Abstract
Monocytoid B cells (MBCs) are a subset of B cells that may be recognized in several reactive and tumoral lymph node conditions, including toxoplasmic lymphadenitis, infectious mononucleosis, and Hodgkin’s lymphoma. Although this is a commonly observed cell population, which has even given its name to a type of lymphoma, MBC lymphoma, scarcely any information is available about the function and characteristics of this cell type. A relationship with marginal zone (MZ) B lymphocytes has been claimed for MBCs, but this has not yet been fully proven. Indeed, specific markers for MBCs are still lacking, which has made it difficult to analyze their relationship with other B cell subpopulations and confirm the existence of tumors deriving from this B cell subset. We used a panel of cell cycle markers to explore the characteristics of MBCs and their relationship with MZ B cells, nodal MZ lymphoma, and splenic MZ lymphoma. We therefore compared the phenotypic profile of MBCs in different conditions with normal MZ B cells within the spleen and mesenteric lymph nodes, with a group of seven cases of nodal MZ/MBC lymphoma and another group of five cases of splenic MZ lymphoma. MBCs were mainly in the G0 to G1 phases, as deduced from the presence of a proportion of between 10 and 35% Ki67-positive cells, whereas very low expression was observed with cyclin A and cyclin B staining. Nests of MBCs were clearly labeled by the expression of p21WAF1, a cyclin-dependent kinase inhibitor (CKI), rarely detectable in benign lymphocytes, and by cyclin E. Basically all MBCs were bcl-2-negative, and high cyclin D2 and cyclin D3 were also detected in these cells, at proportions and intensities above expected levels, when the percentage of proliferating cells was taken into account. p27KIP1 expression was characterized by homogeneous reactivity, higher than that observed in other B cell populations with a relatively high-growth fraction. Immunoglobulin staining showed undetectable light and heavy chains. However, splenic MZ cells, nodal MZ lymphoma, and splenic MZ lymphoma showed a distinct expression of IgM and bcl-2, with high p27 KIP1 nuclear expression and undetectable or low levels of cyclin A, B, E, or D, or p21WAF1 expression. The data from this study show an unexpected immunophenotype in MBCs, different from the one observed in splenic and lymph node MZ B cells. This suggests that either MBCs are a unique B cell population from a distinct cell lineage, or if related to MZ cells, they would represent a definite differentiation stage characterized by a distinctive immunophenotype. They also show so-called MZ/MBC lymphoma to be more closely related to lymph node and splenic MZ B cells, as they do not share the most distinctive features of MBCs.
Monocytoid B cells (MBCs) are a subset of B cells that may be recognized in several reactive and tumoral lymph node conditions, including toxoplasmic lymphadenitis, infectious mononucleosis, and Hodgkin’s lymphoma. 1-5 They are situated in clusters inside and around intermediary sinuses, adjacent to the subcapsular sinus and forming parafollicular rims. The cytological features of MBCs are the presence of abundant pale to clear cytoplasm, medium size, and bland-looking irregular nuclei with inconspicuous nucleoli.
A relationship with marginal zone (MZ) B lymphocytes has been claimed for this B cell subpopulation, based mainly on architectural localization and cytology, but also on the proposed IgM+, IgD− immunophenotype and the results of mutational analysis of rearranged immunoglobulin variable genes (VH). 6-8 Although a clear relationship may be established between splenic MZ cells and Peyer’s patches MZ lymphocytes, based on the presence of an immunophenotype of IgM+, IgD−, alkaline phosphatase+, 4D12+, CD21+, 9 this has not yet been fully proved for MBCs.
Specific markers for MBCs are still lacking, and this makes it difficult to analyze their relationship with other B cell subpopulations and to confirm the existence of tumors derived from this cell subpopulation. This difficulty is reflected in the denomination of the tumors that are thought to derive from MBCs, which have recently been termed MZ B cell lymphoma/MBC lymphoma (MZL/MBCL),. 10-12 This suggests that both subpopulations, MBCs and MZ cells, belong to the same cell lineage, and has in fact led to the use of MZ cells or MBCs as almost equivalent terms. Nevertheless, the only data, which seem to show there is a relationship between MBCs and MZL/MBCL, are morphological, and they are based on the architectural feature of parafollicular localization and the presence of clear cytoplasm.
These difficulties in the identification of MBCs have been used as an explanation for the differences in the frequency of IgH somatic mutations reported in the studies performed to date, 8,13 these differences having been attributed to the technique of identification and microdissection of MBCs.
Immunohistochemical analysis of human tissues has shown that the expression of specific cyclin and cyclin-dependent kinase inhibitors (CKIs) is dependent on tissue and cell type, which provides additional markers for the identification of cell subpopulations. 14,15 Analysis of reactive lymphoid tissue with antibodies for cyclins and CKIs has shown that nontumoral lymphoid tissue shows some peculiarities in the expression of cyclins and CKIs. Thus, in opposition to what is usually observed in epithelial tissues, normal lymphocytes express almost undetectable levels of p21WAF1 expression. 15 As in other tissues, p27KIP1 is expressed by resting cells, and no expression by proliferating cells has been observed. 16 At the same time, lymphoid cells usually show an inverse expression pattern of bcl-2 and Ki67. This has been said to be a result of the division of the lymphocytes in a pool of bcl-2+ long-lived memory cells and another section of bcl-2- short-lived proliferating cells. 17-19 Lymphoid cells do not express cyclin D1 at detectable levels, and only display weak cyclin E expression, whereas they show an intermediate level of expression of cyclin D2 and cyclin D3. These cyclin D2- and cyclin D3-positive cells correspond to the proliferating cells inside and outside the germinal center.
We used a panel of these cell cycle markers to explore the characteristics of MBCs and their relationship with splenic and nodal marginal B cells, as well as nodal MZ B cell lymphoma/MBCL and splenic MZ lymphoma (SMZL).
Materials and Methods
Cases
Six lymph nodes with toxoplasmic lymphadenitis, three cases of nontoxoplasmic lymphadenitis, and four lymph nodes with classical mixed cellularity Hodgkin’s disease were included in this study. All of them showed a conspicuous presence of MBCs, detectable by morphological observation.
For the study of splenic MZ B cells, six splenectomy specimens from cases of idiopathic thrombocytopenic purpure and spleens removed incidentally during abdominal surgery were included. Four cases of mesenteric lymph nodes with MZ B cell hyperplasia were also included, as were seven cases with nodal MZL/MBCL. Tumors were diagnosed in the cervical (three cases), inguinal (two cases), or axillary (two cases) lymph nodes. All cases were considered primary nodal MZL/MBCL, because no evidence was observed of mucosal involvement in any localization. All of these cases showed an IgD− phenotype. On the other hand, we studied five cases of SMZL. The criteria used in the recognition of nodal MZL/MBCL and SMZL were taken from the REAL Classification. 20
Tissues
All specimens had been fixed in 10% buffered formalin and after dehydration steps in alcohol series they were embedded in paraffin.
Immunohistochemical Technique
Paraffin sections (4 mm) were dehydrated and deparaffinized according to standard procedures. Pressure cooker-based antigen retrieval was performed for 3 minutes, with 10 mmol/L of sodium citrate buffer at pH 6. This was followed by incubation with the primary antibodies described in Table 1 ▶ . Immunodetection was performed with biotinylated anti-mouse anti-rabbit immunoglobulins, followed by peroxidase-labeled streptavidin (LSAB-DAKO, Glostrup, Denmark) and diaminobenzidine chromogen as substrate. All immunostaining was performed using the Techmate 500 (DAKO) automatic immunostaining device.
Table 1.
Description of the Primary Antibodies Used in Immunohistochemical Techniques
| Antibody | Manufacturer | Clone | Dilution | Incubation | Control |
|---|---|---|---|---|---|
| Cyclin A | Novocastra (Newcastle upon Tyne, UK) | 6E6 | 1:100 | 40 minutes | Proliferating cells in the tonsil |
| Cyclin B1 | Novocastra | 7A9 | 1:25 | 40 minutes | Proliferating cells in the tonsil |
| Cyclin D1 | Novocastra | DCS-6 | 1:50 | Overnight at 4°C | Mantle cell lymphoma |
| Cyclin D2 | Novocastra | DCS-3.1 | 1:50 | Overnight at 4°C | Germinal center cells in the tonsil |
| Cyclin D3 | Novocastra | DCS-22 | 1:10 | Overnight at 4°C | Sinus lining cells in the spleen |
| Cyclin E | Novocastra | 13A3 | 1:10 | 40 minutes | Trophoblast |
| P21 | Oncogen (Cambridge, UK) | EA10 | 1:25 | 40 minutes | Intestinal epithelium |
| P27 | Transduction Lab. (Lexington, KY) | 57 | 1:1000 | 40 minutes | Quiescent lymphocytes in the tonsil |
| MIB1 | Immunotech (Marseille, France) | MIB1 | 1:50 | 40 minutes | Proliferating cells in the tonsil |
| Bcl-2 | DAKO (Glostrup, Denmark) | 100+124 | 1:25 | 40 minutes | Quiescent lymphocytes in the tonsil |
| IgA | DAKO | Polyclonal | 1:25 | 40 minutes | Tonsillar B cells |
| IgD | DAKO | Polyclonal | 1:200 | 40 minutes | Tonsillar B cells |
| IgM | DAKO | Polyclonal | 1:50 | 40 minutes | Tonsillar B cells |
| IgG | DAKO | Polyclonal | 1:800 | 40 minutes | Tonsillar B cells |
| P53 | DAKO | DO7 | 1:50 | 40 minutes | Germinal center cells in the tonsil |
Immunohistochemistry Evaluation
Those proteins with nuclear staining and cyclin B were quantitatively evaluated. The relative percentage of positive cells was evaluated using a 10 × 10 square grid fitted into the eyepiece of the microscope and with an objective lens of ×40. At least 200 cells were counted in every case. Heavy and light chains, and bcl-2 proteins, which have cytoplasmic expression, were qualitatively evaluated.
Results
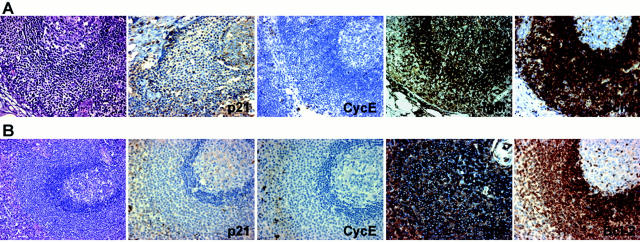
MBCs are mainly in the G0-G1 phases, as is shown by Ki67, cyclin A, and cyclin B staining. Thus, although a relatively high proportion of between 10 and 35% of MBCs were Ki67-positive, the fraction of cyclin A-positive cells was always <5%; and the presence of cyclin B-positive cells, all of them showing cytoplasmic localization, was rarely observed (Figure 1 ▶ and Table 2 ▶ ).
Figure 1.
A: Expression of different cell cycle proteins in MBCs in a case of toxoplasmic lymphadenitis. B: Detail of the expression of IgM, bcl-2, p21WAF1, cyclin E, and p27KIP1.
Table 2.
Phenotypic Profile of MBCs Compared to Other B Cell Subpopulations, Nodal MZL/MBCL, and SMZL
| Antibody | MBCs | MZ B cell in spleen and LN | Nodal MZL/MBCL | SMZL | Other B cell subpopulations |
|---|---|---|---|---|---|
| MIB1 | 10–35% | 5–10% | 20–25% | <25% | G1, S, G2, M phases. GC and other proliferating cells |
| Cyclin A | <5% | 1–2% | <5% | <10% | S, G2, M phases. 30–45% GC cells. |
| Cyclin B | <2% | <2% | <5% | <5% | G2-M phases. 10–15% of GC cells. |
| P21/WAF1 | >40% | <1% | <1% | <5% | Undetectable. EBV+ lymphocytes. |
| P27/KIP1 | 50–80% | 80–90% | 20–50% | >50% | Resting cells (mantle cells and others) |
| Cyclin E | >50% | <1% | <5% | <1% | Scattered GC cells (<10%) |
| Cyclin D1 | <1% | <1% | <1% | <1% | Undetectable. |
| Cyclin D2 | >40% | <1% | <1% | <5% | GC cells |
| Cyclin D3 | 60% | <1% | 5–10% | 5–10% | GC cells |
| Ig | − (IgG−/+) | IgM | + (6 IgM, 1 IgG) | + (4 IgM, 1 IgG) | B cells |
| κλ | − | + | + (7/7)κ | + (4λ/1κ) | B cells |
| Bcl2 | − | + | + | + | Positive in mantle cells. Negative in PC |
| P53 | <1% | <1% | <5% | 0–60% | −/+ in the GC cells |
MZ, marginal zone; LN, lymph node; GC, germinal center; EBV, Epstein-Barr virus; PC, plasma cell.
MBCs also showed some peculiarities in the expression of cyclins and CKIs. Thus, in opposition to what is seen in other lymphoid subpopulations, all MBCs were basically bcl-2-negative, independently of Ki67 expression. Additionally, most MBCs were clearly labeled by the expression of p21WAF1, a CKI rarely detectable in benign lymphocytes. The same situation was observed with cyclin E, which was hardly detectable in isolated germinal center B cells, and frequently expressed in contrast, by MBCs. High levels of cyclin D2 and cyclin D3 protein were also detected in these cells, in a proportion and intensity above expected levels, taking into account the percentage of proliferating cells. p27KIP1 expression in these cells was characterized by homogeneous reactivity, higher than that observed in other B cell populations with a relatively high growth fraction (Figure 1 ▶ and Table 2 ▶ ). Immunoglobulin staining showed undetectable kappa, lambda, IgM, IgA, and IgD, with only weak imprecise IgG staining attributable to the background existent within the lymph node sinuses. No difference was observed in the immunophenotype of MBCs between toxoplasmic lymphadenitis, nontoxoplasmic lymphadenitis, and Hodgkin’s disease.
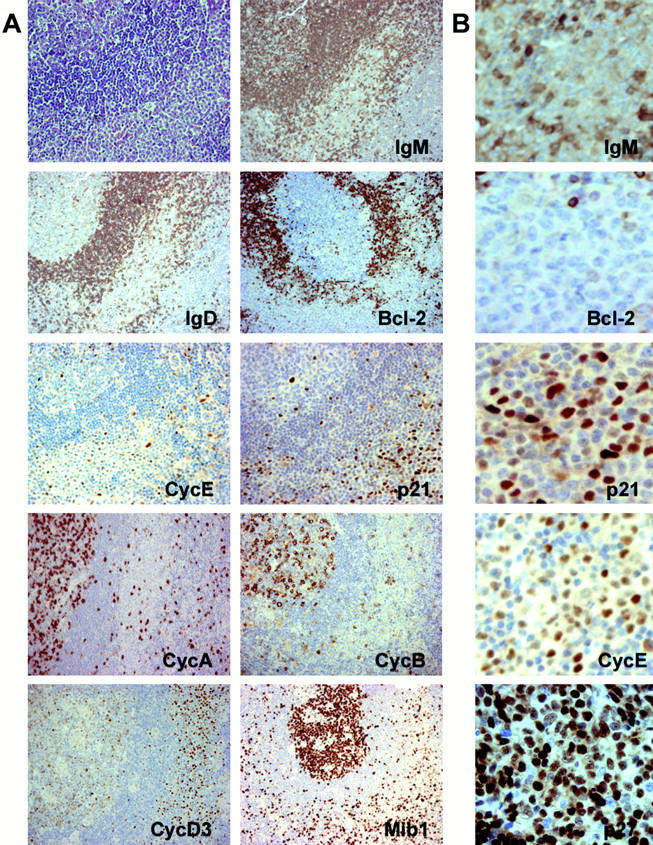
The immunophenotype of MZ cells in mesenteric lymph nodes and spleens differs strongly from that which is observed in MBCs. Thus MZ cells show distinct immunoglobulin staining (IgM), bcl-2 expression, with a low growth fraction, high and homogenous p27KIP1 nuclear expression, and undetectable levels of cyclin A, B, E, or D, or p21WAF1 expression (Figure 2 ▶ and Table 2 ▶ ).
Figure 2.
A: Expression of IgM, bcl-2, p21WAF1, and cyclin E in a case of mesenteric lymph node with MZ B cell hyperplasia. B: Expression of IgM, bcl-2, p21WAF1, and cyclin E in a case of spleen with MZ B cell hyperplasia.
Nodal MZL/MBCL cases display an immunophenotype different from the one observed in MBCs, and similar to that of MZ B cells. Thus, all cases of MZ lymphoma were IgM- or IgG-positive and had a κ-restriction light chain. Most tumoral cells in all cases showed bcl-2 positivity, and essentially lacked any labeling for p53, p21WAF1, cyclin D1, and cyclin D2. The level of expression of cyclin A and cyclin B was low. A low level of expression was also observed with cyclin D3. All but two cases were negative for cyclin E (Figure 3 ▶ and Table 2 ▶ ). SMZL here analyzed coincide in showing the same immunophenotype Ig+, bcl-2+, with very low or absence of p21WAF1, cyclin E, cyclin D2, and cyclin D3 expression. All but one case showed low or negative p53 nuclear expression.
Figure 3.
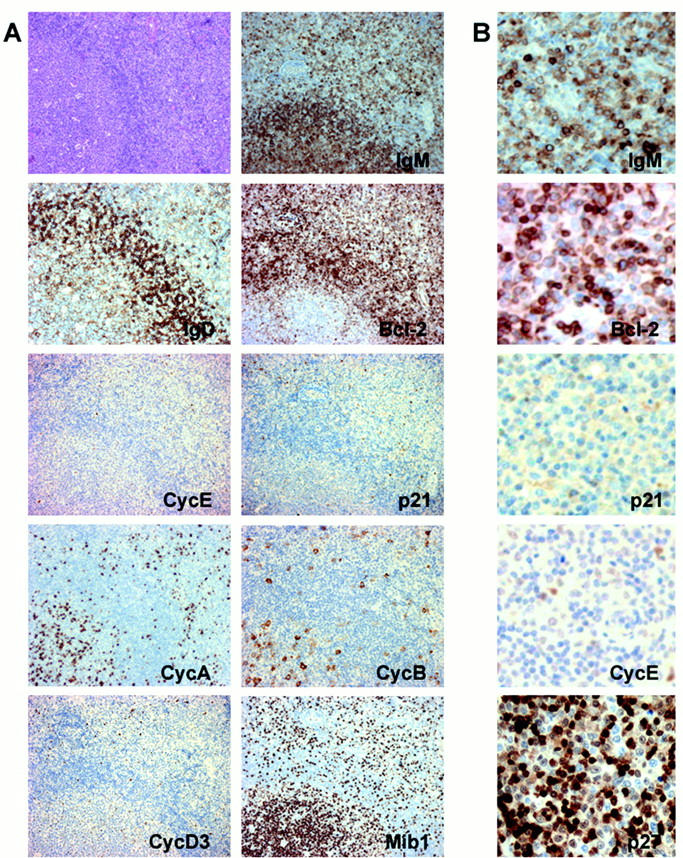
A: Expression of different cell cycle proteins in a case of MZL/MBCL. B: Detail of the expression of IgM, bcl-2, p21WAF1, cyclin E, and p27KIP1.
Discussion
The data of this study show an unexpected immunophenotype in MBCs, different from that which is observed in other B cell subpopulations. This suggests the need to relocate MBCs as a cell subpopulation with a unique phenotype, different from splenic and nodal MZ B cells, whose relationship with other B cell subpopulations needs to be reinvestigated. These observations are also supported by a recent study of Stein and colleagues 13 showing absence of Ig protein and mRNA expression by MBCs.
MBCs nests seem to be mainly formed by cells in the G0 or G1 phases, which usually do not proceed to the S and G2/M phases of the cell cycle, as is shown by the low proportion of cyclin A and cyclin B staining. The relatively high level of expression of p21WAF1 and p27KIP1 would explain the existence of a negative balance of the cell cycle regulators that, despite increased cyclin D2, D3, and E staining, keeps MBCs in the G0/G1 phases, in a similar manner to that which has been described in other cell types. 21
The absence of bcl-2 staining in nonproliferating lymphoid cells is an unusual observation, and confirms previous findings. 22-25 Consistently with the bcl-2- immunophenotype observed in proliferating germinal center B cells, it has been demonstrated that bcl-2 expression confers longer average life on memory cells, protecting them against apoptosis. 17-19 It seems that for MBCs either the expression of bcl-2 is dispensable, or that these Ig-negative cells do not need to survive as memory cells.
These data also show a homogeneous profile in MBCs, and this does not support previous suggestions about the possibly heterogeneous nature of the cells included within these nests. 8
Two different groups 8,13 have recently provided additional data in favor of the hypothesis that MBCs constitute a different cell subpopulation. Although in different proportions, when studying the sequence and mutation of the IgH rearrangements, they found a lower frequency of somatic mutations than is the case in MZ and germinal center B cells. Stein and colleagues 13 investigate the differentiation stage of MBCs in relation to other B cell subsets, showing that the majority of these B cells originate from unmutated naive B cells at a pregerminal center stage of differentiation.
Although their architectural location around lymphoid follicles has suggested that MBCs could be related to lymph node or splenic MZ cells, these data raise additional doubts about the nature of this relationship. Thus MZ cells in the spleen and lymph node are consistently IgM+, bcl-2+ and lack expression of p21WAF1 and cyclin E.
Additionally, we observed moderate to high cyclin D3 expression in MBCs, higher than expected taken into account the growth fraction. Different data suggest that cyclin D3 protein could have a dual role, its expression correlating either with proliferation or the induction and establishment of differentiation, as has been observed in some tissues, including hematopoietic tissue. 26,27 A high level of cyclin D3 in MBCs could be consistent with a hypothetical role of the induction of differentiation in this B cell subpopulation, in a specific tissue microenvironment. Alternatively it could be argued that the immunophenotype of MBCs reflects changes that are secondary to location in a different microenvironment, adjacent to or within the lymph node sinuses, where there is a loss of adhesion to the extracellular matrix.
The recognition of this phenotypic profile in MBCs also makes it possible to reconsider the hypothetical origin of the tumors that used to be classified as MBC lymphoma. Thus the data collected here support the hypothesis that these lymphomas do not share most of the markers observed in MBCs, whereas they do seem to display a very similar immunophenotype to reactive MZ B cells, in both the spleen and lymph nodes, thereby supporting their denomination as MZL. The question of whether there is any tumoral counterpart of MBCs awaits the identification of cases that more closely mimic the morphology and immunophenotype of MBCs.
To summarize, with respect to the relationship between MZ cells and MBCs, the data given here strongly support the hypotheses that either MBCs is a unique B cell population from a different cell lineage, or that if they are related to MZ cells, they would represent a definite differentiation stage characterized by a distinctive immunophenotype.
Acknowledgments
We thank Dr. J. C. Martinez-Montero for his kind help with the immunohistochemical techniques, Dr. Sergio Moreno for his useful suggestions, and Maria Jesús Acuña for excellent technical assistance.
Footnotes
Address reprint requests to Miguel A Piris, “Centro Nacional de Investigaciones Oncológicas Carlos III,” Carretera Majadahonda-Pozuelo Km.2, 28220 Majadahonda (Madrid), Spain. E-mail: mapiris@cnio.es.
Supported by a grant from the Fondo de Investigaciones Sanitarias (FIS 98/993), Ministerio de Sanidad y Consumo, and the Comision Interministerial de Ciencia y Tecnologia (1FD97-0431), Spain.
References
- 1.Stansfeld AG: The histological diagnosis of toxoplasmic lymphadenitis. J Clin Pathol 1961, 14:565-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miettinen M: Histological differential diagnosis between lymph node toxoplasmosis and other benign lymph node hyperplasias. Histopathology 1981, 5:205-216 [DOI] [PubMed] [Google Scholar]
- 3.Stein H, Lennert K, Mason DY, Liangru S, Ziegler A: Immature sinus histiocytes. Their identification as a novel B-cell population. Am J Pathol 1984, 117:44-52 [PMC free article] [PubMed] [Google Scholar]
- 4.Mohrmann RL, Nathwani BN, Brynes BBK, Sheibani K: Hodgkin’s disease occurring in monocytoid B-cell clusters. Am J Clin Pathol 1991, 95:802-808 [DOI] [PubMed] [Google Scholar]
- 5.Ohsawa M, Kanno H, Naka N, Aozasa K: Occurrence of monocytoid B-lymphocytes in Hodgkin disease. Mod Pathol 1994, 7:540-543 [PubMed] [Google Scholar]
- 6.Van der Oord JJ, De Wolf-Peeters C, de Vos R, Desmet VJ: Immature sinus histiocytosis. Light- and electron-microscopic features, immunologic phenotype, and relationship with marginal zone lymphocytes. Am J Pathol 1985, 118:266-277 [PMC free article] [PubMed] [Google Scholar]
- 7.De Wolf-Peeters C, Pittaluga S, Dierlamm J, Wlodarska I, Van Den Berghe H: Marginal zone B-cell lymphomas including mucosa-associated lymphoid tissue type lymphoma (MALT), monocytoid B-cell lymphoma and splenic marginal zone cell lymphoma and their relation to the reactive marginal zone. Leuk Lymphoma 1997, 26:467-478 [DOI] [PubMed] [Google Scholar]
- 8.Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C: Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood 1999, 93:226-234 [PubMed] [Google Scholar]
- 9.Spencer J, Finn T, Pulford K, Mason D, Isaacson P: The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol 1985, 62:607-612 [PMC free article] [PubMed] [Google Scholar]
- 10.Nathwani BN, Drachenberg MR, Hernandez AM, Levine AM, Sheibani K: Nodal monocytoid B-cell lymphoma (nodal marginal-zone B-cell lymphoma). Semin Hematol 1999, 36:128-138 [PubMed] [Google Scholar]
- 11.Campo E, Miquel R, Krenacs L, Sorbara L, Raffeld M, Jaffe ES: Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol 1999, 23:59-68 [DOI] [PubMed] [Google Scholar]
- 12.Hernandez AM, Nathwani BN, Nguyen D, Shibata D, Chuan W, Nichols P, Taylor CR: Nodal benign and malignant monocytoid B cells with and without follicular lymphomas: a comparative study of follicular colonization, light chain restriction, bcl-2, and t (14;18) in 39 cases. Hum Pathol 1995, 26:625-632 [DOI] [PubMed] [Google Scholar]
- 13.Stein K, Hummel M, Korbjuhn P, Foss HD, Anagnostopoulos I, Marafioti T, Stein H: Monocytoid B cells are distinct from splenic marginal zone cells and commonly derive from unmutated naive B cells and less frequently from postgerminal centre B cells by polyclonal transformation. Blood 1999, 94:2800-2808 [PubMed] [Google Scholar]
- 14.Nurse P: A long twentieth century of the cell cycle and beyond. Cell 2000, 100:71-78 [DOI] [PubMed] [Google Scholar]
- 15.Mateo MS, Sáez AI, Sánchez-Beato M, García P, Sánchez-Verde L, Martínez JC, Orradre JL, Piris MA: Expression of p21WAF1/CIP1 in foetal and adult tissues: simultaneous analysis with Ki67 and p53. J Clin Pathol 1997, 50:645-653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Beato M, Sáez AI, Martínez-Montero JC, Mateo MS, Sánchez-Verde L, Villuendas R, Troncone G, Piris MA: Cyclin-dependent kinase inhibitor p27/KIP1 in lymphoid tissue: p27/KIP1 expression is inversely proportional to the proliferative index. Am J Pathol 1997, 151:151-160 [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshino T, Kondo E, Cao L, Takahashi K, Hayashi K, Nomura S, Akagi T: Inverse expression of bcl-2 protein and Fas antigen in lymphoblasts in peripheral lymph nodes and activated peripheral blood T and B Lymphocytes. Blood 1994, 83:1856-1861 [PubMed] [Google Scholar]
- 18.Reed JC: Bcl-2 family proteins: regulators of apoptosis and chemoresistance in hematologic malignancies. Semin Hematol 1997, 34(Suppl. 5):9-19 [PubMed] [Google Scholar]
- 19.Hockenbery DM: The bcl-2 oncogene and apoptosis. Semin Immunol 1992, 4:413-420 [PubMed] [Google Scholar]
- 20.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 21.Orend G, Hunter T, Ruoslahti E: Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene 1998, 16:2575-2583 [DOI] [PubMed] [Google Scholar]
- 22.Tang SC, Visser L, Hepperle B, Hanson J, Poppema S: Clinical significance of bcl-2-MBR gene rearrangement and protein expression in diffuse large-cell non-Hodgkin’s lymphoma: an analysis of 83 cases. J Clin Oncol 1994, 12:149-154 [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Lasota J, Hanau CA, Miettinen M: Bcl-2 oncoprotein is widespread in lymphoid tissue and lymphomas but its differential expression in benign versus malignant follicles and monocytoid B-cell proliferations are of diagnostic value. APMIS 1995, 103:655-662 [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Nakamura S, Itoh H, Yoshida K, Shimizu K, Motoori T, Yamane N, Joshita T, Suchi T: Occurrence of monocytoid B-cells in reactive lymph node lesions. Pathol Res Pract 1998, 194:559-565 [DOI] [PubMed] [Google Scholar]
- 25.Lai R, Arber DA, Chang KL, Wilson CS, Weiss LM: Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol 1998, 11:864-869 [PubMed] [Google Scholar]
- 26.Doglioni C, Chiarelli C, Macrí E, Dei Tos AP, Meggiolaro E, Dalla Palma P, Barbareschi M: Cyclin D3 expression in normal, reactive and neoplastic tissues. J Pathol 1998, 185:159-166 [DOI] [PubMed] [Google Scholar]
- 27.Bartkova J, Lukas J, Strauss M, Bartek J: Cyclin D3: requirement for G1/S transition and high abundance in quiescent tissues suggest a dual role in proliferation and differentiation. Oncogene 1998, 17:1027-1037 [DOI] [PubMed] [Google Scholar]