genes are present in the genomes of all animals which have so far been mapped as well as in the genomes of plants and fungi, indicating that the origins are ancient and precede the divergence of these kingdoms. Plants, fungi and unicellular animals do not, however, have clustered homeobox genes. Shortly after the origins of animals the primordial homeobox gene duplicated to form a protohox cluster of two genes which are still present in cnidara such as hydra (Figure 1). Sponges do not have clustered homeobox genes, suggesting that this duplication occurred before the divergence of the parazoa. This is also reflective of the very simple body structure of sponges compared to other multicellular animals.
Fig 1.
A representative dendrogram illustrating the evolution of Hox clusters. Hox gene clusters are thought to have developed by a process of duplication and divergence from a primordial homeobox gene estimated to have arisen about 1,000 million years ago.
The nematode Caenorhabditis elegans has a single cluster of at least five homeobox genes.2 Amphioxus is a vertebrate-like chordate which has a notochord and segmental muscles derived from somites but does not develop a true vertebral column. It has only one Hox cluster which contains ten Hox genes and this cluster is regarded as being homologous to the ancestral cluster from which all vertebrate Hox clusters were derived. Two duplication events, early in vertebrate evolution, resulted in the four clusters seen in mammals and birds. Loss of some of the Hox genes in each cluster has also occurred with the result that not every primordial vertebral Hox gene is represented in each of the four clusters. Interestingly in some fish, such as zebrafish, a further duplication has occurred resulting in seven clusters.3
Hox GENES IN VERTEBRATES
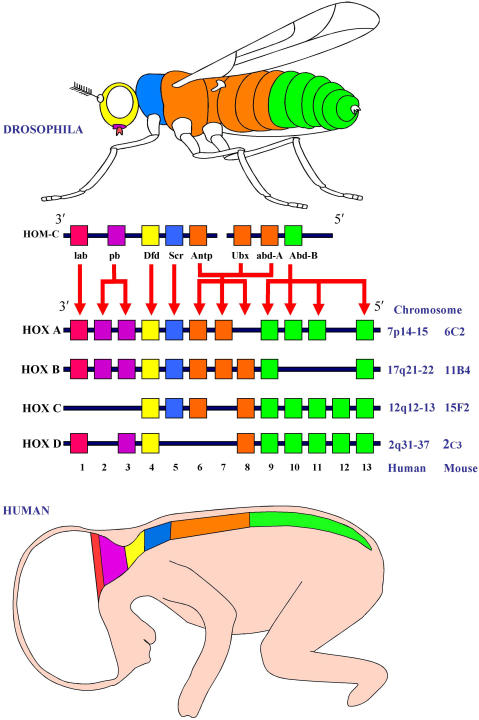
The vertebrate counterparts of the bithorax/antennapedia cluster are the Hox genes, usually found in four clusters (reviewed by Duboule 4). In man the four HOX gene clusters (A-D) are located on different chromosomes, at 7p15, 17q21.2, 12q13, and 2q31. Each cluster consists of 13 paralog groups with nine to eleven members assigned on the basis of sequence similarity and relative position within the cluster. A high degree of homology is evident between the human HOX genes and the Hom-C genes of Drosophila, (Figure 2). Thus the human paralog groups 1–8 are more closely related to antennapedia (Antp), with groups 9–13 more closely related to abdominal-B (abd-B).
Fig 2.
Conservation between the HOM-C and HOX gene clusters.
The four Hox gene clusters found in mammals are conserved from the Drosophila Hom-C complex in terms of nucleotide sequence and colinear expression. During embryonic development, the genes are expressed in a pattern that correlates with the chromosomal positioning, depicted here for human and mouse. The 3′ genes are expressed both earlier and more anteriorly than the 5′ genes.
Hox STRUCTURE
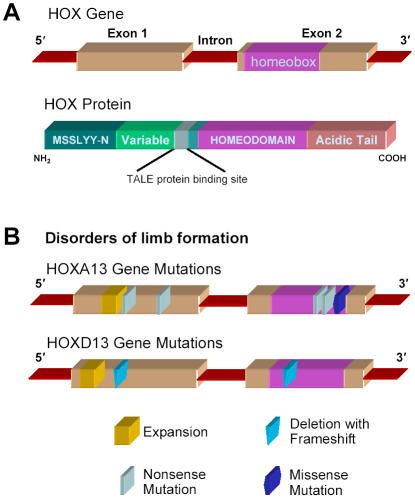
Mammalian Hox genes are small, each containing only two exons and a single intron which varies from less than 200 bases to several kilobases (Figure 3). The homeobox is always present within the second exon in Hox genes and shows a high degree of homology among these genes, especially within paralog groups. The structures of non-Hox homeobox genes are more variable, frequently having the homeobox bridging an exon splicing site.
Fig 3.
HOX gene/protein structure and mutations found in limb malformation.
(A) HOX genes consist of two exons and one intron. Exon 2 contains a 180-nucleotide sequence, termed the homeobox, that encodes a 60-amino acid helix-turn-helix motif, termed the homeodomain, which has DNA-binding activity.
(B) Mutations in HOXA13 and HOXD13 are found in disorders of limb formation, such as hand–foot–genital syndrome (HFGS), synpolydactyly (SPD), and brachydactyly.
Hox proteins have an acidic tail at the C-terminus and a pentamer upstream of the homeodomain that binds the TALE (three amino acid loop extension) proteins which act as cofactors. The homeodomain is a highly conserved motif of 60 amino acids. The function of the homeodomain was suggested by its similarity to the sequence of several prokaryotic gene regulatory proteins which contain a helix-turn-helix DNA binding motif. The homeodomain can be divided into three helical regions. Helix 3 contacts the major groove of DNA while helices 1 and 2 lie above the DNA.5 Further contact of the homeodomain to the DNA is made by the sequence which proceeds helix 1, the N-terminal arm. The binding of Hox cofactors, (exd in Drosophila, Meis or Pbx in mammals) increases the stability of Hox-DNA binding.
Hox GENES AND DEVELOPMENT
The order of expression of HOX genes within a cluster is co-ordinated during development, so that the low number, 3′ genes, are expressed more anteriorly and earlier than the high number, 5′ genes. During embryogenesis, cells require positional information to ensure that uncommitted cells differentiate into tissue appropriate for its location within the developing embryo. Thus groups of cells, known as functional domains, become committed to form body structures such as limbs and organs. There is growing evidence that it is the combination of Hox genes expressed within the functional domains along the AP axis which results in specifying the development of structures within these domains. The possible mechanisms by which this occurs have been reviewed by Kmita and Duboule.6 In both Drosophila and man the spatial patterning corresponds to the relative position on the chromosome, thereby conforming to the “principle of colinearity”.
In the developing vertebrate Hox genes are first expressed during early gastrulation at a stage when the embryo generates its major body axis.7 In a pattern which correlates with the spatial expression of Hox genes, 3′ genes are expressed earlier than 5′ and as the embryo develops more progressively 5′ genes are expressed. This pattern is termed “temporal colinearity” and is evident in other models of development such as haematopoiesis.
HOX GENES AND LIMB DEVELOPMENT
Hox genes define patterns of development in vertebrate limbs. In the chick, at least 23 Hox genes are expressed during limb development, with Hoxa9 expressed in the proximal part of the limbs where the humerus or femur develop. Hoxa9, Hoxa10 and Hoxa11 are expressed in the forelimb where the radius and ulna (or tibia and fibula) develop. Hoxa9 to Hoxa13 are expressed in the wrist (or ankle) and the digits. A similar pattern of expression was found for the Hoxd genes whereas the expression of the Hoxc cluster was more complex. These observations illustrate that complicated networks of gene expression are involved in organ development, and suggest that functional redundancy among the Hox genes may mask the effects of under-expression or mutations in individual Hox genes. However a number of abnormalities in human limb formation have been described and recently these have been linked to specific Hox genes.
Synpolydactyly (SPD), a rare, dominantly inherited limb malformation with a distinctive combination of syndactyly (fusion of digits) and polydactyly (extra digits), is caused by mutations in HOXD13. SPD typically consists of 3/4-finger and 4/5-toe syndactyly, with a duplicated digit in the syndactylous web. Affected family members often show variable expression of the disorder due to incomplete penetrance. The molecular basis of SPD was identified during a study of affected individuals in an isolated Turkish village.8 The SPD locus was mapped to chromosome 2q31, where the HOXD gene cluster is located.9 In normal individuals exon 1 of HOXD13 contains an imperfect trinucleotide repeat sequence encoding a 15-residue polyalanine tract, and in subsequent studies each affected family displayed an expansion of this repeat, resulting in 7, 8 or 10 additional residues being expressed, see Figure 3B.
Brachydactyly, in which there is shortening of the digits, is rare in patients who are homozygous for SPD.10 Two patients out of 128 screened for unselected congenital limb abnormalities requiring reconstructive surgery, were found to have a novel mutation within the HOXD13 homeodomain (Ile314Leu). In further investigations specific mutations in HOXD13 were linked with different combinations of limb disorders.11
Hypodactyly, a semi-dominant syndrome of loss of digit development, has been studied in mice. Animals with homozygous hypodactyly have a profound deficit in digital arch formation associated with a deletion in exon 1 in Hoxa13.12 This leads to a translational frame-shift resulting in the loss of wild-type Hoxa13 protein and the production of a novel, stable protein in the limb buds of mutant mice. Mortlock and Innis have linked hypodactyly to a strikingly similar human disorder – hand-foot-genital syndrome (HFGS), which differs from SPD because the deformities of the hands and feet are fully penetrant, bilateral and symmetrical, and uniform in their severity.13
The first HOXA13 mutation associated with HFGS was a nonsense mutation in exon 2 which leads to the conversion of a tryptophan residue in the homeodomain to a stop codon, truncating the protein by 20 amino acids.13 Some patients with HFGS also harbour expansions of the polyalanine tract of HOXA13, similar to those found in HOXD13 of SPD.14,15 A missense mutation in exon 2 is associated with an exceptionally severe form of HFGS.14 Two unrelated boys had deletions at 2q24.1-q31 and 2q31.1-q32.2, regions that include HOXD3 and HOXD13, associated with severe limb and genital abnormalities.16 Other patients, in whom the entire HOXD cluster is deleted, have a mild SPD phenotype attesting to the inherent redundancy in the HOX gene network.
HOX GENES AND LUNG DEVELOPMENT
Lung development is dependent upon the coordinated expression of a large number of genes in a manner tightly controlled both in time and space. Expression studies in fetal human and rodent lungs have demonstrated high expression of 3′ Hox genes in clusters A and B.17, 18 There is a marked decrease in expression of most of these genes as lung development progresses suggesting that they are involved in the early stages of lung morphogenesis, such as airway branching. However some Hox genes, for example Hoxa5, continue to be expressed at high levels throughout development and may be required for pulmonary maturation.19
Abnormal expression of HOX genes is associated with several congenital lung abnormalities e.g. HOXB5 is over-expressed in both bronchopulmonary sequestration 20 and congenital cystic adenomatoid malformation.18 These disorders are characterised by deregulated patterns of morphogenesis in primordial lung tissue. Persistent high levels of HOXB5 expression, beyond the early stages of lung development, result in primitive lung morphology. Altered patterns of HOX gene expression have also been demonstrated in several acquired disorders including emphysema, primary pulmonary hypertension and lung carcinomas.21,22
Murine models in which Hox gene expression has either been reduced or deleted provide strong evidence for the role of these genes in structural development of the respiratory system and regulation of pulmonary surfactant production. The degree of branching morphogenesis is decreased following reduction in Hoxb5 levels by antisense oligonucleotides.23 Furthermore, Hoxa5 knock-out mice develop to full term but die in the early neonatal period due to tracheal occlusion, reduced expression of surfactant proteins and lung pathology similar to surfactant-deficient respiratory distress syndrome in preterm human neonates.24
HOX GENES AND LEUKAEMIA
Multiple HOX genes of clusters A, B and C, but not D, are expressed in haematopoietic stem cells. Down-regulation or many HOX genes occurs as cells within a given lineage differentiate. For example, Care et al. demonstrated that peripheral T lymphocytes which were stimulated to proliferate using phytohaemagglutinin showed a rapid induction wave of Hox genes from Hoxb1 to Hoxb9, i.e. in the 3′ to 5′ direction.25
Perturbation of the process of cell differentiation by reciprocal chromosomal translocations can lead to the development of leukaemia. Such translocations lead to the creation of fusion genes, and may involve individual HOX genes or regulators of HOX gene activity. Thus translocations involving t[(7;11)(p15;p15)] or t[(2;11)(q31;p15)] have been described in which the HOXA9 or HOXD13 genes, respectively, are fused with the NUP98 nucleoporin gene in rare cases of acute myeloid leukaemia (AML). More frequently rearrangements of the mixed-lineage leukaemia gene MLL1, a positive regulator of cell specific HOX gene expression, have been found associated with aggressive acute leukaemias in both children and adults. Both types of translocation lead to gain of function, affecting the normal processes of differentiation of the pluripotent stem cells or the committed lymphoid or myeloid progenitors by deregulating the HOX gene expression patterns.
Rearrangements involving MLL and its 39 partner genes identified to date, are associated with approximately 5% of patients suffering from AML and 22% of those with acute lymphoblastic leukaemia (ALL).26 To investigate the t[(11;19)(p22;q23)] translocation which gives rise to the MLL-ENL fusion protein, commonly found in infant acute leukaemias of both myeloid and lymphoid lineage, Horton et al, established a tetracycline-regulable system of MLL-ENL expression in primary haematopoietic cells.27 Utilising a real-time quantitative PCR system 28 they were able to measure the expression of all 39 murine Hox genes and showed for the first time that reduced Hox gene expression is specific to loss of MLL-ENL and is not a consequence of differentiation. They concluded that MLL-ENL is required to initiate and maintain immortalisation of myeloid progenitors and may contribute to the development of leukaemia by aberrantly sustaining the expression of a “Hox code ” consisting of Hoxa4 to Hoxa11.
HOX GENES AND CANCER
Numerous studies have been undertaken to examine the differences in HOX gene expression between normal and neoplastic tissue, but the functional relationship with the malignant phenotype has remained elusive as reviewed by Abate-Shen.29 Some investigators have explored the postulate that Hox genes expressed during embryogenesis but down-regulated during adult life are re-expressed in neoplasia-the so called “oncology recapitulates ontology” hypothesis. During embryogenesis a fine balance exists between cell proliferation and differentiation which is essential for normal development of the fetus. In contrast in cancer the balance between the two processes goes awry as reviewed by Grier et al.30
Neoplastic growth in mammary epithelial cells is associated with increased expression of human growth hormone (hGH). Utilising human mammary carcinoma cells, Zhang and colleagues found that hGH production increased the expression and transcriptional activity of HOXA1.31 Furthermore overexpression of HOXA1 in mammary carcinoma cells resulted in up-regulation of Bcl-2, an anti-apoptotic factor, and increased total cell numbers. Interestingly HOXA1 also enhanced anchorage-independent cell proliferation and caused oncogenic transformation of the cells, rendering them capable of aggressive tumour formation. Furthermore overexpression of HOXA1 abrogated the response of the mammary carcinoma cells to daunorubicin. Taken together these observations serve to exemplify the effect of overexpression of a single gene HOXA1, and indicate that changes of expression of multiple Hox genes may substantially dysregulate cellular processes in neoplasia.
Epithelial ovarian cancers (EOCs) arise from the simple epithelium lining the ovarian surface. Major EOC subtypes show morphological features that resemble müllerian duct-derived epithelia of the reproductive tract. Recently Cheng and colleagues presented strong evidence that lineage infidelity of epithelial ovarian cancers is controlled by HOXA genes that specify regional identity in the reproductive tract.32 They found that the HOX genes which normally regulate müllerian duct differentiation are not expressed in normal ovarian surface epithelium, but are expressed in EOC subtypes according to the pattern of müllerian-like differentiation of the cancers. Furthermore overexpression of Hoxa9, Hoxa10 and Hoxa11 gave rise to papillary tumours resembling serous, endometrioid-like and mucinous-like EOCs respectively. These observations support the contention that alteration of expression of genes in the HOX network that controls the patterning of the reproductive tract could explain the morphological heterogeneity of EOCs.
CONCLUSIONS
Attempts to understand the role of HOX genes in both normal and abnormal development and malignant transformation will be enhanced by the identification of their upstream regulators and downstream target genes. Whereas MLL fusion genes have provided some useful insights in the molecular mechanisms involved in leukaemogenesis much work remains to be done to identify specific gene products involved in the HOX network which might ultimately become feasible targets for therapeutic intervention. More research is also needed to explore the role of HOX genes in developmental processes.
Acknowledgments
This work was supported by the Research and Development Office of the Health and Personal Social Services in Northern Ireland, the Northern Ireland Leukaemia Research Fund and the Elimination of Leukaemia Fund. The authors have no conflict of interest.
Based on an Invited Lecture “Physiology and Pathophysiology of HOX genes in embryonic development” given by Professor Terry Lappin to the British Association for Perinatal Medicine at their Annual Scientific Meeting in Belfast on 9th September, 2005.
REFERENCES
- 1.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–70. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 2.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368(6466):32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- 3.Schilling TF, Knight RD. Origins of anteroposterior patterning and Hox gene regulation during chordate evolution. Philos Trans R Soc Lond B Biol Sci. 2001;356(1414):1599–613. doi: 10.1098/rstb.2001.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992;14(6):375–84. doi: 10.1002/bies.950140606. [DOI] [PubMed] [Google Scholar]
- 5.Lewin B. Genes VII. Oxford: Oxford University Press; 2000. Homeodomains bind related targets in DNA; pp. 660–62. [Google Scholar]
- 6.Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301(5631):331–33. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- 7.Duboule D. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development. 1994;(Suppl):135–42. [PubMed] [Google Scholar]
- 8.Sayli BS, Akarsu AN, Sayli U, Akhan O, Ceylaner S, Sarfarazi M. A large Turkish kindred with syndactyly type II (synpolydactyly). 1. Field investigation, clinical and pedigree data. J Med Genet. 1995;32(6):421–34. doi: 10.1136/jmg.32.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarfarazi M, Akarsu AN, Sayli BS. Localization of the syndactyly type II (synpolydactyly) locus to 2q31 region and identification of tight linkage to HOXD8 intragenic marker. Hum Mol Genet. 1995;4(8):1453–58. doi: 10.1093/hmg/4.8.1453. [DOI] [PubMed] [Google Scholar]
- 10.Muragaki Y, Mundlos S, Upton J, Olsen BR. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science. 1996;272(5261):548–51. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- 11.Johnson D, Kan SH, Oldridge M, Trembath RC, Roche P, Esnouf RM, Giele H, Wilkie AO. Missense mutations in the homeodomain of HOXD13 are associated with brachydactyly types D and E. Am J Hum Genet. 2003;72(4):984–97. doi: 10.1086/374721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortlock DP, Post LC, Innis JW. The molecular basis of hypodactyly (Hd): a deletion in Hoxa 13 leads to arrest of digital arch formation. Nat Genet. 1996;13(3):284–89. doi: 10.1038/ng0796-284. [DOI] [PubMed] [Google Scholar]
- 13.Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15(2):179–80. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- 14.Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, Beemer FA, Hennekam RC, Scambler PJ. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000;67(1):197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utsch B, Becker K, Brock D, Lentze MJ, Bidlingmaier F, Ludwig M. A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-foot-genital syndrome: proper function of poly (A)-harbouring transcription factors depends on a critical repeat length? Hum Genet. 2002;110(5):488–94. doi: 10.1007/s00439-002-0712-8. [DOI] [PubMed] [Google Scholar]
- 16.Del Campo M, Jones MC, Veraksa AN, Curry CJ, Jones KL, Mascarello JT, Ali-Kahn-Catts Z, Drumheller T, McGinnis W. Monodactylous limbs and abnormal genitalia are associated with hemizygosity for the human 2q31 region that includes the HOXD cluster. Am J Hum Genet. 1999;65(1):104–110. doi: 10.1086/302467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollard R, Dziadek M. Homeobox genes from clusters A and B demonstrate characteristics of temporal colinearity and differential restrictions in spatial expression domains in the branching mouse lung. Int J Dev Biol. 1997;41(5):655–66. [PubMed] [Google Scholar]
- 18.Golpon HA, Geraci MW, Moore MD, Miller HL, Miller GJ, Tuder RM, Voelkel NF. HOX genes in human lung: altered expression in primary pulmonary hypertension and emphysema. Am J Pathol. 2001;158(3):955–66. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim C, Nielsen HC. Hoxa-5 in mouse developing lung: cell-specific expression and retinoic acid regulation. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L863–71. doi: 10.1152/ajplung.2000.279.5.L863. [DOI] [PubMed] [Google Scholar]
- 20.Volpe MV, Archavachotikul K, Bhan I, Lessin MS, Nielsen HC. Association of bronchopulmonary sequestration with expression of the homeobox protein Hoxb-5. J Pediatr Surg. 2000;35(12):1817–9. doi: 10.1053/jpsu.2000.19266. [DOI] [PubMed] [Google Scholar]
- 21.Calvo R, West J, Franklin W, Erickson P, Bemis L, Li E, Helfrich B, Bunn P, Roche J, Brambilla E, Rosell R, Gemmill RM, Drabkin HA. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci USA. 2000;97(23):12776–81. doi: 10.1073/pnas.97.23.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res Part A Clin Mol Teratol. 2003;67(8):550–6. doi: 10.1002/bdra.10086. [DOI] [PubMed] [Google Scholar]
- 23.Volpe MV, Vosatka RJ, Nielsen HC. Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta. 2000;1475(3):337–45. doi: 10.1016/s0304-4165(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 24.Aubin J, Lemieux M, Tremblay M, Berard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192(2):432–45. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- 25.Care A, Testa U, Bassani A, Tritarelli E, Montesoro E, Samoggia P, Cianetti L, Peschle C. Coordinated expression and proliferative role of HOXB genes in activated adult T lymphocytes. Mol Cell Biol. 1994;14(7):4872–77. doi: 10.1128/mcb.14.7.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Braekeleer M, Morel F, Le Bris MJ, Herry A, Douet-Guilbert N. The MLL gene and translocations involving chromosomal band 11q23 in acute leukemia. Anticancer Res. 2005;25(3B):1931–44. [PubMed] [Google Scholar]
- 27.Horton SJ, Grier DG, McGonigle GJ, Thompson A, Morrow M, De Silva I, Moulding DA, Kioussis D, Lappin TR, Brady HJ, Williams O. Continuous MLL-ENL expression is necessary to establish a “Hox Code” and maintain immortalization of hematopoietic progenitor cells. Cancer Res. 2005;65(20):9245–52. doi: 10.1158/0008-5472.CAN-05-1691. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A, Quinn MF, Grimwade D, O'Neill CM, Ahmed MR, Grimes S, McMullin MF, Cotter F, Lappin TR. Global down-regulation of HOX gene expression in PML-RARalpha + acute promyelocytic leukemia identified by small-array real-time PCR. Blood. 2003;101(4):1558–65. doi: 10.1182/blood.V101.4.1558. [DOI] [PubMed] [Google Scholar]
- 29.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2(10):777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 30.Grier DG, Thompson A, Kwasniewska A, McGonigle GJ, Halliday HL, Lappin TR. The pathophysiology of HOX genes and their role in cancer. J Pathol. 2005;205(2):154–71. doi: 10.1002/path.1710. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Zhu T, Chen Y, Mertani HC, Lee KO, Lobie PE. Human growth hormone-regulated HOXA1 is a human mammary epithelial oncogene. J Biol Chem. 2003;278(9):7580–90. doi: 10.1074/jbc.M212050200. [DOI] [PubMed] [Google Scholar]
- 32.Cheng W, Liu J, Yoshida H, Rosen D, Naora H. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med. 2005;11(5):531–37. doi: 10.1038/nm1230. [DOI] [PubMed] [Google Scholar]