Abstract
Proliferation of mesangial cells is a hallmark of glomerular disease, and understanding its regulatory mechanism is clinically important. Previously, we demonstrated that the product of growth arrest-specific gene 6 (Gas6) stimulates mesangial cell proliferation through binding to its cell-surface receptor Axl in vitro. We also showed that warfarin and the extracellular domain of Axl conjugated with Fc portion of human IgG1 (Axl-Fc) inhibit mesangial cell proliferation by interfering the Gas6/Axl pathway in vitro. In the present study, therefore, we examined in vivo roles of Gas6 and Axl in an experimental model of mesangial proliferative glomerulonephritis induced by the injection of anti-Thy1.1 antibody (Thy1 GN). In Thy1 GN, expression of Gas6 and Axl was markedly increased in glomeruli, and paralleled the progression of mesangial cell proliferation. Administration of warfarin or daily injection of Axl-Fc inhibited mesangial cell proliferation, and abolished the induction of platelet-derived growth factor-B mRNA and protein in Thy1 GN. Moreover, the anti-proliferative effect of warfarin was achieved at lower concentrations than those in routine clinical use. These findings indicate that the Gas6/Axl pathway plays a key role in mesangial cell proliferation in vivo, and could be a potentially important therapeutic target for the treatment of renal disease.
Excessive proliferation of mesangial cells is often found in many types of glomerular disease, and is usually associated with matrix expansion, leading to the development of glomerular sclerosis. 1-3 Mesangial cells proliferate in response to a variety of growth factors and cytokines, such as platelet-derived growth factor (PDGF), basic fibroblast growth factor, and interleukin-6. 4-12 Because the proliferation of mesangial cells seems to be an important pathological event that precedes glomerular sclerosis, 1-3 several studies have attempted to suppress mesangial cell proliferation by inhibiting specific mitogens. 13-15 However, interventions targeting these inhibitors to the site of proliferation are difficult, and have been clinically used in only restricted situations.
Gas6 is a vitamin K-dependent growth-potentiating factor for smooth muscle cells 16-19 and its activities depend on γ-carboxylation of glutamate residues at its N terminus. 20,21 Recently we demonstrated that Gas6 works as an autocrine growth factor for mesangial cells through its cell surface receptor Axl. 22 We have also shown that warfarin and the extracellular domain of Axl conjugated with the Fc portion of human IgG1 (Axl-Fc) inhibit mesangial cell proliferation by interfering the Gas6/Axl pathway. 22 These two inhibitors exert their activities in different ways; warfarin inhibits mesangial cell proliferation possibly by inhibiting γ-carboxylation of Gas6, whereas Axl-Fc blocks the binding of Gas6 to the cell surface Axl by scavenging Gas6. We have also shown the possibility that effective concentrations of warfarin as an anti-proliferative agent are much below those used as an anticoagulant. 23 Therefore, one of the aims of the current study is to confirm the in vivo role of Gas6 and its receptor Axl, and to examine the effect of warfarin and Axl-Fc as anti-proliferative agents in experimental mesangial proliferative glomerulonephritis caused by the injection of anti-Thy1.1 antibody, Thy1 glomerulonephritis (Thy1 GN). 24,25 We also examined whether the expression of growth factors that stimulate mesangial cell proliferation in Thy1 GN can be modulated when the Gas6/Axl pathway is interfered.
Materials and Methods
Animals
Wistar rats (8 to 12 weeks old, 180 to 200 g) were purchased from Shimizu Laboratory Animal Center (Hamamatsu, Japan). Rats were housed under specific pathogen-free conditions at the Animal Facilities of Kyoto University, Faculty of Medicine. All animal experiments were performed in accordance with institutional guidelines, and the Review Board of Kyoto University granted an ethical permission to this study.
Induction of Experimental Mesangial Proliferative Glomerulonephritis (Thy1 GN)
Thy1 GN was induced by a single intravenous injection of mouse anti-Thy1.1 monoclonal antibody OX-7 (1 mg/kg body weight; Cedarlane, Hornby, Canada) as described elsewhere. 24,25 These rats were sacrificed to obtain renal specimens, total glomerular RNA, and protein at days 3, 5, 8, and 15 (n = 6 per group). Six rats were injected with vehicle only and sacrificed as controls.
Isolation of Glomeruli
Glomeruli were isolated from the renal cortex of rats using the differential thieving method. 26,27 The purity of the glomeruli was >90%.
Northern Blot Analysis
Total RNA was extracted from rat glomerular preparations by Trizol (GibcoBRL, Paisley, Scotland). Glomerular denatured RNA (20 μg) was electrophoresed through formaldehyde 1% agarose gel, and transferred to a nylon membrane (Schleiecher & Schuell, Keene, NH) as previously described. The membranes were hybridized with the SalI/EcoRI 0.5-kb fragment of rat Gas6 cDNA, and the PstI/EcoRI 1.3-kb fragment of human PDGF-B cDNA (kindly provided by T. Collins, Boston, MA) radiolabeled with [32P]-dCTP (10 mCi/ml; Amersham Pharmacia Biotechnology, Buckinghamshire, UK) by random primer extension. 26,27 All Northern blots were repeated at least three times with RNA from different sets of animals.
Western Blot Analysis
Isolated glomeruli were suspended in lysis buffer (0.1 ml of 50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 0.5% Triton X, 1 mmol/L ethylenediaminetetraacetic acid, 10 μg/ml aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride) for 1 hour. After centrifugation of the samples, the supernatants were used as total cell lysates. Protein concentrations were measured by DC protein assay (Bio-Rad Laboratories, Richmond, CA). Sixty μg of each sample was applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were transferred to nitrocellulose filters (Schleiecher & Schuell). The blots were subsequently incubated with rabbit anti-Gas6 or anti-Axl polyclonal antibody, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham Pharmacia Biotechnology). The final reaction was developed by the chemiluminescent system (Amersham Pharmacia Biotechnology). All Western blots were repeated at least three times from different sets of animals.
Histological Examination
Kidney tissues from each animal were processed for evaluation by light and immunofluorescence microscopy. For light microscopy, the tissues were fixed in methyl Carnoy’s solution and were embedded in paraffin. Sections (3 μm) were stained with periodic acid-Schiff. For immunofluorescence microscopy, the tissues were snap-frozen in cold acetone in OCT compound (Miles Inc., Elkhart, IN), and cryostat sections (4 μm) were stained using indirect immunofluorescence procedure with the following primary antibodies: rabbit polyclonal antibodies against rat Gas6 (1:100 dilution), 18,22,28 human Axl (1:100 dilution), 29,30 proliferating cell nuclear antigen (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), rat PDGF-B (1:100 dilution) (Santa Cruz Biotec.), rat type I collagen (1:100 dilution), 31 rat type III collagen (1:100 dilution; Chemicon International, Inc., Temecula, CA), rat type IV collagen (1:500 dilution), 31 rat fibronectin (1:100 dilution; Calbiochem, La Jolla, CA), and rat laminin B2 (1:100 dilution) 31 and goat polyclonal antibody against rat CD68 (1:100 dilution) (Santa Cruz Biotechnology). To detect mesangial cells in glomeruli, sections were incubated with biotin-conjugated OX-7 (1:100 dilution) (Cedarlane), and treated with fluorescein isothiocyanate-avidin (1:100 dilution; Cappel, Aurora, OH). Detection of α-smooth muscle actin (a marker of activated mesangial cells) 32 was done by a direct immunofluorescence procedure with fluorescein isothiocyanate-conjugated monoclonal antibody against α-smooth muscle actin (1A4; Sigma Chemical Co., St. Louis, MO). Specificity of the procedures was confirmed by substituting the primary antibody with equivalent concentrations of irrelevant murine monoclonal antibodies or normal rabbit/goat IgG.
Semiquantitative Histological Study
For quantitation of proliferating cells [proliferating cell nuclear antigen (PCNA)-positive cells], a blinded observer evaluated more than 15 consecutive cross sections of cortical glomeruli in each specimen and mean values per glomerulus were calculated. Glomerular expression of PDGF-B and type IV collagen was graded semiquantitatively at five levels: 0, very weak or absent staining; 1+, positive staining in <25% of the glomerular tuft; 2+, 25 to 49% of glomerular tuft; 3+, 50 to 75% of the glomerular tuft; and 4+, >75% of the glomerular tuft stained. 33
Immunofluorescent Double Staining
To determine the localization of Gas6 and Axl, double immunostaining for α-smooth muscle actin and Gas6 or Axl was done. Both primary antibodies (Gas6/Axl and 1A4) were incubated overnight at 4°C followed by the incubation with rhodamine-conjugated anti-rabbit IgG (Chemicon International Inc.). Double immunostaining for CD68 and PCNA was done in the same way. Specificity was confirmed by the negative results when replacing either one of the primary antibodies with irrelevant mouse monoclonal antibody or rabbit preimmune IgG. Specimens were observed with a Zeiss microscope equipped with proper filters.
Protocol of the Treatment with Warfarin in Thy1 GN
Dosage and time of administration of warfarin potassium (provided by Esai Co. Ltd., Tokyo, Japan) were determined based on the results of preliminary studies. When rats were administered with 0.25 and 0.5 mg/ml of warfarin in drinking water, the serum concentrations of warfarin gradually increased during the first 5 days, and reached a plateau value required to abrogate mesangial cell proliferation in vitro, previously described. 22 In these concentrations in drinking water, no remarkable bleeding tendency or anemia was encountered. Based on these results, rats were treated with warfarin in drinking water (0, 0.25, or 0.5 mg/L) from 5 days before the initiation of Thy1 GN to the day of sacrifice. Rats were divided into three groups: a group without treatment, a group treated with 0.25 mg/L warfarin, and a group treated with 0.5 mg/L warfarin. In each group, rats were sacrificed at day 0, 3, 5, 8, and 15 (n = 6 for each group). Blood was collected at sacrifice and prothrombin times, hematocrits, and serum concentrations of warfarin were assessed as described. 23 Before sacrifice, a 24-hour urine collection for creatinine and albumin measurement (Nephrat; Exocell Inc., Philadelphia, PA) was obtained from each rat as described previously. 27
Treatment with Axl-Fc in Thy1 GN
A construct to fuse the extracellular domain of Axl and Fc portion of IgG1 was described previously. 19 A control plasmid containing the Fc portion was made by ligating the 105-bp signal sequence of Axl and Fc portion directly. Expression vectors of Axl-Fc and Fc were transiently transfected into COS-7 cells and the culture supernatant was collected after 48 hours to purify recombinant Axl-Fc and Fc using Protein A agarose (Roche Diagnostics, Mannheim, Germany) as previously described. 19 Purity of these recombinant proteins was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Two hundred μg of recombinant Axl-Fc or Fc was intravenously administered to rats (n = 6 for each group) once a day from 24 hours after the injection of anti-Thy 1.1 antibodies to day 7. In this experiment, rats were sacrificed at day 8, and a 24-hour urine collection was obtained before sacrifice as described.
Statistical Analysis
Statistical analyses of serum concentrations of warfarin, prothrombin times, and urinary albumin/creatinine index were done by Student’s t-test. Numbers of PCNA-positive cells per glomeruli, and grading of expression of PDGF-B and type IV collagen were analyzed by two-way repeated analysis of variance followed by the Fisher’s post hoc test. P values <0.01 were considered significant. Data are expressed as means ± SD. Analysis was performed by simple regression using StatView program (Abacus Concepts Inc., Barkeley, CA).
Results
Expression of Gas6 and Axl in Thy1 GN
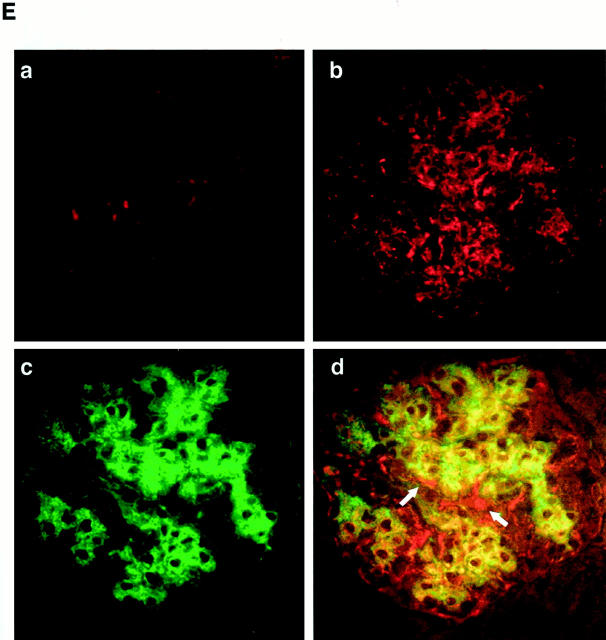
In Thy1 GN, proliferation of mesangial cell begins at day 2, peaks at day 8, and subsides in 15 days after injection of the antibody. First, to examine whether expression of Gas6 and Axl is correlated with mesangial proliferation, glomerular expression of Gas6 and Axl in Thy1 GN was determined. Signal intensity of Northern blot is determined by NIH image, and is normalized to 28S ribosomal RNA. Glomerular expression of Gas6 mRNA at day 0 was very scarce, however, the expression increased, peaking at day 8 (2.3-fold), and returned to the basal level at day 15, when mesangial-cell proliferation subsided (Figure 1A) ▶ . Expression of Gas6 protein also increased by 3.8-fold (at day 5) and 6.6-fold (at day 8) at maximum, and returned to the basal level at day 15 (Figure 1B) ▶ . Next, we examined the glomerular expression of Axl. Two major immunoreactive proteins of approximately 140 kd (full length) and 120 kd (splice variant) were detected, which are compatible with our previous studies in mesangial cells. Expression of Axl increased by 3.2-fold (day 5), and 2.9-fold (day 8), and resolved at day 15 (Figure 1C) ▶ . Next we studied the localization of Gas6 and Axl by immunostaining. At day 0, Gas6 was hardly detected in glomeruli (Figure 1D, a) ▶ , however in the glomerulus of day 8, Gas6 was extensively expressed in a typically expanded mesangial pattern (Figure 1D, b) ▶ . No significant staining was detected in any sections treated with irrelevant antibody or anti-Gas6 antibody preincubated with an excess amount of recombinant Gas6 (data not shown). Simultaneously, double immunostaining for Gas6 and α-smooth muscle actin was done to determine whether activated mesangial cells express Gas6. Figure 1D, d ▶ , demonstrates that the majority of Gas6-positive cells at day 8 expressed α-smooth muscle actin, indicating that Gas6 appears to be produced predominantly by mesangial cells in this experimental model. A minor portion of glomerular cells was Gas6-positive and α-smooth muscle actin-negative (arrows), and Gas6-negative and α-smooth muscle actin-positive cells (asterisks) at the hylus of glomerulus seemed to be smooth muscle cells in the arteriole. Similar to the results of Gas6, Axl was hardly detected in the glomeruli at day 0 (Figure 1E, a) ▶ , but at day 8, glomerular cells were highly positive for Axl (Figure 1E, b) ▶ . No significant staining was detected in any sections treated with irrelevant antibody or anti-Axl antibody preincubated with an excess amount of recombinant Axl-Fc (data not shown). Finally, double immunostaining for Axl and α-smooth muscle actin was performed. Figure 2E, d ▶ , demonstrates that the majority of Axl-positive cells at day 8 expressed α-smooth muscle actin. A minor portion of glomerular cells was positive for Axl and negative for α-smooth muscle (arrows).
Figure 1.
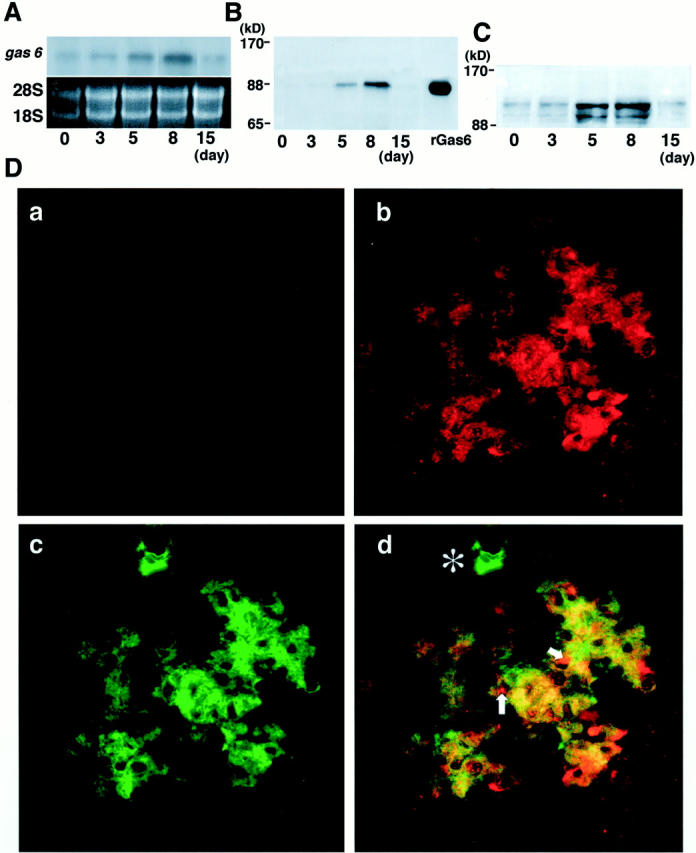
Expression of Gas6 and Axl in Thy1 GN. A: A representative Northern blot for gas6 mRNA and corresponding 18S and 28S RNA. Expression of gas6 mRNA is peaked at day 8. B: Expression of Gas6 protein by Western blot analysis. Purified Gas6 protein (30 ng) is used as a positive control (right lane). Expression of Gas6 is peaked at day 8. C: Expression of Axl protein by Western blot analysis. Expression of 140-kd and 120-kd proteins corresponding to the full-length Axl and smaller alternative spliced protein is increased at day 5 and at day 8. D: Double immunostaining for Gas6 (rhodamine in red in a, b, and d) and α-smooth muscle actin (fluorescein isothiocyanate in green in c and d) in glomeruli of rats injected with anti-Thy1.1 antibody at day 0 (a) and day 8 (b, c, and d). Gas6 and α-smooth muscle actin are co-localized (yellow in d) in mesangial cells. A site indicated by asterisk is only positive for α-smooth muscle actin. Note that some inner sites of glomerular capillary walls (arrows) are only positive for Gas6. Original magnification, ×200. E: Double immunostaining for Axl (rhodamine in red in a, b, and d) and α-smooth muscle actin (fluorescein isothiocyanate in green in c and d) in glomeruli of rats injected with Thy1.1 antibody at day 0 (a) and day 8 (b, c, and d). Axl and α-smooth muscle actin are distributed in the same site of the glomerulus (yellow in d) in an expanded mesangial pattern. Some outer sites of the glomerular capillary wall (arrows) and some Bowman’s capsular epithelial cells are only positive for Axl. Original magnification, ×200.
Figure 2.
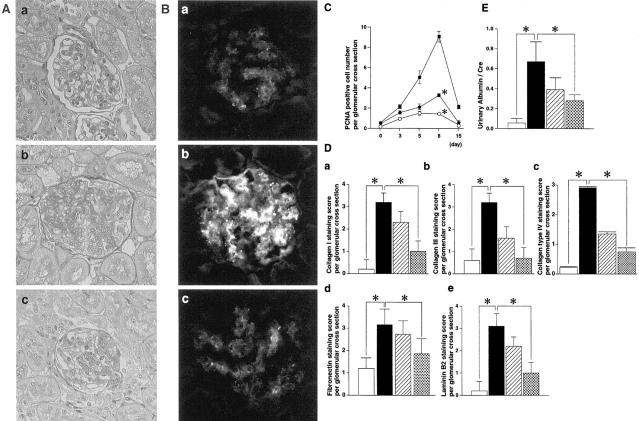
Inhibitory effects of warfarin on Thy1 GN. Effects of warfarin treatment on glomerular cell proliferation (A) and glomerular expression of OX-7 (B). Representative glomeruli of day 0 (a), day 8 of Thy1 GN (b), and day 8 of Thy1 GN with warfarin treatment (0.5 mg/ml) (c) are shown. A: PAS staining. B: Immunofluorescent staining for OX-7. Original magnification, ×200. C: PCNA expression in glomeruli of Thy1 rats. PCNA-positive cell numbers per glomerular cross-section are counted as described in Materials and Methods. Closed squares, nontreated Thy1 rats; closed circles, Thy1 rats treated with 0.25 mg/L of warfarin; open circles, Thy1 rats treated with 0.5 mg/L of warfarin. *, P < 0.001 versus nontreated Thy1 rats. D: Expression of extracellular matrix protein in glomeruli of Thy1 rats at day 8. Collagen type I (a), type III (b), type IV (c), fibronectin (d), and laminin B2 (e) staining scores per glomerular cross-section are counted as described in Materials and Methods. Open bar, control rats (day 0); closed bar, nontreated Thy1 rats; hatched bar, Thy1 rats treated with 0.25 mg/L of warfarin; dotted bar, Thy1 rats treated with 0.5 mg/L of warfarin in D and E. *, P < 0.001 versus nontreated Thy1 rats. E: Urinary albumin excretion standardized by urinary creatinine of Thy1 rats at day 8. *, P < 0.001 versus nontreated Thy1 rats.
Low-Dose Warfarin Inhibits Glomerular Cell Proliferation in Vivo
Because expression of Gas6 and Axl was induced dramatically in parallel with disease severity of Thy1 GN, the Gas6/Axl pathway seems to play an important role in the development of glomerulonephritis. Therefore, we examined whether inhibiting this pathway might be beneficial in treating this experimental glomerulonephritis. We administered warfarin in drinking water at various concentrations (0, 0.25, or 0.5 mg/ml). Serum concentrations of warfarin in these rats were 0.28 ± 0.05 μmol/L (0.25 mg/L) and 1.23 ± 0.4 μmol/L (0.5 mg/L) (Table 1) ▶ , which were within the serum concentrations that inhibit mesangial cell proliferation in vitro. Significant prolongation of prothrombin times, anemia (Table 1) ▶ , or bleeding tendency was not observed in rats during the whole period of warfarin treatment.
Table 1.
Serum Concentrations of Warfarin, Prothrombin Time, and Hematocrit of Thy1 Rats Treated with Warfarin
| Warfarin concentration in drinking water (mg/l) | 0 | 0.25 | 0.5 |
|---|---|---|---|
| Serum warfarin concentration (μmol/L) | 0 | 0.28 ± 0.05* | 1.23 ± 0.4* |
| Prothrombin time (seconds) | 12.63 ± 0.51 | 13.33 ± 0.28 | 14.10 ± 1.49 |
| Hematocrit (%) | 48.4 ± 1.0 | 49.6 ± 1.2 | 47.4 ± 1.9 |
Data are expressed as means ± SD (n = 6 for each group).
*P < 0.001 versus nontreated Thy1 rats.
Mesangial cell proliferation and mesangial matrix expansion on day 8 in Thy1 GN was significantly reduced by warfarin treatment (Figure 2A) ▶ . Expression of OX-7 was also reduced in glomeruli of Thy1 GN treated with warfarin (Figure 2B, c) ▶ . To examine the effect of warfarin on glomerular cell proliferation, the number of PCNA-positive cells were counted. The number of PCNA-positive cells in the glomeruli of rats treated with warfarin was significantly reduced in a dose-dependent manner at each point studied (Figure 2C) ▶ . To examine the participation of infiltrating macrophages in the number of PCNA-positive cells per glomerulus, double immunostaining of PCNA and CD68 was performed. The number of PCNA/CD68-positive cells was 0.03 + 0.18 at day 0, 0.30 + 0.46 at day 3, 0.90 + 0.53 at day 5, 1.83 + 0.63 at day 8, and 0.37 + 0.63 when treated with warfarin (0.5 mg/L). We also examined the expression of extracellular matrix protein, such as collagen type I, type III, type IV, fibronectin, and laminin B2. By semiquantitave analysis, Thy1 rats had a substantial increase in immunostaining of these extracellular matrix proteins at day 8, whereas in the rats treated with warfarin, expression of these extracellular matrix proteins was markedly decreased (69% reduction in collagen type I, 78% in collagen type III, 75% in collagen type IV, 41% in fibronectin, and 68% in laminin B2 with 0.5 mg/L of warfarin treatment) (Figure 2D) ▶ . These data indicate that the reduction of glomerular cell proliferation was associated with a decrease in matrix expansion. Finally, we assessed the urinary protein excretion. Figure 2E ▶ shows that warfarin treatment significantly inhibited urinary albumin excretion at day 8 in a dose-dependent manner.
Axl-Fc Inhibits Glomerular Cell Proliferation in Vivo
Next, we examined the effect of another inhibitor of the Gas6/Axl pathway, Axl-Fc in Thy1 GN. Axl-Fc is supposed to capture Gas6 and block its binding to endogenous cell surface Axl. Rats were daily injected with vehicle, Axl-Fc, or Fc for 24 hours after the administration of anti-Thy1.1 antibody. A significant reduction of mesangial cells and matrix expansion in the glomeruli of Thy1 rats was observed when treated with Axl-Fc but not with Fc (Figure 3A) ▶ . Differences in the expansion of mesangial cells were further confirmed by immunostaining for OX-7 (Figure 3B) ▶ . Expression of OX-7 in glomeruli was significantly reduced when treated with Axl-Fc, but not with Fc. The number of PCNA-positive cells in the glomeruli of rats treated with Axl-Fc was also significantly reduced (89%) (Figure 3C) ▶ , but not with Fc. These data indicate that Axl-Fc could inhibit mesangial cell proliferation. Finally, we examined urinary protein excretion. Treatment with Axl-Fc significantly inhibited urinary albumin excretion at day 8, but not with Fc (Figure 3D) ▶ .
Figure 3.
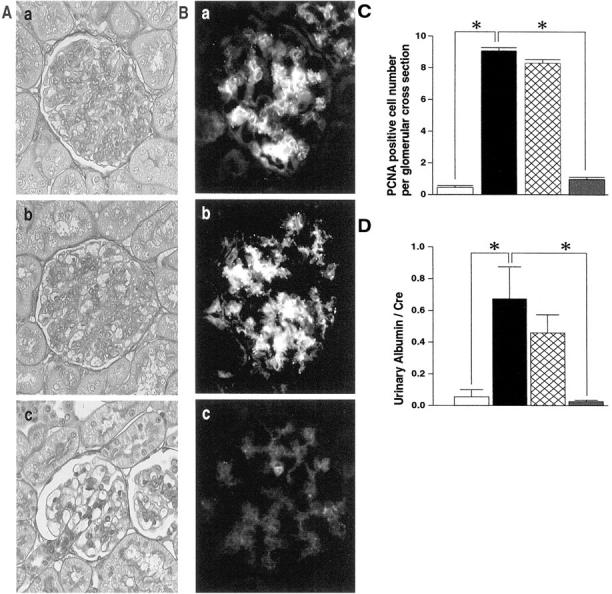
Inhibitory effects of Axl-Fc on Thy1 GN. Effects of Axl-Fc treatment on glomerular cell proliferation (A) and expression of OX-7 (B). A representative glomerulus at day 8 of Thy1 GN is shown. a, control; b, treatment with Fc; c, treatment with Axl-Fc in A and B. A: PAS staining. B: Immunofluorescent staining for OX-7. Original magnification, ×200. C: The number of PCNA-positive cells per glomeruli. PCNA-positive cell numbers per glomerular cross-section are counted as described in Materials and Methods. Open bar, control rats (day 0); closed bar, nontreated Thy1 rats; double-hatched bar, Thy1 rats treated with Fc; shaded bar, Thy1 rats treated with Axl-Fc in C and D. *, P < 0.001 versus nontreated Thy1 rats. D: Urinary albumin excretion standardized by urinary creatinine of Thy1 rats at day 8. *, P < 0.001 versus nontreated Thy1 rats.
Inhibition of the Gas6/Axl Pathway Reduces the Expression of PDGF-B in Thy1 GN
Finally, we tried to determine whether the inhibition of the Gas6/Axl pathway could affect the expression of other growth factors, such as PDGF-B that are known to play critical roles in Thy1 GN. 6-8 Expression of PDGF-B mRNA was induced in Thy1 GN, whereas the induction was abolished when treated with warfarin or Axl-Fc (Figure 4A) ▶ . Expression of PDGF-B protein in glomeruli was also abolished in Thy1 GN treated with warfarin or Axl-Fc (Figure 4B) ▶ . Semiquantitation for localization for PDGF-B was shown in Figure 4C ▶ (62% reduction in warfarin treatment, 79% reduction in Axl-Fc treatment, respectively).
Figure 4.
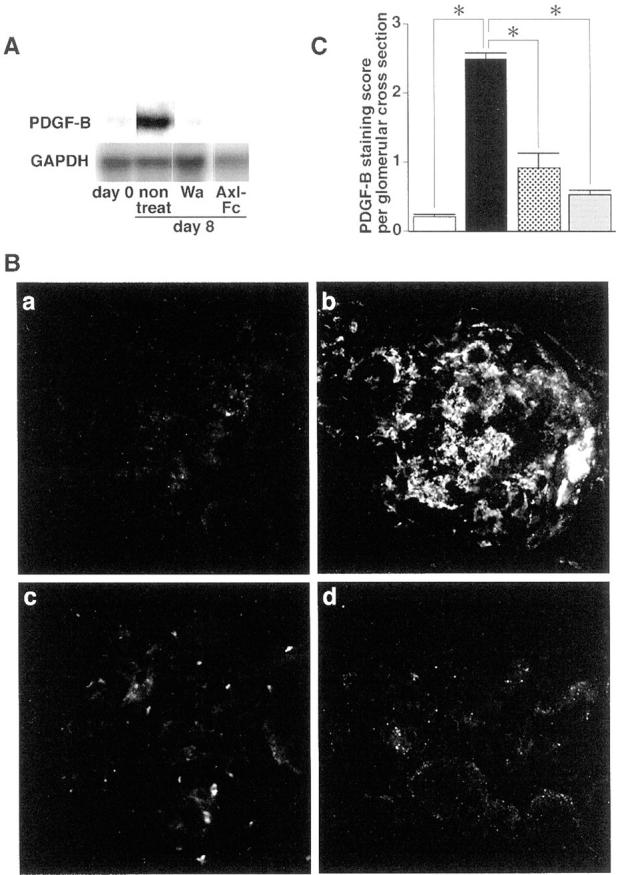
Inhibitory effects of warfarin and Axl-Fc on expression of PDGF-B in Thy1 GN. A: Northern blot analysis of glomerular RNA of Thy1 rats at day 0, Thy1 rats without treatment (non treat), Thy1 rats treated with 0.5 mg/L warfarin (Wa), and Thy1 rats treated with Axl-Fc (Axl-Fc) at day 8. Shown are representative Northern blots for PDGF-B mRNA and corresponding GAPDH mRNA. B and C: Effects of warfarin and Axl-Fc treatment on glomerular expression of PDGF-B. B: Representative glomeruli of Thy1 rats at day 0 (a), day 8 (b), Thy1 GN treated with 0.5 mg/L of warfarin (c), Thy1 GN treated with Axl-Fc (d) at day 8. Immunofluorescent staining for PDGF-B. Original magnification, ×200. C: PDGF-B-staining score per glomerular cross-section is counted as described in Materials and Methods. Open bar, control rats (day 0); closed bar, nontreated Thy1 rats; dotted bar, Thy1 rats treated with warfarin (0.5 mg/L) (day 8); shaded bar, Thy1 rats treated with Axl-Fc (day 8). *, P < 0.001 versus nontreated Thy1 rats.
Discussion
In this study, we have demonstrated that expression of Gas6 and Axl was increased in an established model of glomerulonephritis, and that treatment with warfarin and Axl-Fc can significantly inhibit mesangial cell proliferation and reduce severity of glomerular injury in vivo. We have also shown that treatment with warfarin or Axl-Fc reduced the expression of PDGF-B in Thy1 GN, indicating that the Gas6/Axl pathway can affect PDGF-B production in vivo. These findings led us to conclude that the Gas6/Axl pathway modulates growth factor production, regulates mesangial cell proliferation, and thus plays a critical role in Thy1 GN.
Expression of Gas6 and Axl was dramatically increased in Thy1 GN in parallel with the degree of mesangial cell proliferation. Double-staining immunohistochemistry indicated that most part of Gas6 and Axl was co-localized mainly in mesangial cells. Thus, it is conceivable that Gas6 is secreted from mesangial cells to stimulate the mesangial cell surface receptor, Axl, in an autocrine and paracrine manner in vivo.
We have already shown that warfarin and Axl-Fc block the mesangial proliferation induced by Gas6 in vitro. 22 We extended these in vitro results to in vivo experiments. We administered these two inhibitors of the Gas6/Axl pathway to Thy1 rats to examine their anti-proliferative effects. Both agents significantly inhibited glomerular cell proliferation demonstrated histologically, and by the number of PCNA-positive cells. Warfarin treatment also significantly suppressed extracellular matrix accumulation and urinary protein excretion. Therefore, we concluded that warfarin and Axl-Fc inhibited mesangial cell proliferation and attenuated glomerulonephritis in Thy1 GN by blocking the Gas6/Axl pathway.
Because warfarin is an inhibitor of γ-carboxylation, 34 there is a possibility that warfarin might prevent γ-carboxylation of unknown vitamin K-dependent growth factors other than Gas6. However, because Axl-Fc is supposed to be a specific scavenger of Gas6, it may well be that the Gas6/Axl pathway plays an important role in mesangial cell proliferation in vivo.
One of the important findings in this study is a new aspect of warfarin as an anti-proliferative agent. Warfarin has long been used as an anticoagulant to prevent thrombosis and embolism, 35,36 and patients prescribed this agent are monitored by measuring prolongation of prothrombin times to achieve its anticoagulant effect. These patients have to be monitored for the risk of bleeding. 37 However, the anti-proliferative effect of warfarin was achieved at serum concentrations of 0.28 to 1.23 μmol/L, which were significantly lower than the ordinary therapeutic concentrations as an anticoagulant (4 to 5 μmol/L). 23 The prothrombin times of rats treated with warfarin in our experiments were not significantly prolonged, and no bleeding tendency or anemia was observed, whereas mesangial cell proliferation was significantly reduced.
The benefit of restricting the amount of warfarin is not only to prevent bleeding tendency, but also to reduce the incidence of osteoporosis, 38 because long-term prescription of warfarin is known to cause osteoporosis by inhibiting γ-carboxylation of matrix Gla proteins in the bone. Lower doses of warfarin are also beneficial in terms of drug interaction, because warfarin is known to affect drug absorption from the intestine. 39
Despite our findings about the efficacy of warfarin on reducing mesangial cell proliferation, clinical studies have not provided sufficient evidence about the beneficial action of warfarin on glomerulonephritis. 40-43 One of the reasons for this discrepancy might be that all studies so far have investigated the combination of warfarin with dipyridamole or even with cyclophosphamide. Further, in these studies, anticoagulant doses of warfarin are used except the recent report of Woo and colleagues 44 regarding the benefit of low-dose warfarin on mesangial proliferative glomerulonephritis. Therefore, the adverse effect of warfarin might overcome its beneficial effect. Moreover, these studies include cases with advanced forms of glomerulonephritis with severe matrix expansion and glomerulosclerosis without active proliferation. An anti-proliferative agent might not be able to exert its effect in such cases. We speculate that low-dose warfarin should be prescribed at the earlier phase of mesangial cell proliferation to exert its effectiveness.
Our data strongly support a notion that Axl-Fc could be a candidate for therapeutic agents for glomerulonephritis. This fusion protein inhibited mesangial cell proliferation, reduced urinary protein excretion, and restored renal function possibly by inhibiting the binding of Gas6 to the cell surface Axl. 28 Axl-Fc might be better anti-proliferative agent than warfarin, because it specifically inhibits the Gas6/Axl pathway with fewer side effects.
Despite the existence of many potent growth factors known to stimulate mesangial cell proliferation in Thy1 GN, 4-12 inhibition of the Gas6/Axl pathway results in a significant reduction of mesangial cell proliferation. Therefore, we examined the expression of other growth factors in the glomeruli treated with warfarin/Axl-Fc. Blocking the Gas6/Axl pathway abolished the induction of PDGF-B mRNA and protein in Thy1 GN. Therefore, it is conceivable that Gas6 stimulates mesangial cell proliferation not only directly, but also by modulating the expression of other potent growth factors. However, reduced PDGF-B expression in the glomeruli treated with warfarin or Axl-Fc might be because of reduced mesangial cell contents. Therefore, research is now in progress to identify the regulatory mechanism by which Gas6 modulates PDGF-B production in mesangial cells.
In summary, we have presented several lines of evidence that the Gas6/Axl pathway plays a critical role in Thy1 GN, and agents that block this pathway would be potent inhibitors of mesangial cell proliferation in vivo. We also demonstrated that low-dose warfarin is effective in blocking mesangial proliferation and restoring renal function, without altering hemostasis. Finally, identification the Gas6/Axl pathway as a critical mediator of mesangial cell proliferation provides another target for the treatment of human glomerular diseases.
Acknowledgments
We thank Dr. Israel F. Charo for critical reading of our manuscript, Harunobu Ozaki and Masaru Yoshida for their thoughtful advice, and Toshiko Hori and Hideo Uchiyama for their excellent technical assistance.
Footnotes
Address reprint requests to M. Yanagita, M.D., Department of Geriatric Medicine, Kyoto University School of Medicine, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan. E-mail: motoy@kuhp.kyoto-u.ac.jp.
Supported by Grants-in Aid from the Ministry of Education, Science, Sports, and Culture of Japan (04263104, 054040439, 0557052, 04304051, 08407026); the International Scientific Research Program grants 05044163, 07044254, and 09044293 from the Japanese Ministry of Education, Science, Sports, and Culture; a research grant for health sciences from the Japanese Ministry of Health and Welfare; and grants 5A-2 and A8-1 for cardiovascular diseases from the Japanese Ministry of Health and Welfare.
References
- 1.Striker LJ, Doi T, Elliot S, Striker GE: The contribution of glomerular mesangial cells to progressive glomerulosclerosis. Semin Nephrol 1989, 9:318-328 [PubMed] [Google Scholar]
- 2.Fogo A, Ichikawa I: Evidence for the central role of glomerular growth promoters in the development of sclerosis. Semin Nephrol 1989, 9:329-342 [PubMed] [Google Scholar]
- 3.Couser WG, Johnson RJ: Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis 1994, 23:193-198 [DOI] [PubMed] [Google Scholar]
- 4.Floege J, Topley N, Resch K: Regulation of mesangial cell proliferation. Am J Kidney Dis 1991, 17:673-676 [DOI] [PubMed] [Google Scholar]
- 5.Floege J, Eng E, Young BA, Johnson RJ: Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl 1993, 39:S47-S54 [PubMed] [Google Scholar]
- 6.Johnson RJ, Floege J, Couser WG, Alpers CE: Role of platelet-derived growth factor in glomerular disease. J Am Soc Nephrol 1993, 4:119-128 [DOI] [PubMed] [Google Scholar]
- 7.Floege J, Eng E, Young BA, Alpers CE, Barrett TB, Bowen-Pope DF, Johnson RJ: Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest 1993, 92:2952-2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankland SJ, Pippin J, Flanagan M, Coats SR, Nangaku M, Gordon KL, Roberts JM, Couser WG, Johnson RJ: Mesangial cell proliferation mediated by PDGF and bFGF is determined by levels of the cyclin kinase inhibitor p27Kip1. Kidney Int 1997, 51:1088-1099 [DOI] [PubMed] [Google Scholar]
- 9.Floege J, Eng E, Lindner V, Alpers CE, Young BA, Reidy MA, Johnson RJ: Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest 1992, 90:2362-2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Issandou M, Darbon JM: Basic fibroblast growth factor stimulates glomerular mesangial cell proliferation through a protein kinase C-independent pathway. Growth Factors 1995, 5:255-264 [DOI] [PubMed] [Google Scholar]
- 11.Ruef C, Budde K, Lacy J, Northemann W, Baumann M, Sterzel RB, Coleman DL: Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int 1990, 38:249-257 [DOI] [PubMed] [Google Scholar]
- 12.Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ: Interleukin-6 exacerbates glomerulonephritis in (NZB x NZW)F1 mice. Am J Pathol 1994, 144:927-937 [PMC free article] [PubMed] [Google Scholar]
- 13.Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med 1992, 175:1413–1416 [DOI] [PMC free article] [PubMed]
- 14.Floege J, Ostendorf T, Janssen U, Burg M, Radeke HH, Vargeese C, Gill SC, Green LS, Janjic N: Novel approach to specific growth factor inhibition in vivo: antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am J Pathol 1999, 154:169-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichler RH, Bassuk JA, Hugo C, Reed MJ, Eng E, Gordon KL, Pippin J, Alpers CE, Couser WG, Sage EH, Johnson RJ: SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-medicated mesangial cell proliferation in vitro. Am J Pathol 1996, 148:1153-1167 [PMC free article] [PubMed] [Google Scholar]
- 16.Manfioletti G, Brancolini C, Avanzi G, Schneider C: The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol 1993, 13:4976-4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF: The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 1995, 80:661-670 [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Higashino K, Kikuchi N, Kishino J, Nomura K, Fujita H, Ohara O, Arita H: Vascular smooth muscle cell-derived, Gla-containing growth-potentiating factor for Ca(2+)-mobilizing growth factors. J Biol Chem 1995, 270:5702-5705 [DOI] [PubMed] [Google Scholar]
- 19.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K: Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 1996, 271:30022-30027 [DOI] [PubMed] [Google Scholar]
- 20.Nakano T, Kawamoto K, Kishino J, Nomura K, Higashino K, Arita H: Requirement of gamma-carboxyglutamic acid residues for the biological activity of Gas6: contribution of endogenous Gas6 to the proliferation of vascular smooth muscle cells. Biochem J 1997, 323:387-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe K, Nagata K, Ohashi K, Nakano T, Arita H, Mizuno K: Roles of gamma-carboxylation and a sex hormone-binding globulin-like domain in receptor-binding and in biological activities of Gas6. FEBS Lett 1997, 408:306-310 [DOI] [PubMed] [Google Scholar]
- 22.Yanagita M, Ishii K, Ozaki H, Arai H, Nakano T, Ohashi K, Mizuno K, Kita T, Doi T: Mechanism of inhibitory effect of warfarin on mesangial cell proliferation. J Am Soc Nephrol 1999, 10:2503-2509 [DOI] [PubMed] [Google Scholar]
- 23.Holford NH: Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet 1986, 11:483-504 [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa Y, Takenaka M, Matsuoka Y, Imai E, Hori M: Quantitation of mRNA expression in glomeruli using laser-manipulated microdissection and laser pressure catapulting. Kidney Int 2000, 57:717-723 [DOI] [PubMed] [Google Scholar]
- 25.Bokemeyer D, Ostendorf T, Kunter U, Lindemann M, Kramer HJ, Floege J: Differential activation of mitogen-activated protein kinases in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol 2000, 11:232-240 [DOI] [PubMed] [Google Scholar]
- 26.Ziswiler R, Steinmann-Niggli K, Kappeler A, Daniel C, Marti HP: Mycophenolic acid: a new approach to the therapy of experimental mesangial proliferative glomerulonephritis. J Am Soc Nephrol 1998, 9:2055-2066 [DOI] [PubMed] [Google Scholar]
- 27.Pippin JW, Qu Q, Meijer L, Shankland SJ: Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J Clin Invest 1997, 100:2512-2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohashi K, Nagata K, Toshima J, Nakano T, Arita H, Tsuda H, Suzuki K, Mizuno K: Stimulation of sky receptor tyrosine kinase by the product of growth arrest-specific gene 6. J Biol Chem 1995, 270:22681-22684 [DOI] [PubMed] [Google Scholar]
- 29.Avanzi GC, Gallicchio M, Bottarel F, Gammaitoni L, Cavalloni G, Buonfiglio D, Bragardo M, Bellomo G, Albano E, Fantozzi R, Garbarino G, Varnum B, Aglietta M, Saglio G, Dianzani U, Dianzani C: GAS6 inhibits granulocyte adhesion to endothelial cells. Blood 1998, 91:2334-2340 [PubMed] [Google Scholar]
- 30.Melaragno MG, Wuthrich DA, Poppa V, Gill D, Lindner V, Berk BC, Corson MA: Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ Res 1998, 83:697-704 [DOI] [PubMed] [Google Scholar]
- 31.Doi T, Striker LJ, Kimata K, Peten EP, Yamada Y, Striker GE: Related articles glomerulosclerosis in mice transgenic for growth hormone. Increased mesangial extracellular matrix is correlated with kidney mRNA levels. J Exp Med 1991, 173:1287-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM: Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 1991, 87:847-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ: Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and bFGF in vivo. Kidney Int 1995, 48:1846-1856 [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Suttie JW: N-glycosylation contributes to the intracellular stability of prothrombin precursors in the endoplasmic reticulum. Thromb Res 1999, 96:91-98 [DOI] [PubMed] [Google Scholar]
- 35.Gallus AS, Baker RI, Chong BH, Ockelford PA, Street AM: Consensus guidelines for warfarin therapy. Recommendations from the Australasian Society of Thrombosis and Haemostasis. Med J Aust 2000, 172:600-605 [PubMed] [Google Scholar]
- 36.Schulman S, Lindmarker P: Incidence of cancer after prophylaxis with warfarin against recurrent venous thromboembolism. Duration of Anticoagulation Trial. N Engl J Med 2000, 342:1953-1958 [DOI] [PubMed] [Google Scholar]
- 37.Dager WE, Branch JM, King JH, White RH, Quan RS, Musallam NA, Albertson TE: Optimization of inpatient warfarin therapy: impact of daily consultation by a pharmacist-managed anticoagulation service. Ann Pharmacother 2000, 34:567-572 [DOI] [PubMed] [Google Scholar]
- 38.Booth SL, Mayer J: Warfarin use and fracture risk. Nutr Rev 2000, 58:20-22 [DOI] [PubMed] [Google Scholar]
- 39.Williams D, Kelly A, Feely J: Drug interactions avoided—a useful indicator of good prescribing practice. Br J Clin Pharmacol 2000, 49:369-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolin L, Courteau M: Management of IgA nephropathy: evidence-based recommendations. Kidney Int Suppl 1999, 70:S56-S62 [DOI] [PubMed] [Google Scholar]
- 41.Yoshikawa N, Ito H: Combined therapy with prednisolone, azathioprine, heparin-warfarin, and dipyridamole for paediatric patients with severe IgA nephropathy—is it relevant for adult patients? Nephrol Dial Transplant 1999, 14:1097-1099 [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu M, Ito K, Iitaka K, Koitabashi Y, Yamaoka K, Nakagawa K, Nakamura H, Matsuyama S, Seino Y, Takeda N, Hattori S, Ninomiya M: A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol 1999, 10:101-109 [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman SW, Moorthy AV, Dreher WH, Friedman A, Varanasi U: Prospective trial of warfarin and dipyridamole in patients with membranoproliferative glomerulonephritis. Am J Med 1983, 75:920-927 [DOI] [PubMed] [Google Scholar]
- 44.Woo KT, Lee GS, Pall AA: Dipyridamole and low-dose warfarin without cyclophosphamide in the management of IgA nephropathy. Kidney Int 2000, 57:348-349 [DOI] [PubMed] [Google Scholar]