Abstract
Although periodontitis is a chronic inflammatory disease caused by a group of so-called periodontopathic bacteria, autoimmune mechanisms have also been implicated in the disease process. Recently, a unique subset of lymphocytes designated natural killer (NK) T cells expressing the Vα24JαQ invariant T cell receptor (TCR) has been reported to have a regulatory role in certain autoimmune diseases. Therefore, we investigated the proportion of the invariant Vα24JαQ TCR within the Vα24 T cell population in periodontitis lesions and gingivitis lesions using single-strand conformation polymorphism methodology. NK T cells were identified with a specific JαQ probe whereas the total Vα24 TCR was identified using an internal Cα probe. NK T cells were a significant proportion of the total Vα24 population both in periodontitis lesions and to a lesser extent in gingivitis lesions but not in the peripheral blood of either periodontitis patients or nondiseased controls. Using immunohistochemistry, some of Vα24+ cells in the periodontitis lesions seemed to associate with CD1d+ cells, which are specific antigen-presenting cells for NK T cells. Although the mechanism underlying the elevation of NK T cells in periodontitis and in gingivitis lesions remains unclear, it can be postulated that NK T cells are recruited to a play regulatory role in the immune response to bacterial infection.
Chronic inflammatory periodontal disease manifests clinically as at least two distinct entities. Evidence based on microbiological, immunological, and animal model studies has shown that some forms of periodontal disease in adults can remain stable throughout many years and not endanger the life of the dentition (gingivitis), whereas other forms, despite extensive treatment, continue to breakdown, leading ultimately to tooth loss (periodontitis). 1 Although periodontal bacteria are the causative agents in periodontitis, subsequent progression and disease severity are thought to be determined by the host immune response in which many cell types notably polymorphonuclear leukocytes, macrophages, lymphocytes, and fibroblasts are involved. 2 Whereas T cells dominate the gingivitis lesion, the periodontitis lesion contains large numbers of B cells and plasma cells together with significant numbers of T cells. 3,4 A regulating role for T cell subsets has been suggested in the rat model, 5 and a T-cell regulatory imbalance in human chronic periodontal disease has also been demonstrated. 6,7
Recently a unique lymphocyte population designated natural killer T cells (NK T cells) has been characterized. NK T cells express common markers for NK cells and the invariant Vα-Jα T cell receptor (TCR) both in mice and humans, 8-13 Human invariant Vα24JαQ T cells are homologous to the murine Vα14Jα281 NK 1.1+ T cells that have a TCR α chain in which the Vα14 segment is rearranged to pair with Jα281 with no N-region diversity. 14-16 These NK T cells have functionally important roles in vivo. A direct relationship exists between a deficiency in NK T cells and susceptibility to type 1 diabetes in nonobese diabetic mice 17-20 and in humans. 21 A deficiency in NK T cells has also been implicated in some other autoimmune diseases including autoimmune gastritis 22 and lupus-like disease 23,24 in mice and in humans with systemic sclerosis. 25 These studies suggest a role for NK T cells in the regulation of autoimmune diseases.
We have previously demonstrated that the frequency of seropositivity and the antibody titer to human heat shock protein (hsp) 60 and Porphyromonas gingivalis GroEL, a periodontopathic bacterial homologue of human hsp60, were significantly higher in periodontitis patients than in periodontally healthy controls. 26 Furthermore, affinity-purified serum antibodies to human hsp60 and P. gingivalis GroEL from selected patients reacted with P. gingivalis GroEL and human hsp60, respectively, indicating cross-reactivity of antibodies. In addition we found a higher frequency of hsp60- and P. gingivalis GroEL-reactive T cell clones in peripheral blood mononuclear cells (PBMCs) of periodontitis controls compared with periodontally healthy patients. Analysis of the nucleotide sequences of the CDR3 region in the T-cell receptor β-chain clearly demonstrated that the identical T cell receptors were used between human hsp60-reactive peripheral blood T cells of periodontitis patients and periodontitis lesion-infiltrating T cells of the same patients (Yamazaki K, Ohsawa Y, Tabeta K, Ito H, Ueki K, Yoshie H, Seymour GJ, manuscript in preparation). These results suggest that an immune response based on the molecular mimicry between P. gingivalis GroEL and human hsp60 may play some role in periodontitis. Heat shock proteins, particularly the hsp60 family of proteins, are thought to play important roles in the causal relationship between microbial infections and autoimmunity because of conservation of the amino acid sequence during evolution and their strong immunogenicity. To date, there have been a number of reports regarding the role of hsps and autoimmune diseases. 27
These studies led us to speculate that NK T cells may play an important role in regulating the autoimmune response in chronic inflammatory periodontal disease (periodontitis). Therefore, in the present study we investigated the frequency of NK T cells in both gingivitis and periodontitis lesions and demonstrated that the frequency of invariant Vα24JαQ TCR-expressing T cells is higher in periodontitis tissues and to a lesser extent in gingivitis tissues than in autologous peripheral blood, suggesting a preferential accumulation of NK T cells in chronic inflammatory periodontal disease tissues.
Materials and Methods
Patients, Controls, and Specimen Collection
Gingival tissue samples were obtained at the time of periodontal surgery (flap surgery) from 15 patients with moderate to severe periodontitis (mean age, 40.4 ± 9.9 years; range, 26 to 55 years) referred to the periodontal clinic of Niigata University Dental Hospital. All patients were classified as having chronic periodontitis with no systemic disorders. The mean probing depth, probing attachment level, and bone resorption were 6.5 ± 1.4 mm (range, 4 to 9 mm), 7.5 ± 1.7 mm (range, 4 to 10 mm), and 58.2 ± 27.1% (range, 10 to 100%), respectively. Approximately 100 mg of tissue containing the area of inflammatory cell infiltrate from each specimen was immediately frozen in liquid nitrogen and stored at −80°C until RNA separation. PBMCs were separated by Ficoll-Paque density gradient centrifugation from 10 ml of autologous peripheral blood. PBMCs were also separated from 12 periodontally healthy controls (mean age, 38.0 ± 7.7 years; range, 30 to 49 years) with probing attachment level <4 mm and minimal bone resorption at all sites. A small piece of marginal gingival tissue was also obtained from the second or third molar site from nine of the controls. Previous studies have shown that apparently clinically healthy gingiva display histological evidence of inflammation similar to that seen in marginal gingivitis. 28 Subsequently these tissues were referred to as gingivitis tissues. Informed consent was obtained from all patients and controls before inclusion in the study.
RNA Separation and cDNA Synthesis
Total RNA from gingival tissues and PBMCs was separated by using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. The RNA samples were further purified by successive treatment with DNase I (Life Technologies, Inc., Gaithersburg, MD), phenol/chloroform/isoamylalcohol (Life Technologies, Inc.) and ethanol sedimentation.
The first strand cDNA was synthesized using M-MLV reverse transcriptase (Life Technologies, Inc.) and 50 μmol/L of random hexanucleotides (Takara Shuzo Co., Ltd., Shiga, Japan) from 2 μg of total RNA in the reaction buffer (Life Technologies, Inc.) containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, supplemented with 0.5 U RNase inhibitor, 0.1 mol/L dithiothreitol, and dNTP (each at 0.5 mmol/L). The reaction mixture was incubated at 37°C for 60 minutes and then heated at 95°C for 5 minutes.
Polymerase Chain Reaction (PCR) Amplification of TCR Vα24 Gene
PCR amplification of cDNA was performed using oligonucleotide primers specific for Vα24 (5′-GATATACAGCAACTCTGGATGCA-3′) 14 and Cα (5′-AATAGGTCGACAGACTTGTCACTGGA-3′). 29 The reaction mixture, prepared on ice contained 1× EX Taq buffer (Takara Shuzo Co., Ltd., Shiga, Japan), 0.2 mmol/L of each dNTP, 0.4 μmol/L of each primer, 2.4 μl of cDNA, and 0.35 U of EX Taq DNA polymerase (Takara Shuzo Co.) in a total volume of 15 μl. The PCR reaction was performed using a DNA thermal cycler (PCR Thermal Cycler MP; Takara Shuzo Co.). The amplification cycle profile was as follows: denaturation at 94°C for 10 seconds, annealing at 60°C for 20 seconds, and extension at 72°C for 30 seconds. The durations of denaturation in the first cycle and extension in the last cycle were extended for 7 minutes.
Single-Strand Conformation Polymorphism (SSCP) Analysis
After 35 cycles of amplification, the amplified TCR Vα24 gene was diluted (1:39) in a denaturing solution (95% formamide, 10 mmol/L ethylenediaminetetraacetic acid, 0.1% bromophenol blue, 0.1% xylenecyanol) and kept at 90°C for 2 minutes. The diluted samples (2 μl) were electrophoresed in nondenaturing 4% polyacrylamide gels containing 10% glycerol. The gel was run at 35 W constant power for 100 minutes. After electrophoresis, the DNA was transferred to Immobilon-S (Millipore Intertech, Bedford, MA), and hybridized with biotinylated JαQ probe (5′-ACCCTGGGGAGGCTATACTT-3′), streptavidin, biotinylated alkaline phosphatase, and a chemiluminescent substrate system (Phototope Star Detection Kit; New England Biolabs, Beverly, MA). The membrane was then exposed to X-ray film (RX-U; Fuji Photo Film Co., Ltd., Tokyo, Japan). The membrane was reprobed using biotinylated common Cα probe (5′-GAACCCTGACCCTGCCGTGTACC-3′) and visualized as for JαQ probe.
Image Analysis
The X-ray films were photographed, and their image data were analyzed using computer software (NIH image version 1.62, Research Services Branch, National Institutes of Health, Bethesda, MD). To improve the accuracy of the analysis, the relative amount of Vα24JαQ gene expression was calculated as the ratio to the total Vα24 gene expression that is the amount of Vα24-Cα PCR product. Briefly, using gel plotting macros, the total area of bands and smear of each lane on the gels hybridized with internal Cα probe was calculated and the area of Vα24JαQ was divided by the total area. The relative expression of invariant TCR Vα24JαQ gene to total TCR Vα24 was compared between the gingival tissue and peripheral blood samples.
Sequence Analysis of TCR Vα24 Gene
A small area of the dried SSCP gel, corresponding to the position of the invariant Vα24JαQ TCR, was cut out from selected samples. The gel piece was immersed in 50 μl of 10 mmol/L Tris-HCl and 0.1 mmol/L of ethylenediaminetetraacetic acid in a centrifuge microtube and was heated at 80°C for 20 minutes. The extract was vortexed and centrifuged. The supernatant was then subjected to a second PCR amplification for 40 cycles. Amplified DNA was purified by agarose gel electrophoresis and the subsequent use of DNA purification kit. The recovered DNA fragments were subcloned into pCR 2.1 vector and transfected into TOP10F′ (Invitrogen Co., San Diego, CA). After blue/white screening of recombinant plasmids on X-galactoside/isopropyl-thiogalactoside indicator plate, single, white colonies were picked and grown for 12 hours at 37°C on LB broth. After purification of plasmid, the correct inserts in positive clones were confirmed by PCR amplification with Vα24 and Cα primers, and were used for automated sequencing (Pharmacia Biotech, Uppsala Sweden). A clone bearing invariant TCR Vα24JαQ sequence was used as a control in subsequent experiments.
Immunohistochemistry
To estimate the proportion of TCR Vα24-bearing T cells in periodontitis tissues, gingival specimens were collected from a further seven patients, and serial cryostat sections were prepared. The clinical profile of these patients was similar to those analyzed for Vα24JαQ gene expression. The inflammatory gingival tissues were taken so as to analyze the same area as the gene expression being analyzed. Monoclonal anti-Vα24 (Clone NOR3.2; Cosmo Bio Co., Ltd., Tokyo, Japan), anti-CD1d (Pharmingen, San Diego, CA) and anti-CD3 (DAKO, Glostrup, Denmark) were used for single staining by an avidin-biotin-immunoperoxidase (ABC-PO) method. Double staining of Vα24 and CD1d was performed by using combined an ABC-PO method and an alkaline-phosphatase anti-alkaline-phosphatase (APAAP) method.
After rehydration in 0.05% Tris-buffered saline (pH 7.6) and blocking with normal rabbit serum (DAKO), the sections were incubated with primary monoclonal antibody (mAb) at a predetermined dilution followed by rabbit anti-mouse immunoglobulins (DAKO) and finally with monoclonal mouse APAAP (DAKO). Color was developed with an alkaline-phosphatase substrate III kit (Vector, Burlingame, CA). For double staining, the sections were first incubated with monoclonal anti-Vα24 as first primary mAb at a predetermined dilution followed by biotinylated horse anti-mouse IgG (Vector) and finally with ABC-PO. After color development using 0.005% 3,3-diaminobenzidine in Tris-HCl buffer (pH 7.2) containing 0.01% hydrogen peroxide, an APAAP method using monoclonal anti-CD1d as a second primary mAb followed. Incubation for 30 minutes at room temperature was followed by washing for 10 minutes in Tris-buffered saline (pH 7.6). Nuclei were counterstained with hematoxylin. Endogenous peroxidase and alkaline phosphatase activities were blocked by 0.17% NaN3 and 1 mmol/L levamisole, respectively.
Cell Analysis
The total number of Vα24-positive and CD3-positive cells were counted on each section at a magnification of ×400. The proportion of Vα24-positive cells to the total number of CD3-positive cells was calculated. Counts were repeated three times and minimal variation was confirmed.
Statistical Analysis
The relative expression of invariant TCR Vα24JαQ gene was compared between gingival tissues and peripheral blood samples, and between patients and controls. The data were analyzed using unpaired t-test. The statistical significance risk rate was set at P < 0.05.
Results
Relative Expression of TCR Vα24JαQ in Gingival Tissue and Peripheral Blood
In a preliminary experiment, we examined whether a probe for JαQ can in fact detect invariant TCR Vα24JαQ sequence. The small area of dried SSCP gel, corresponding to the position of the band was cut out. DNA extracted from a piece of gel was amplified by PCR using Vα24 and Cα primers, purified by agarose gel electrophoresis and a subsequent DNA purification kit. The DNA was subcloned using a commercial kit (Invitrogen Co., San Diego, CA) and sequenced. As shown in Table 1 ▶ , all clones demonstrated Vα24JαQ sequence specific to NK T cells, suggesting that this method is appropriate.
Table 1.
TCR Vα24 CDR3 Sequences and Frequencies of Jα Segments from Gingival Tissues of Selected Patients
| Patient number | Vα24 | Jα | Cα | Frequency |
|---|---|---|---|---|
| 9 | CVVS | DRGSTLGRLYFGRGTQLTVWPD | IQN | 3/3 |
| 13 | CVVS | DRGSTLGRLYFGRGTQLTVWPD | IQN | 4/4 |
The small area of dried SSCP gel, corresponding to the position of the band, to which JαQ-specific probe has hybridized, was cut out and DNA was extracted. After purification of DNA and subsequent PCR amplification using Vα24 and Cα primers, the amplified products were subcloned and sequenced. Sequence is expressed as single letter codes of amino acids.
Clonality of Vα24-Bearing T Cells in Peripheral Blood and Gingival Tissues
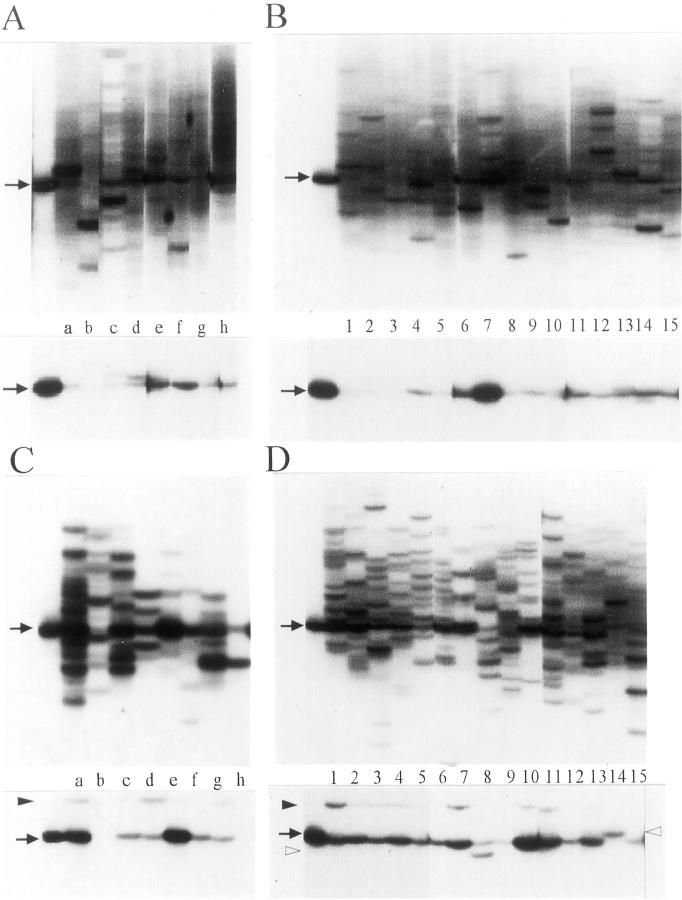
We tried to identify the clonality of Vα24+ T cells in gingival tissues and peripheral blood of both periodontitis patients and controls using an internal Cα probe that can detect all of the Vα24+ T cells. As shown in the top panels of Figure 1, A and B ▶ , samples of peripheral blood from both controls and periodontitis patients demonstrated a few bands on a smear background or a dense smear without appreciable bands. This indicates either relatively few clonotypes are present in peripheral blood or heterogeneity of the Vα24+ T cells. Because there was no difference in the number of clonotypes between periodontitis patients and controls, it seems that clonality of peripheral blood T cells could not be affected by oral bacteria.
Figure 1.
Demonstration of the Vα24+ T cell clonalities and the invariant Vα24JαQ TCR in peripheral blood of controls (A), peripheral blood of periodontitis patients (B), gingival tissue of controls (C), and gingival tissue of periodontitis patients (D). Total RNA was extracted from PBMCs and gingival tissues and then subjected for RT-PCR-SSCP analysis. The top and bottom panels demonstrate the total Vα24+ TCR SSCP profiles as detected with a Cα probe and the invariant Vα24JαQ band as detected with the JαQ-specific probe, respectively. The cloned Vα24JαQ DNA fragment as a positive control was applied on the far left lanes. The alphabetical code and the numerical code correspond to each control (A and C) and each patient (B and D), respectively, implying that blood samples and gingival tissues were taken from same subjects and patients. Arrows indicate the position for the invariant Vα24+JαQ TCR. The unique bands were indicated by arrowhead. Another unique band appeared in patients 8 and 14 was indicated by open arrowhead.
In contrast, a marked increase in the number of Vα24 clonotypes, which is identifiable as a number of distinct bands, was found in both the periodontitis and gingivitis tissues (Figure 1, C and D ▶ ; top). This indicates that the clonality of Vα24-bearing T cells did not differ between periodontitis tissues and gingivitis tissues.
Detection of the Invariant Vα24JαQ TCR
After removing the Cα probe, the membrane was re-hybridized with the biotinylated probe for the invariant Vα24JαQ sequence. As shown in bottom panels of Figure 1 ▶ , the invariant Vα24JαQ TCR was detected in peripheral blood and gingival tissue samples from both controls and periodontitis patients. However, the frequency of samples containing the Vα24JαQ TCR was lower in peripheral blood than gingival tissue samples (Figure 1, A and B) ▶ . Although there was no difference in the frequency and the density of bands corresponding to the invariant Vα24JαQ in the peripheral blood of patients and controls, those of gingival tissue samples were higher in periodontitis tissues compared with gingivitis tissues. In gingivitis tissues, two samples (controls b and h) demonstrated absence of the band corresponding to the invariant Vα24JαQ TCR. Although the density of the invariant Vα24JαQ TCR was higher in gingival tissues than in peripheral blood of controls a and e, it did not demonstrate a big difference between the gingival tissues and peripheral blood of controls c, d, f, and g. Therefore, although the intensity of a band does not necessarily represent the absolute number of clones, these results generally indicate that the frequency of the invariant Vα24JαQ-bearing NK T cells increased in periodontitis lesions and to a lesser extent in gingivitis lesions. Several faint bands appeared at different positions of the invariant Vα24JαQ in most gingival tissue samples (Figure 1, C and D ▶ ; bottom). Although these bands were at a similar position (arrowhead), another unique band did appear in patients 8 and 14 (open arrowhead).
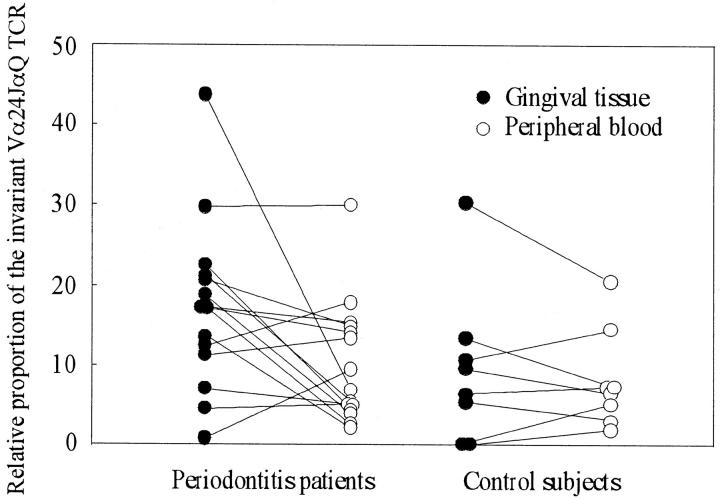
To compare the proportion of the invariant Vα24JαQ-bearing NK T cells within the Vα24 T cells between periodontitis and gingivitis lesions, semiquantitative analysis was performed. As shown in Figure 2 ▶ , relative gene expression of Vα24JαQ was slightly but significantly higher in periodontitis tissues than gingivitis tissues (P = 0.016). No significant difference was observed either for peripheral blood between patients and controls or between gingivitis tissues and peripheral blood. Thus, it is apparent that an increased proportion of Vα24JαQ NK T cells in gingival tissue is a characteristic feature in both periodontitis lesions and gingivitis lesions.
Figure 2.
Relative proportion of the invariant Vα24JαQ TCR in total Vα24 population. SSCP profiles were analyzed on a computer software (NIH image version 1.62) and relative area and density of Vα24JαQ band to the total area and density of Vα24 TCR was calculated in each lane. The data were expressed as mean ± SD. Relative proportion of the Vα24JαQ TCR in the total Vα24 population was significantly higher in periodontitis tissues than either in gingivitis tissues (P = 0.016) or in peripheral blood of both controls (P = 0.015) and periodontitis patients (P = 0.036).
Immunohistology of the Periodontitis Tissues
As with the previous reports, the dominant cell types were B cells and plasma cells. However, a significant number of T cells were also observed. The CD3+ cells formed clusters or were scattered beneath the pocket epithelium (data not shown).
Vα24+ cells and CD1d+ cells were found in all of the tissues examined. The proportion of Vα24+ cells to CD3+ cells in periodontitis tissue was the same as that of peripheral blood, which had been determined in a preliminary experiment in four out of seven specimens. In three cases, a very high proportion of Vα24+ cells was observed (Table 2) ▶ . However, we could not find any relationship between the proportion of Vα24+ cells and clinical condition. Although the specificity of the antibody is not for the invariant Vα24JαQ but for the variable Vα24 chain, it is possible that these high proportions of Vα24+ cells reflect the accumulation of NK T cell population.
Table 2.
Frequency of Vα24+ Cells to CD3+ Cells in Periodontitis Lesions
| Patient no. | Number of CD3+ cells | Number of Vα24+ cells | Proportion of Vα24+ cells within CD3+ population (%) |
|---|---|---|---|
| 16 | 1890 | 46 | 2.43 |
| 17 | 2040 | 13 | 0.64 |
| 18 | 3848 | 170 | 4.42 |
| 19 | 4032 | 10 | 0.25 |
| 20 | 2399 | 8 | 0.33 |
| 21 | 1289 | 58 | 4.50 |
| 22 | 3441 | 17 | 0.49 |
Total number of positive cells for each antibody was counted on neighboring sections.
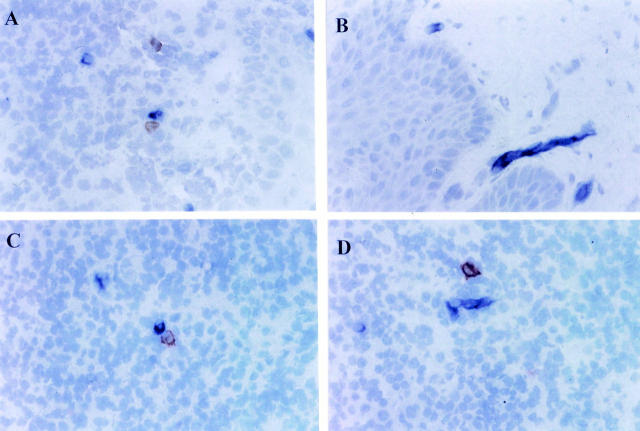
Anti-CD1d antibody reacted with the cells in the inflammatory cell infiltrate and with spindle shape cells, in noninflamed connective tissue beneath the oral epithelium. Double staining revealed that Vα24+ cells and CD1d+ cells were in close proximity with direct cell-cell contact sometimes being observed. In most cases however, Vα24+ cells and CD1d+ cells were stained separately (Figure 3, A–D) ▶ .
Figure 3.
Expression of Vα24 and CD1d in periodontitis tissues. Double staining of Vα24 and CD1d was performed by using combined an ABC-PO method and an alkaline-phosphatase anti-alkaline-phosphatase (APAAP) method on cryostat sections. Vα24+ cells appeared as brown and CD1d+ cells appeared as blue. Anti-CD1d antibody reacted with either cells in the inflammatory infiltrate (A) or cells with spindle shape beneath the oral epithelium (B). Although direct cell-cell contact between Vα24+ cells and CD1d+ cells was observed in some sections (C), they were also stained separately (D). Original magnification, ×132.
Discussion
Periodontitis is a chronic inflammatory disease caused by a group of gram-negative bacteria. However, the immune response to self-antigens such as collagen type I, a major component of the periodontium, has also been considered as one of the pathogenic pathways. High titers of anti-collagen type I antibody are found in the sera 30 and collagen type I-specific T cell clones can be identified in the inflamed gingival tissues of periodontitis patients. 31 Recently, we demonstrated that self-hsp60 might also be a target for an autoimmune response in periodontitis because of molecular mimicry between human hsp60 and its bacterial homologue GroEL. 26 As numerical or functional defects in NK T cells have been reported in systemic as well as organ-specific autoimmune diseases, 17-25 it was of particular interest to investigate the status of NK T cells in periodontitis.
In the present study, we used SSCP methodology to analyze the invariant Vα24JαQ TCR within the Vα24 population. Most recently, Illés and colleagues 32 also applied the SSCP method and demonstrated a great reduction of Vα24JαQ NK T cells in the peripheral blood of multiple sclerosis patients. We have used this method previously to analyze the Vβ repertoire of infiltrating T cells in periodontitis lesion. 33,34 It has been reported that PCR-SSCP analysis can detect an accumulation of T cell clonotypes in heterogeneous populations at a frequency of one in several thousands, which is more sensitive than limiting dilution analysis previously used to estimate the frequency of antigen-specific T cell populations. 35
If there is an autoimmune aspect to periodontitis, it follows that a reduction in NK T cells might be involved in an elevated humoral immune response to self hsp60 as well as periodontopathic bacterial GroEL. However, it is of note that the results did not support this concept. In the present study, we clearly demonstrated that the T cells bearing the invariant Vα24JαQ TCR are the dominant clone within the Vα24+ population in periodontitis tissues, which is considered to be the progressive lesion and less dominant in gingivitis tissues, a possible stable lesion. Although one or two additional faint bands were detected in some samples, there was no difficulty in identifying the band as the invariant TCR in a given sample by comparison with the positive control. The additional bands appeared at the same position across the samples of periodontitis tissues (Figure 1D ▶ , bottom). As these bands were detected by the JαQ-specific probe, they are also considered to be the invariant TCR. Interestingly, Kent and colleagues 36 examined 126 T cell clones bearing the invariant and variant Vα24JαQ CDR3 region and demonstrated that 15 clones possessed no N-region diversity, but bore either a nucleotide sequence that resulted in an amino acid substitution at the C-terminal amino acid in the Vα24 segment or the first amino acid in the JαQ segment. These nucleotide substitutions may also have occurred in the patients we examined. However, direct sequence of the DNA extracted from these bands is required to prove the band in fact represents the invariant Vα24JαQ TCR.
To the best of our knowledge, this is the first study to show Vα24JαQ NK T cells in bacterial infection-related chronic inflammatory lesions. Although the role of NK T cells in chronic inflammatory periodontal disease is not known, considering the function of NK T cells as regulators of autoimmune responses, they may play a role in controlling the tissue destruction mediated by autoreactive T cells and B cells. In addition to elevated humoral immune response to self-hsp60, we found that hsp60-reactive T cells accumulated in periodontitis lesions (Yamazaki K, Ohsawa Y, Tabeta K, Ito H, Ueki K, Yoshie H, Seymour GJ, manuscript in preparation). Therefore, the high proportion of the invariant Vα24JαQ T cells in periodontitis tissues that is supposed to be a progressive lesion and in stable gingivitis tissues may be explained by the idea that NK T cells accumulate to control autoimmune response in each lesion by differential functions. Alternatively, because the NK T cells have the capacity to secrete rapidly both interleukin-4 (IL-4) and interferon-γ (IFN-γ) without priming but become polarized for the production of IL-4 after stimulation 13,37 they may regulate the Th1/Th2 balance of CD4+ T helper cells in the lesion. In this regard, it has been reported that both Th1-type 38 and Th2-type 4 responses are predominant in the periodontitis lesion suggesting that the activation stage of NK T cell may explain these contradictory reports. In one case of controls, the invariant Vα24JαQ TCR was higher in gingival tissue than in peripheral blood. Although the underlying mechanism is not known, this may be indicative of the conversion from a stable lesion to a progressive lesion. 1 Although the proportion of invariant Vα24JαQ TCR was statistically higher in periodontitis tissues compared with gingivitis tissues, they were still present in the majority of gingivitis tissues and the proportion of Vα24JαQ TCR within Vα24+ cells was relatively similar between periodontitis and gingivitis. As the T cells in the gingivitis lesion and the periodontitis lesion are phenotypically similar 39 but functionally different, 4 it would be reasonable to consider that the cytokine profile by NK T cells can be different in two distinct disease entities.
In the immunohistochemical study, we could identify Vα24+ cells in periodontitis tissues. The antibody used has a specificity for variable α24 chain not for rearranged Vα24JαQ. Therefore, the positive cells may include not only NK T cells but also other Vα24+ cells. The proportion of Vα24+ cells to CD3+ cells was variable from 0.25 to 4.5%. This wide variation may reflect different disease activity of the lesions although the clinical profiles are similar. It is likely that different T cell subsets predominate at different stages of disease. 40
In vitro studies demonstrated that NK T cells specifically recognize glycolipid α-galactosylceramide and its synthetic homologue and that this recognition requires expression of the MHC class I-like molecule CD1d. 13,37,41-44 In periodontitis lesions, morphologically distinct cell types expressed CD1d antigen. Some of these cells seemed to interact with Vα24+ cells. However, the ligand for the human NK T cells has not been fully elucidated. Although it has been shown that the reactivity of mouse Vα14+ NK T cells for CD1d is greatly augmented by the addition of the glycosphingolipid α-galactosylceramide antigens 41,43 the vast majority of glycosphingolipids in mammalian cells have a β rather than α linkage at the 1 position of the hexose to the sphingosine base. Phytosphingolipids with an α-linkage of the sugar are not known to be abundant in microorganisms. 45 Therefore, it remains to be determined whether α-galactosylceramide or some other compound(s) is the natural ligand(s) responsible for NK T cell activation. Detection of antigens being capable of binding to CD1d and stimulating NK T cells in periodontopathic bacteria would be of particular interest.
In summary, we demonstrated an elevation of Vα24JαQ NK T cells in the gingival lesion of periodontitis patients and to a lesser extent in that of gingivitis patients as compared with peripheral blood of either periodontitis patients or nondiseased controls. This finding is of particular interest, because autoimmune mechanisms are thought to be involved in the destructive periodontal disease process. Although the reason for the elevated proportion of the NK T cells remains unclear, it is postulated that NK T cells are recruited to down-regulate the autoimmune response against self-components such as hsp60. At the same time, they may control cellular and humoral immune responses by producing different cytokines in gingivitis and periodontitis lesions, respectively. To clarify these issues, further study is needed.
Acknowledgments
We thank G. J. Seymour (Oral Biology and Pathology, Department of Dentistry, The University of Queensland) for critical reading of this manuscript.
Footnotes
Address reprint requests to Dr. Kazuhisa Yamazaki, Dept. of Periodontology, Faculty of Dentistry, Niigata University, 5274, Gakkocho-Dori 2-ban-cho, Niigata 951-8514, Japan. E-mail: kaz@dent.niigata-u.ac.jp.
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (grants 10470458,10357020,and 10307054).
References
- 1.Seymour GJ: Possible mechanisms involved in the immunoregulation of chronic inflammatory periodontal disease. J Dent Res 1987, 66:2-9 [DOI] [PubMed] [Google Scholar]
- 2.Seymour GJ, Gemmell E, Reinhardt RA, Eastcott J, Taubman MA: Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodont Res 1993, 28:478-486 [DOI] [PubMed] [Google Scholar]
- 3.Mackler BF, Frostad KB, Robertson PB, Levy BM: Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodont Res 1977, 12:37-45 [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki K, Nakajima T, Hara K: Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol 1995, 99:384-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamashita K, Eastcott JW, Taubman MA, Smith DJ, Cox DS: Effect of adoptive transfer of a cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect Immun 1991, 59:1529-1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole KL, Seymour GJ, Powell RN: Phenotypic and functional analysis of T-cells extracted from chronically inflamed human periodontal tissues. J Periodontol 1987, 58:569-573 [DOI] [PubMed] [Google Scholar]
- 7.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP: Phenotypic analysis of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol 1987, 7:235-245 [DOI] [PubMed] [Google Scholar]
- 8.Lantz O, Bendelac A: An invariant T cell receptor a chain is used by a unique subset of MHC class I specific CD4+ and CD4−CD8− T cells in mice and humans. J Exp Med 1994, 180:1097-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A: Mouse NK1+ T cells. Curr Opin Immunol 1995, 7:367-374 [DOI] [PubMed] [Google Scholar]
- 10.Bix M, Locksley RM: Natural T cells: cells that co-express NKPR-1 and TCR. J Immunol 1995, 155:1020-1022 [PubMed] [Google Scholar]
- 11.MacDonald HR: NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med 1995, 182:633-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicari A, Zlotnik A: Mouse NK1.1+ T cells: a new family of T cells. Immunol Today 1996, 17:71-76 [DOI] [PubMed] [Google Scholar]
- 13.Bendelac A, Rivera MN, Park SH, Roark JH: Mouse CD1-specific NK1 T cells—development, specificity, and function. Ann Rev Immunol 1997, 15:535-562 [DOI] [PubMed] [Google Scholar]
- 14.Porcelli S, Yockey CE, Brenner MB, Balk SP: Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J Exp Med 1993, 178:1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A: An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J Exp Med 1994, 180:1171-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Exley M, Garcia J, Balk SP, Porcelli S: Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J Exp Med 1997, 186:109-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gombert JM, Herbelin A, Tancredebohin E, Dy M, Carnaud C, Bach JF: Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur J Immunol 1996, 26:2989-2998 [DOI] [PubMed] [Google Scholar]
- 18.Baxter AG, Kinder SJ, Hammond KJL, Scollay R, Godfrey DI: Association between αβTCR+CD4−CD8− T cell deficiency and IDDM in NOD/Lt mice. Diabetes 1997, 46:572-582 [DOI] [PubMed] [Google Scholar]
- 19.Godfrey DI, Kinder SJ, Silveria P, Baxter A: Flow cytometric study of T cell development in NOD mice reveals a deficiency in αβTCR+CD4−CD8− thymocytes. J Autoimmun 1997, 10:279-285 [DOI] [PubMed] [Google Scholar]
- 20.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG: α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med 1998, 187:1047-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA: Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature 1998, 391:177-181 [DOI] [PubMed] [Google Scholar]
- 22.Hammond K, Cain W, van Driel I, Godfrey D: Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol 1998, 10:1491-1499 [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Dennert G: The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type-1 positive cells: evidence for their suppressive action on bone mallow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med 1993, 177:155-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, Koseki H, Taniguchi M: Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol 1996, 156:4035-4040 [PubMed] [Google Scholar]
- 25.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M: Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med 1995, 182:1163-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabeta K, Yamazaki K, Hotokezaka H, Yoshie H, Hara K: Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin Exp Immunol 2000, 120:285-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mollenhauser J, Schulmeister A: The humoral immune response to heat shock proteins. Experientia 1992, 48:644-649 [DOI] [PubMed] [Google Scholar]
- 28.Seymour GJ, Gemmell E, Walsh LJ, Powell RN: Immunohistological analysis of experimental gingivitis in humans. Clin Exp Immunol 1988, 71:132-137 [PMC free article] [PubMed] [Google Scholar]
- 29.Oksenberg JR, Stuart S, Begovich AB, Bell RB, Erlich HA, Steinman L, Bernard CCA: Limited heterogeneity of rearranged T-cell receptor Vα transcripts in brains of multiple sclerosis patients. Proc Natl Acad Sci USA 1990, 345:344-346 [DOI] [PubMed] [Google Scholar]
- 30.Hirsch HZ, Tarkowski A, Miller EJ, Gay S, Koopman WJ, Mestecky J: Autoimmunity to collagen in adult periodontal disease. J Oral Pathol 1988, 17:456-459 [DOI] [PubMed] [Google Scholar]
- 31.Wassenaar A, Reinhardus C, Thepen T, Abraham-Inpijn L, Kievits F: Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect Immun 1995, 63:2147-2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illés Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T: Differential expression of NK T cell Vα24JαQ invariant TCR chain in the lesion of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol 2000, 164:4375-4381 [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki K, Nakajima T, Ohsawa Y, Tabeta K, Yoshie H, Sakurai K, Seymour GJ: Selective expansion of T cells in gingival lesions of patients with chronic inflammatory periodontal disease. Clin Exp Immunol 2000, 120:154-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osawa Y, Yamazaki K, Nakajima T, Hara K: Clonal accumulation of T-cells bearing Vβ6 T-cell receptor in chronic inflammatory periodontal disease. Oral Microbiol Immunol 2000, 15:211-217 [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Sakoda H, Nakajima T, Kato T, Okubo M, Dohi M, Mizushima Y, Ito K, Nishioka K: Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol 1992, 4:1219-1223 [DOI] [PubMed] [Google Scholar]
- 36.Kent SC, Hafler DA, Strominger JL, Wilson SB: Noncanonical Vα24JαQ T cells with conservative a chain CDR3 region amino acid substitutions are restricted by CD1d. Hum Immunol 1999, 60:1080-1089 [DOI] [PubMed] [Google Scholar]
- 37.Hong S, Scherer DC, Singh N, Mendiratta SK, Serizawa I, Koezuka Y, Van Kaer L: Lipid antigen presentation in the immune system: lessons learned from CD1d knockout mice. Immunol Rev 1999, 169:31-44 [DOI] [PubMed] [Google Scholar]
- 38.Okada H, Murakami S, Kitamura M, Nozaki T, Kusumoto Y, Hirano H, Shimauchi H, Shimabukuro Y, Saho T: Diagnostic strategies of periodontitis based on the molecular mechanisms of periodontal tissue destruction. Oral Dis 1996, 2:87-95 [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki K, Nakajima T, Aoyagi T, Hara K: Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodont Res 1993, 28:324-334 [DOI] [PubMed] [Google Scholar]
- 40.Mathur A, Michalowicz BS: Cell-mediated immune system regulation in periodontal diseases. Crit Rev Oral Biol Med 1997, 8:76-89 [DOI] [PubMed] [Google Scholar]
- 41.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M: CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 1997, 278:1626-1629 [DOI] [PubMed] [Google Scholar]
- 42.Joyce S, Wood AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR: Natural ligand of mouse CD1d: cellular glycophosphatidylinositol. Science 1998, 279:1541-1544 [DOI] [PubMed] [Google Scholar]
- 43.Burdin N, Kronenberg M: CD1-mediated immune responses to glycolipids. Curr Opin Immunol 1999, 11:326-331 [DOI] [PubMed] [Google Scholar]
- 44.Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD: CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NK T cells. Science 1999, 283:225-229 [DOI] [PubMed] [Google Scholar]
- 45.Brossay L, Kronenberg M: Highly conserved antigen-presenting function of CD1d molecules. Immunogenetics 1999, 50:146-151 [DOI] [PubMed] [Google Scholar]