Abstract
Mesenchymal stem cells (MSCs) have been exploited as cellular vectors to treat a wide array of diseases but the mechanisms responsible for their therapeutic effect remain indeterminate. Previously, we reported that MSCs inhibit bleomycin (BLM)-induced inflammation and fibrosis within the lungs of mice. Interrogation of the MSC transcriptome identified interleukin 1 receptor antagonist (IL1RN) as a potential mediator of this effect. Fractionation studies indicated that MSCs are the principal source of IL1RN in murine bone marrow and that its expression is restricted to a unique subpopulation of cells. Moreover, MSC-conditioned media was shown to block proliferation of an IL-1α-dependent T cell line and inhibit production of TNF-α by activated macrophages in vitro. Studies conducted in mice revealed that MSC administration was more effective than recombinant IL1RN delivered via adenoviral infection or osmotic pumps in inhibiting BLM-induced increases in TNF-α, IL-1α, and IL1RN mRNA in lung, IL1RN protein in bronchoalveolar lavage (BAL) fluid, and trafficking of lymphocytes and neutrophils into the lung. Therefore, MSCs protect lung tissue from BLM-induced injury by blocking TNF-α and IL-1, two fundamental proinflammatory cytokines in lung. Identification of IL1RN-expressing human MSC subpopulations may provide a novel cellular vector for treating chronic inflammatory diseases in humans.
Keywords: bleomycin, inflammation
Interstitial lung diseases (ILDs) are a heterogeneous group of >150 disorders characterized by epithelial injury, fibroblast proliferation, expansion of the lung matrix, and dyspnea. Among these diseases, idiopathic pulmonary fibrosis (IPF) is the most frequent and lethal. The median survival of IPF patients is 3–5 years irrespective of whether they receive therapy (1), and recent studies suggest that its incidence and prevalence is increasing (2). Inflammatory responses in IPF are mediated by release from activated macrophages and other leukocytes of the proinflammatory cytokines IL-1 and TNF-α (3). These cytokines induce endothelial cells to express adhesion molecules and chemokines that attract other white cells from the blood to the site of injury (4, 5). IL-1 and TNF-α also stimulate proliferation of endothelial cells and fibroblasts that increase the blood supply at the site of injury and repair damage by formation of scar tissue (6).
The IL-1 family includes the structurally related proteins IL1-α, IL-1β, and interleukin 1 receptor antagonist (IL1RN) that bind to the same receptor. However, IL1RN functions as a competitive inhibitor of IL-1α and IL-1β (7). Various studies have shown that the IL-1 family plays an important role in ILD. For example, injection of recombinant IL-1 protein into rodent tracheas promotes acute alveolar leakage and neutrophil inflammation in lung (8, 9). Moreover, IL-1 expression levels in lung are correlated with the development of pulmonary fibrosis in rodents exposed to bleomycin (BLM) or radiation (10, 11) and up-regulated in fibro-proliferative areas within the lungs of idiopathic pulmonary fibrosis patients (12). In addition, patients with a particular IL1RN polymorphism demonstrate a higher risk for fibrosing alveolitis, indicating that an imbalance between IL-1 and IL1RN activity also contributes to ILD (13). The results are consistent with studies demonstrating that IL1RN administration blocks lung fibrosis induced in mice by exposure to BLM or silica (14).
Over the past decade, stem cells from adult bone marrow have been exploited as therapeutic vectors to treat a wide variety of diseases (15, 16). However, limited information exists regarding the therapeutic potential of these cells in lung diseases. For example, several studies have demonstrated that hematopoietic stem cells (17) or marrow-derived stromal cells (18) contribute to airway and distal (type II) alveolar epithelium, but the capacity of these cells to ameliorate disease has been largely unexplored. Recently, we reported that mesenchymal stem cells (MSCs) administered to mice challenged with BLM engrafted at low levels but significantly reduced the extent of inflammation and fibrosis in lungs (19). Importantly, MSCs were efficacious in ameliorating lung injury only when administered at the time of BLM challenge and not at later time points. This result suggested their therapeutic effect was attributed to the production of soluble factors that modulate inflammation. Herein, we report the identification of murine and human MSC subpopulations that secrete high levels of IL1RN. We also provide in vitro and in vivo data that production of IL1RN by MSCs protects mice from BLM-induced lung injury by blocking the production and/or activity of TNF-α and IL-1α, the predominant proinflammatory cytokines in lung tissue. Our discovery of human MSC subpopulations that express IL1RN provides a strong rationale for developing MSC-based therapies to treat ILD.
Results
Identification of an IL1RN-Expressing Subpopulation of MSCs.
To identify proteins expressed by murine MSCs that may modulate the inflammatory response, we interrogated their transcriptome, which we catalogued (20) by serial analysis of gene expression (SAGE). This SAGE library consisted of 59,007 sequenced SAGE tags that corresponded to 5,737 identifiable, unique mRNAs. Characterizing these expressed mRNAs based on gene ontologies revealed that MSCs expressed transcripts encoding regulatory proteins involved in angiogenesis, cell motility and communication, hematopoiesis, neural activities, and immunity and defense. In the latter case, numerous SAGE tags corresponding to IL1RN were identified, and one such tag ranked 208th in abundance of 15,815 distinct tag sequences (Table 1). Because of its antagonistic effects on IL-1 activity, we pursued the characterization of IL1RN expression in murine MSCs.
Table 1.
SAGE tags reliably mapping to IL1RN mRNA
| SAGE tag sequence | Count | Reliable unigene clusters matched to tag |
|---|---|---|
| CTACGTATTG | 33 | Mm.882; IL1RN |
| TGTGCTCAGG | 5 | Mm.882; IL1RN |
| Mm.319011; Pftk1 | ||
| TCGGCAAGAG | 1 | Mm.882; IL1RN |
| GCTTCAGAGG | 1 | Mm.882; IL1RN |
| Mm.37341; Anapc7 | ||
| subunit 7 | ||
| TTCTGAGACC | 1 | Mm.882; IL1RN |
| Mm.95281: Wdr17 |
Listed in order of abundance are the SAGE tags catalogued from the murine MSC transcriptome that reliably mapped to mRNA sequences encoding IL1RN. The SAGE tag CTACGTATTG ranked 208th in abundance of 15, 815 distinct tag sequences. In addition, this SAGE tag ranked 98th of 8,133 tags that mapped reliably to a unique mRNA sequence. Pftk1, PFTAIRE protein kinase 1; Anapc7, anaphase promoting complex; Wdr17, WD repeat domain 17.
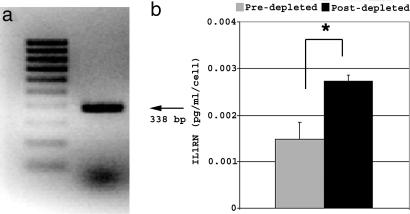
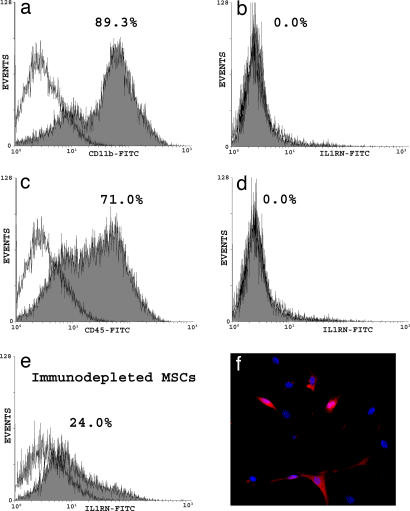
Screening by PCR of a murine MSC cDNA library validated that SAGE tags mapping to IL1RN corresponded to expressed mRNAs encoding this protein (Fig. 1a). Additionally, ELISA analysis of conditioned media revealed that enrichment of murine MSCs by immunodepletion (21) afforded a significant increase (P = 0.00067) in the amount of secreted IL1RN on a per cell basis compared with unfractionated, plastic adherent cells (Fig. 1b). These data were consistent with the failure of hematopoietic lineages enriched by immunodepletion for expression of CD11b, CD45 (Fig. 2 a and c), and CD34 (data not shown) to express detectable levels of IL1RN protein (Fig. 2 b and d). In contrast, ≈24% of immunodepleted murine MSCs were found to express IL1RN (Fig. 2e). Immunostaining showed that IL1RN expression was restricted to a specific subpopulation of cells (Fig. 2f). Therefore, this unique MSC subpopulation represents the predominant source of IL1RN protein in murine bone marrow.
Fig. 1.
Murine MSCs express IL1RN mRNA and protein. (a) Screening of a murine MSC cDNA library by PCR yielded a 338-bp fragment corresponding to IL1RN mRNA. (b) Levels of secreted IL1RN protein were quantified by ELISA on a per-cell basis from unfractionated plastic adherent marrow cells (predepleted) or murine MSCs enriched by immunodepleted. Plotted values (mean ± SD) represent duplicates from four separate experiments. ∗, P < 0.0007.
Fig. 2.
Distribution of IL1RN in murine bone marrow. (a–e) FACS analysis revealed that CD11b (a) and CD45 (c) positive cells recovered from magnetic beads after immunodepletion lacked expression of IL1RN protein (b and d), but 24% of immunodepleted murine MSCs expressed IL1RN (e). (f) Immunostaining of murine MSCs with an anti-IL1RN antibody (red) and DAPI (blue) confirmed that IL1RN expression was restricted to a specific subpopulation of cells.
IL1RN Expressed by MSCs Blocks Antagonizes IL-1α Function.
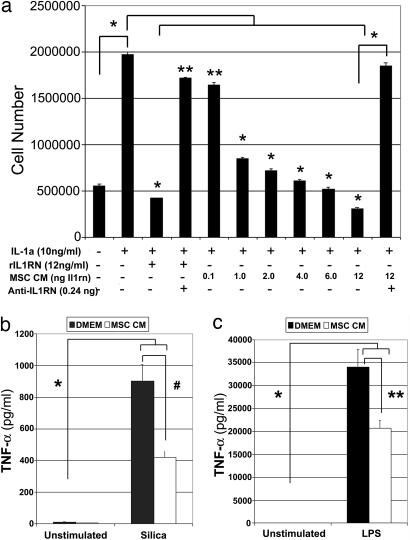
To determine whether IL1RN expressed by MSCs is biologically active, we assayed its ability to inhibit proliferation of the IL-1α-dependent helper T lymphocyte cell line D10.G4.1. Initially, we confirmed that recombinant IL1RN protein inhibited in a dose-dependent manner proliferation of D10.G4.1 cells induced by IL-1α and that this activity could be blocked by a neutralizing anti-IL1RN antibody [supporting information (SI) Fig. 7]. Subsequently, we showed that the stimulatory affect of IL-1α on D10.G4.1 cell proliferation was also significantly inhibited (P < 1 × 10−10) by MSC-conditioned media in a dose-dependent manner (Fig. 3a). The inhibitory effect of conditioned media was correlated with its content of IL1RN protein and proportional to that measured for recombinant IL1RN protein. Moreover, treating MSC-conditioned media with a neutralizing anti-IL1RN antibody abolished its inhibitory effect on IL-1α-induced cell proliferation (Fig. 3a). Collectively, these data demonstrate that IL1RN is the principal IL-1α antagonist secreted by murine MSCs.
Fig. 3.
IL1RN secreted by MSCs antagonizes IL-1α activity and blocks release of TNF-α from activated macrophages. (a) Equivalent numbers of D10.G4.1 cells were cultured for 5 days in media alone or media supplemented with the indicated concentrations of IL-1α, recombinant IL1RN (rIL1RN), MSC-conditioned media (CM), or a neutralizing IL1RN antibody (anti-IL1RN), and then the total yield of cells for each condition was determined by counting. ∗, P < 1 × 10−10; ∗∗, P < 0.005. (b and c) Levels of TNF-α secreted by the macrophage cell line RAW-264.7 after exposure to silica (b) or LPS (c) in the presence or absence of MSC-conditioned media (≈3 ng of IL1RN) was quantified by ELISA. ∗, P < 0.000005; ∗∗, P < 0.0005; #, P < 0.001. Plotted values (mean ± SD) represent duplicates from three separate experiments.
IL1RN Expressed by MSCs Blocks Release of TNF-α from Activated Macrophages.
In related experiments, we found that MSC-conditioned media also inhibited the capacity of RAW-264.7 macrophages to secrete TNF-α in response to exposure to silica or LPS. Specifically, RAW-264.7 cells exposed to 20 μg/cm2 of silica (Fig. 3b) or 1 ng/ml LPS (Fig. 3c) for 4 h secreted significantly higher amounts of TNF-α into the media compared with nonstimulated cells (P < 0.000005). In contrast, when the exposures were performed in the presence of MSC-conditioned media, the amount of TNF-α secreted by RAW-264.7 cells in response to silica or LPS was reduced 54% (904.1 vs. 421.1 pg/ml) and 39.3% (34,040 vs. 20,652 pg/ml), respectively (Fig. 3 b and c). The inhibitory effect on TNF-α secretion was significantly reversed by preincubation of the MSC-conditioned media with a recombinant neutralizing anti-IL1RN antibody (data not shown). Whether reduction in TNF-α protein secretion is due to effects on gene transcription or translation is unknown at this time. Nevertheless, because macrophage activation contributes significantly to the inflammatory response, these data reveal another mechanism by which MSCs may limit inflammation at sites of tissue injury.
MSCs Modulate the Inflammatory Response in Lung in Vivo.
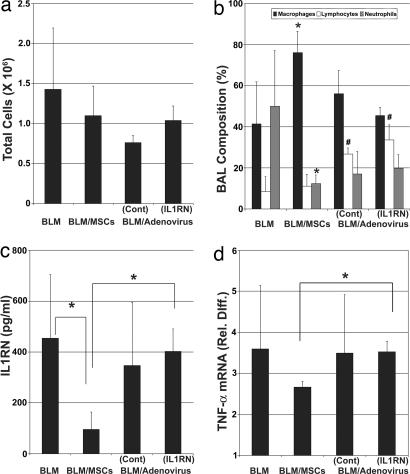
To determine whether MSCs alter the inflammatory response in vivo, mice were administered 0.1 unit of BLM via the endotracheal route and then infused with murine MSCs (500,000 cells in 20 μl of PBS) through the jugular vein. Alternatively, as a substitute for MSCs, 1 × 109 adenoviral particles harboring either an empty expression vector or one encoding IL1RN were instilled into the trachea of some mice before BLM exposure. The total number of cells contained in BAL fluid obtained from mice 3 days after exposure to BLM was not significantly different (P = 0.15) between treatment groups (Fig. 4a). However, BAL fluid from mice administered BLM and MSCs contained a significantly greater number of macrophages (P = 0.039) and significantly fewer neutrophils (P = 0.034) compared with that of animals exposed to BLM alone (Fig. 4b). In contrast, BAL fluid from adenovirus-infected animals showed no significant difference in the number of macrophages (P > 0.2) or neutrophils (P > 0.05) but did contain significantly more lymphocytes (P < 0.005) compared with that from animals exposed to BLM. Differences in lymphocyte levels likely reflects a confounding effect of viral infection, because animals exposed to virus harboring the empty or IL1RN-expressing vector both showed elevated lymphocyte counts in their BAL fluid.
Fig. 4.
MSC administration alters BLM-induced inflammatory responses in lung. BAL fluid harvested from mice 3 days after BLM challenge was analyzed for total cell number (a), cell composition (b), and levels of IL1RN protein (c). Total RNA harvested from lung tissue was analyzed by real-time PCR to quantify TNF-α mRNA levels (d). Reported values represent the average ± SD (bars) calculated from animals within each treatment group. ∗, P < 0.05; ∗∗, P < 0.01; #, P < 0.005; n = 5 mice per group.
ELISA analysis also revealed that BAL fluid from animals administered BLM and MSCs contained significantly lower levels of IL1RN protein compared with that from animals challenged with BLM alone (P = 0.032) or animals infected with the IL1RN-expressing adenovirus (P = 0.014) (Fig. 4c). In addition, TNF-α mRNA levels expressed in lung were shown by real-time PCR to be lower in MSC-treated animals compared with those of all other experimental groups. This difference was statistically significant (P = 0.01) when compared directly with animals infected with the IL1RN-expressing adenovirus (Fig. 4d). Therefore, MSC administration inhibited BLM-induced increases in cytokine mRNA and protein levels in lung and impeded trafficking of immune cells to this tissue. In most cases, the effects of MSCs were more pronounced than those of recombinant IL1RN delivered to lung by adenoviral infection.
MSCs Block Up-Regulation of IL-1α Gene Expression Induced in Lung Tissue by BLM Exposure.
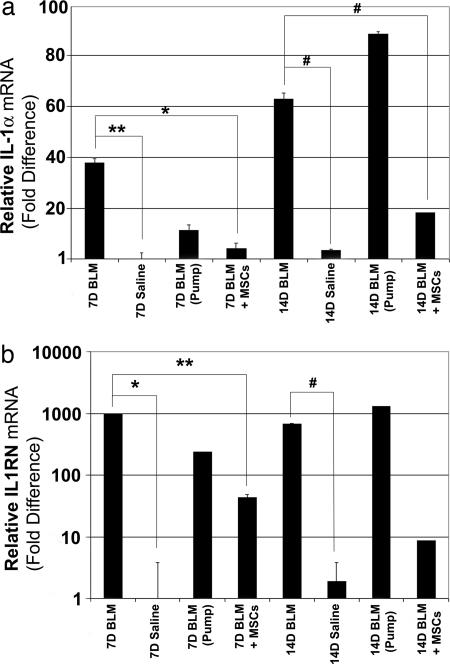
In a second series of experiments, we evaluated how BLM exposure with or without MSC administration altered IL-1α mRNA levels in lung. In these studies, some mice were also administered recombinant IL1RN protein systemically by osmotic minipumps throughout the duration of the experiment. Levels of IL-1α mRNA were significantly elevated in the lungs of mice at 7 days (38.6-fold) and 14 days (14.5-fold) after BLM exposure compared with control animals challenged with saline alone (Fig. 5a). However, systemic administration of IL1RN only modestly inhibited BLM-induced increases in IL-1α mRNA levels at 7 days after exposure and had no affect at 14 days. In contrast, MSC administration significantly reduced expressed levels of IL-1α mRNA induced in lung by BLM exposure by 7.3- and 3.3-fold at 7 and 14 days after exposure, respectively.
Fig. 5.
MSC administration inhibits BLM-induced up-regulation of IL-1α and IL1RN mRNA in lung. Relative expression levels of IL-1α (a) and IL1RN (b) mRNA in lung at 7 and 14 days after BLM exposure was quantified by real-time PCR, using the relative Ct method. Plotted values represent the average ± SD (bars) calculated from animals within each treatment group and then normalized to 7 day saline control treated animals (n = 3–7 mice). ∗, P < 0.0002; ∗∗, P < 0.01; #, P < 0.05.
Levels of IL1RN mRNA were also significantly elevated in the lungs of mice at 7 (971-fold) and 14 (358-fold) days after BLM exposure compared with saline-treated controls (Fig. 5b). Systemic administration of recombinant IL1RN failed to alter the effect of BLM on IL1RN expression levels in lung in any significant way. However, MSC administration significantly inhibited (P < 0.05) BLM-induced increases in IL1RN mRNA levels in lung tissue at 7 days after exposure. The inhibitory effect of MSCs was still apparent by 14 days after exposure but was no longer statistically significant. Therefore, MSC administration inhibited BLM-induced increases in both IL1RN mRNA and protein in lung tissue and BAL fluid, respectively.
Genomic DNA obtained from the lungs of all but one female mouse administered male MSCs (n = 6–9 mice per group) was determined by real-time PCR (22) to contain appreciable levels of male DNA, which on average represented 0.54% and 0.01% of the total DNA content of lung at 7 and 14 days after exposure, respectively (data not shown). Although this represented a >40-fold difference in engraftment levels between time points, these values were not significantly different (P = 0.075). Therefore, female mice challenged with BLM retained measurable levels of engrafted male MSCs in lung tissue over the time course of the experiment, providing further evidence that the cells directly effected changes in cytokine mRNA and protein levels.
Identification of an IL1RN-Expressing Subpopulation of Human MSCs.
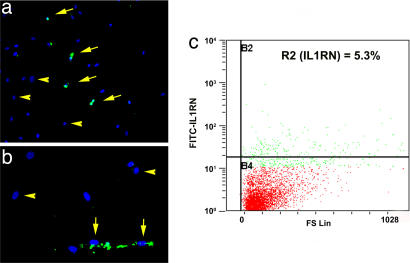
Interrogation of the human MSC transcriptome (23) also revealed a single SAGE tag (TGCCTGTAAT) corresponding to IL1RN. Although a total of six such tags were in our library, it reliably mapped to a large number of genes in addition to IL1RN. However, immunostaining of human MSC populations revealed a distinct subpopulation of IL1RN-expressing cells (Fig. 6a). FACS analysis confirmed that ≈5% of human MSCs expressed IL1RN protein (Fig. 6b). Therefore, the ability of MSCs to express IL1RN is conserved among different species.
Fig. 6.
IL1RN-expressing human MSCs. Photomicrographs of human MSCs immuno-stained with an anti-IL1RN antibody (green) and DAPI (blue). (Magnifications: a, ×200; b, ×400) IL1RN-expressing and nonexpressing MSCs are demarked by arrows and arrow heads, respectively. (c) FACS analysis of human MSCs revealed that ≈5% of cells (R2 gate) expressed IL1RN protein.
Discussion
Our study identifies a subpopulation of MSCs that secrete high levels of IL1RN, which was shown to antagonize the function of IL-1α and block production of TNF-α from activated macrophages in vitro. Considering that TNF-α and IL-1α lie at the nexus of the inflammatory response, these findings reveal a novel mechanism by which MSCs protect lung tissue from BLM-induced injury (SI Fig. 8). Moreover, our results showed that MSC administration was more effective than recombinant IL1RN, delivered systemically or by viral infection, in inhibiting immune cell trafficking and cytokine expression levels in lung in response to BLM exposure. The enhanced effectiveness of MSCs likely reflects their ability to traffic to and engraft within the lungs for prolonged periods after systemic injection (22, 24, 25), a process that may be enhanced by the accumulation of hyaluronan degradation products in the inflamed lung (26, 27). The latter is consistent with our studies showing that MSC engraftment levels are elevated in lung by BLM exposure (19), which may effectively target IL1RN to sites of lung injury.
Paradoxically, our findings indicate that endogenous IL1RN produced in the inflamed lung was not as effective as MSCs in modulating the inflammatory response to BLM. This may be explained by the fact that MSCs engrafted in lung produce IL1RN constitutively during the initial phases of the inflammatory response, whereas local production only occurs later in response to inflammation. IL-1α is know to positively regulate expression of IL1RN, providing a means to counterbalance its potent activity in vivo (28). In this context, IL1RN expression in tissues reflects the amount of IL-1 activity induced by tissue injury. However, there exists in cells a significant delay between exposure to IL-1 and expression of IL1RN protein (29), and our own work shows that IL-1α induces in MSCs a significant increase in IL1RN secretion only after 72 h of exposure (SI Fig. 9). Therefore, high endogenous IL1RN levels in lung reflect an ongoing inflammatory response, which is down-regulated only after significant tissue damage occurs. In contrast, MSCs protect lung tissue from bleomycin-induced injury by preventing the inflammatory response. This effect is consistent with the fact that MSC administration inhibited collagen accumulation and blocked matrix metalloproteinase activation in the lungs of mice challenged with BLM (19).
Our data also show that MSCs block recruitment of lymphocytes and neutrophils into the injured lung, which is consistent with our previous histological findings (19). Neutrophils contribute to parenchymal injury by producing toxic reactive oxygen intermediates, cytokines, and secreting proteolytic enzymes that alter the lung cytoarchitecture (30, 31). In addition, lymphocytes produce secondary immune effectors, such as IL-6, and induce epithelial cytotoxicity (32). Recent studies indicate that TNF-α also contributes to the pathophysiology of ILD by inducing apoptosis of epithelial cells by direct activation of the TNFR and indirectly by stimulating expression of IL-1 (29). Therefore, MSCs may directly enhance epithelial cell survival by blocking downstream effects of TNF-α and IL-1. This outcome has important implications, because injury to the alveolar epithelium has serious clinical consequences, including dysregulation of surfactant production and exposure of the basement membrane, which causes further macrophage activation.
It is well established that TNF-α and IL-1 also function as potent bone-resorbing factors (33, 34), the effects of which can be modulated by IL1RN (35). Therefore, IL1RN-expressing MSC subpopulations identified herein may represent a unique subtype of stromal cell that plays an important role in modulating bone turnover in vivo. This would explain why IL1RN expression by MSCs is conserved across species. These results are consistent with our previous study showing the cellular composition of marrow stroma and its associated functions are more complex than previously envisioned (20). Our data indicating that IL1RN-expressing subpopulations are less abundant in human vs. murine populations may reflect differences in the anatomical location and methods used to isolate and culture expand the cells. Nevertheless, the existence of IL1RN-expressing MSCs in human bone marrow may provide a novel cellular vector for treating chronic inflammatory diseases in humans, particularly those affecting the lung.
Methods
Isolation of MSCs.
Murine MSCs were isolated from 4 week-old, male Balb/C mice and purified by immunodepletion as described in ref. 21. In some experiments CELLection Dynabeads (Dynal Biotech, Brown Deer, WI) were used to recover immunodepleted cell lineages. Human MSCs were isolated from small volume (2- to 5-ml) bone marrow aspirates obtained from healthy donors (23) as approved by the Institutional Review Board of Tulane University.
ELISAs.
Murine plastic adherent marrow cells or MSCs (50,000–200,000 cells) were cultured for 5–7 days in α-MEM media (Invitrogen, Carlsbad, CA) supplemented with 10% FCS (Atlanta Biological, Atlanta, GA), 100 units per ml penicillin, and 100 μg/ml streptomycin. Cells were washed with HBSS and maintained in 1 ml of serum-free α-MEM for 24 h, and the conditioned media were collected. Levels of IL1RN in conditioned media were quantified by using the Mouse Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
Immunostaining.
Murine MSCs (2 × 104 cells per 0.4 cm2) were fixed for 15 min at room temperature, washed, fixed for 30 min in blocking buffer (PBS containing 0.1% BSA, 5% Tween-20, and 20% donkey sera), incubated overnight at 4°C with a 1:100 dilution of an anti-mouse IL1RN antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and then 1 h at room temperature with a 1:2,000 dilution of an Alexa Fluor 546 donkey anti-goat IgG(H+L) (Molecular Bioprobes, Eugene, OR). Slides were counterstained with DAPI, photographed with a Leica RX-DMV fluorescent microscope (Meyer Instruments, Houston, TX) with a Cooke Sensicam digital camera (Hamamatsu, Bridgewater, NJ), and rendered by using Slidebook software (3I, Denver, CO).
FACS Analysis.
Murine MSCs (3 × 105) were incubated in 1 ml of fixation buffer (Santa Cruz Biotechnology) for 30 min, washed, and incubated for 15 min with a 1:60 dilution of a rat anti-mouse CD16/CD32 antibody (BD Biosciences, San Jose, CA) at 4°C in the dark. Cells were then incubated 15 min in permeabilization buffer for 1 h with a 1:100 dilution of an anti-IL1RN antibody and then for 20 min with a 1:200 dilution of a FITC-conjugated donkey anti-goat antibody (Santa Cruz Biotechnology). Cells were washed and resuspended in 500 μl of wash buffer, and the extent of cell labeling was evaluated by using a Beckman Coulter Model Epics XL flow cytometer (Beckman Coulter, Fullerton, CA). Isotype controls were run in parallel, using the same concentration of each antibody tested. Human MSCs were processed similarly except that use of the anti-CD16/CD32 antibody was omitted.
PCR.
SAGE tags corresponding to IL1RN were validated by screening a murine MSC cDNA library (20) by PCR, using the following primers: 5′-AGGCCCCACCACCAGCTT TGAGTC-3′ and 5′-TCACCCAGATGGCAGAGGCAACAA-3′. An aliquot (50-μl) of the pooled cDNA phage library was boiled for 5 min, and then 500 ng was used as input in a PCR (100 μl) containing 100 pmol of forward and reverse gene-specific primers, 1× PCR buffer, 0.2 mM dNTPs, and 0.5 μl of TaqDNA polymerase (Qiagen, Valencia, CA). After an initial denaturation step at 94°C for 3 min, reactions were amplified for 30 cycles at 94°C for 30 sec, 59.9°C for 45 sec, and 72°C for 90 sec, followed by a final incubation at 72°C for 7 min. PCR products were gel-purified by using GeneElute columns (Sigma, St. Louis, MO) and cloned by using the AdvanTAge PCR cloning kit (Clontech, Palo Alto, CA). Plasmid DNA was isolated and sequenced to confirm the identity of the PCR product.
T-Cell Proliferation and Macrophage Activation Assays.
D10.G4.1 and RAW-264.7 cell lines were maintained as described by the supplier (ATCC, Manassas, VA). D10.G4.1 cells (500,000) were cultured with the indicated concentration of recombinant mouse IL-1α alone or in combination with varying amounts of recombinant mouse IL1RN, a neutralizing anti-IL1RN antibody (R&D Systems) or MSC-conditioned media. Cultures were maintained for 3–5 days with media changes each day, after which the total number of viable cells were determined by staining with Tryptan blue and counting on a hemocytometer. RAW-264.7 cells were exposed to 1 μg/ml LPS (Sigma) or 20 μg/cm2 silica (provided by A. Ghio, Environmental Protection Agency, Chapel Hill, NC) for 4 h in the presence of MSC-conditioned media preincubated with or without a neutralizing anti-IL1RN antibody. Subsequently, levels of TNF-α secreted from cells were quantified by ELISA as described in ELISAs. All assays were performed in quadruplicate.
BLM Exposures and Tissue Processing.
Anesthetized female 6- to 10-week-old C57BL/6 mice were exposed to 0.1 unit of BLM as described in ref. 19. Murine MSCs (5 × 105 in 200 μl of PBS) were injected into the jugular vein immediately after BLM administration. Some mice were infected with 1 × 109 particles of a retrovirus encoding human IL1RN (provided by P. Robbins, University of Pittsburgh, Pittsburgh, PA) or had an osmotic pump (ALZET, Cupertino, CA) that secreted 0.5 mg/kg/hr of recombinant human IL1RN (ProSpec-Tany TehcnoGene, Rehovot, Israel) surgically implanted into their peritoneal cavity 1 day before BLM exposure. Animals were killed at 3, 7, or 14 days after BLM exposure, at which time BAL fluid was collected. The left lung was then removed, flash-frozen in liquid nitrogen, and used to harvest genomic DNA and total RNA, using the AllPrep DNA/RNA Mini Kit (Qiagen). All experiments involving live animals were approved by the animal use and care committee of Tulane University Health Sciences Center and the University of Pittsburgh.
Quantification of Cytokine mRNA and Protein Levels in Vivo.
Cytospins of BAL fluid were stained with Diff Quick (Fisher Scientific, Pittsburgh, PA), and cell differentials were determined by counting 400 consecutive cells. Total RNA (25 ng) prepared from lung was converted to cDNA and amplified by PCR, using the TaqMan EZ RT-PCR kit, a 7900 HT sequence detector and the following Assay-On-Demand Taqman probes: TNF-α (Mm0043258), IL-1α (Mm00439620), and IL1RN (Mm00446185) (Applied Biosystems, Foster City, CA). Transcript levels were quantified by using the relative Ct method with GAPDH mRNA as an internal control. Genomic DNA (25 ng) isolated from lung tissue was also analyzed by real-time PCR to quantify levels of engrafted MSCs as described in ref. 24.
Statistical Analysis.
All values were expressed as the mean ± SD. Differences between treatment groups were measured by Student's t test or ANOVA with Fisher's PLSD test for pair-wise comparison (Statview 4 software; Abacus Concept, Piscataway, NJ). P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health Grants R01-NS39033-01A2, R01-NS39033-02S1, P40-RR17447, and P01-HL075161 (to D.G.P.) and R01-HL071953 and ES010859 (to L.A.O.); the Louisiana Gene Therapy Research Consortium (New Orleans, LA); and the Health Care Company (Nashville, TN).
Abbreviations
- BAL
bronchoalveolar lavage
- BLM
bleomycin
- IL1RN
interluekin 1 receptor antagonist
- ILD
interstitial lung disease
- MSC
mesenchymal stem cell
- SAGE
serial analysis of gene expression.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704421104/DC1.
References
- 1.American Thoracic Society. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Am J Respir Crit Care Med. 2006;74:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Phan SH. Biol Signals. 1996;5:232–239. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- 4.Furie MB, Randolph GJ. Am J Pathol. 1995;146:1287–1301. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneider NC, Leger AJ, Kuliopulos A. FEBS J. 2006;273:4416–4424. doi: 10.1111/j.1742-4658.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 6.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. J Clin Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arend WP. J Clin Invest. 1991;88:1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hybertson BM, Lee YM, Repine JE. Ann NY Acad Sci. 1997;832:266–273. doi: 10.1111/j.1749-6632.1997.tb46253.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee YM, Hybertson BM, Cho HG, Terada LS, Cho O, Repine AJ, Repine JE. Am J Physio Lung Cell Mol Physiol. 2000;279:L75–L80. doi: 10.1152/ajplung.2000.279.1.L75. [DOI] [PubMed] [Google Scholar]
- 10.Phan SH, Kunkel SI. Exp Lung Res. 1992;18:29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- 11.Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Radiat Res. 1996;145:762–767. [PubMed] [Google Scholar]
- 12.Pan LH, Ohtani H, Yamauchi K, Nagura H. Pathol In. 1996;46:91–99. doi: 10.1111/j.1440-1827.1996.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 13.Whyte M, Hubbard R, Meliconi R, Whidborne M, Eaton V, Bingle C, Timms J, Duff G, Facchini A, Pacilli A, et al. Am J Respir Crit Care Med. 2000;162:755–758. doi: 10.1164/ajrccm.162.2.9909053. [DOI] [PubMed] [Google Scholar]
- 14.Piguet PF, Vesin C, Grau GE, Thompson RC. Cytokine. 1993;5:57–61. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- 15.Minguell JJ, Erices A. Expt Biol Med. 2006;231:39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 16.Phinney DG, Isakova I. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 17.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 18.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Development (Cambridge, UK) 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Hill K, Michelson C, DuTreil M, Hughes C, Humphries S, Wilkinson R, Baddoo M, Bayly E. Stem Cells. 2006;24:186–198. doi: 10.1634/stemcells.2004-0236. [DOI] [PubMed] [Google Scholar]
- 21.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 22.McBride C, Gaupp D, Phinney DG. Cytotherapy. 2003;5:7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- 23.Tremain N, Korkko J, Ibberson D, Kopen GC, DiGirolamo C, Phinney DG. Stem Cells. 2001;19:408–418. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 24.Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Respir Res. 2005;6:125–138. doi: 10.1186/1465-9921-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 26.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 28.Juge-Aubry CE, Somm E, Chicheportiche R, Burger D, Pernin A, Cuenod-Pittet B, Quinodoz P, Giusti V, Dayer J-M, Meier CA. J Clin Endocrin Metabol. 2004;89:2652–2658. doi: 10.1210/jc.2003-031219. [DOI] [PubMed] [Google Scholar]
- 29.Janes KA, Gaudet S, Albeck JG, Nielsen UB, Lauffenburger DA, Sorger PK. Cell. 2006;124:1225–1239. doi: 10.1016/j.cell.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 30.Strausz J, Muller-Quernheim J, Steppling H, Ferlinz R. Am Rev Respir Dis. 1990;141:124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- 31.Gadek JE, Kelman JA, Fells G, Weinberger SE, Horwitz AL, Reynolds HY, Fulmer JD, Crystal RG. N Engl J Med. 1979;301:737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- 32.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, et al. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. J Biol Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo JA, Naprta A, Rao Y, Alander C, Glaccum M, Widmer M, Gronowicz G, Kalinowski J, Pilbeam CC. Endocrinology. 1998;139:3022–3025. doi: 10.1210/endo.139.6.6128. [DOI] [PubMed] [Google Scholar]
- 35.Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, et al. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.