Abstract
To investigate whether caveolin-1 (cav-1) may modulate inducible nitric oxide synthase (iNOS) function in intact cells, the human intestinal carcinoma cell lines HT29 and DLD1 that have low endogenous cav-1 levels were transfected with cav-1 cDNA. In nontransfected cells, iNOS mRNA and protein levels were increased by the addition of a mix of cytokines. Ectopic expression of cav-1 in both cell lines correlated with significantly decreased iNOS activity and protein levels. This effect was linked to a posttranscriptional mechanism involving enhanced iNOS protein degradation by the proteasome pathway, because (i) induction of iNOS mRNA by cytokines was not affected and (ii) iNOS protein levels increased in the presence of the proteasome inhibitors N-acetyl-Leu-Leu-Norleucinal and lactacystin. In addition, a small amount of iNOS was found to cofractionate with cav-1 in Triton X-100-insoluble membrane fractions where also iNOS degradation was apparent. As has been described for endothelial and neuronal NOS isoenzymes, direct binding between cav-1 and human iNOS was detected in vitro. Taken together, these results suggest that cav-1 promotes iNOS presence in detergent-insoluble membrane fractions and degradation there via the proteasome pathway.
An important bioactive signaling molecule, NO, is critically implicated in a variety of functions such as vasodilatation, neurotransmission, host defense, and iron metabolism (1). NO is endogenously produced by NO synthases (NOSs), a family of enzymes that currently includes three different isoenzymes in mammals (2). The inducible isoenzyme (iNOS) was isolated originally from mouse macrophages but is now known to be expressed in a wide variety of cell types (3). In contrast to the other family members endothelial (eNOS) and neuronal NOS (nNOS), iNOS is not constitutively expressed in cells. Furthermore, under standard culture conditions, iNOS produces micromolar NO concentrations, whereas Ca2+-controlled NO production by either eNOS or nNOS is in the picomolar to nanomolar range (4–6). Because of this property, iNOS expression and activity have also been linked to a number of human pathologies and particularly to cancer. Increased levels of NOS expression and/or activity have been observed in human breast (7), central nervous system (8), and colon tumors (9). In addition, exposure of cells to NO together with superoxide leads to mutation in the p53 tumor-suppressor gene contributing to an endogenous mechanism of genomic alteration (10). Thus, because iNOS is increased in early colon adenomas (9), NO represents a candidate endogenous carcinogen that either generates or selects for the high frequency of p53 mutations that may be of importance at the transition from adenoma to carcinoma in situ (11).
Within cells, NOS enzymes are generally evenly distributed between the cytosol and membrane fractions (6). In the case of eNOS, presence at the membrane is linked to acylation by palmitic and myristic acid residues, modifications that not only target proteins to the plasma membrane, but also are held responsible for protein accumulation in detergent-insoluble membrane fractions (12, 13). In cells expressing caveolin-1 (cav-1), plasma-membrane sections displaying such properties are detectable at the cell surface as flask-shaped invaginations of 50–100 nm and referred to as caveolae (14). When isolated from cells that do not express cav-1, detergent-insoluble membrane microdomains, rich in glycosphingolipids and cholesterol, have been designated caveolae-like fractions. Interestingly, both caveolae and caveolae-like fractions of the plasma membrane contain a large number of signaling molecules, including heterotrimeric G proteins, steroid receptor coactivator family kinases, and the aforementioned NOS isoenzymes (14).
Cav-1 coimmunoprecipitates with eNOS in cultured bovine endothelial cells (15, 16) and inhibits both eNOS and nNOS activity in vivo, as well as in vitro via interaction with the NOS caveolin-binding motif (17–20). Despite the presence of a similar motif in iNOS, the existence of an inhibitory interaction between iNOS and caveolins remains a controversial issue (18, 19). This possibility is particularly relevant to human colon-tumor biology in which elevated iNOS activity (9) and reduced cav-1 levels (21) are thought to favor tumor formation.
Here, the consequences of enhanced cav-1 expression for iNOS were investigated in the human carcinoma lines HT29 and DLD1 where cav-1 expression levels are low (21) and iNOS expression can be stimulated by the addition of IL-6, IFNγ, and IL-1β (22). In both cell lines, increased presence of cav-1 after transfection reduced iNOS activity on cytokine induction. Unexpectedly, however, the loss of iNOS activity was linked to a decrease in iNOS protein levels as a consequence of proteolytic degradation via the proteasome pathway in cav-1-containing detergent-insoluble fractions. These results uncover an unexpected mechanism by which cav-1 regulates iNOS and shed light on how cav-1 may function as a tumor suppressor in particular in colon carcinomas (21).
Experimental Procedures
Antibodies and Reagents.
Human iNOS antiserum-1 was a kind gift of R. Mumford (Merck). Rabbit polyclonal anti-cav-1 antibody (C13630) was purchased from Transduction Laboratories (Lexington, KY); polyclonal anti-extracellular signal-regulated kinase (ERK)1/2 (K-23) was purchased from Santa Cruz Biotechnology, and rabbit anti-actin and rabbit anti-protein kinase C (PKC)-α was purchased from Sigma. Anti-rabbit horseradish peroxidase-conjugated second antibodies were obtained from Amersham Pharmacia. Cytokines were purchased from Roche Molecular Biochemicals; electrophoresis reagents were purchased from Bio-Rad; and other reagents not specifically mentioned were of the highest quality available from either Fluka or Sigma.
Cell Culture.
HT29 cells and DLD1 cells were cultured at 37°C in 5% CO2 in DMEM or RPMI medium 1640, respectively, supplemented with 10% (vol/vol) heat-inactivated FBS (all from GIBCO), 2 mM of l-glutamine (Integra Biosciences, Wallisellen, Switzerland), and penicillin/streptomycin (from GIBCO). HT29 and DLD1 cells stably transfected with placIOP-cav-1, a plasmid that allows isopropyl β-d-thiogalactoside (IPTG)-inducible expression of cav-1 (21), were cultured as parental cells.
iNOS Induction.
To induce iNOS expression, subconfluent cultures were exposed to a mix of cytokines consisting of 100 units/ml IFNγ, 200 units/ml IL-6, and 0.5 ng/ml IL-1β for the designated periods of time, in general 15–24 h. In experiments with protease inhibitors, medium with cytokines was removed 15 h after induction, and cells were grown in fresh medium in the presence or absence of inhibitors for an additional 9 h. N-acetyl-Leu-Leu-Norleucinal (ALLN), (2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methyl-butane ethyl ester (E64D), and lactacystin (from E. J. Corey, Harvard University, Cambridge, MA) were used at the final concentrations of 10 μM, 50 μM, and 10 μM, respectively.
Preparation of Cellular Extracts.
Cells were lysed in 50 mM Hepes, pH 7.4/10% (vol/vol) glycerol/0.1 mM 4-(2-aminoethyl)-benzensulfonyl fluoride (Roche Molecular Biochemicals)/50 mM NaF/1 mM DTT/10 μg/ml leupeptin/5 μg/ml pepstatin/3 μg/ml aprotinin/1 mM sodium vanadate (Merck). After 3 freeze/thaw cycles, the protein concentration of homogenates was determined by using the bicinchoninic acid (BCA) reagent (Pierce).
Isolation of Detergent-Insoluble Microdomains.
Caveolae-like membrane microdomains were isolated by sucrose-gradient fractionation in the presence of Triton X-100 starting from two 15-cm dishes of 80% confluent cells as described (23) with some modifications. Briefly, cells of two confluent 15-cm dishes were lysed in 2 ml of MNE buffer (25 mM Mes, pH 6.5/150 mM NaCl/2 mM EDTA) containing 1% Triton X-100 and protease inhibitors (10 μg/ml benzamidine/2 μg/ml antipain/1 μg/ml leupeptin) for 20 min on ice. Cells were homogenized further with 10 gentle strokes of a loose fitting type A pestle in a Dounce homogenizer. The homogenate was mixed with 2 ml of 90% (wt/vol) sucrose in MNE. A discontinuous sucrose gradient was formed by overlaying sequentially 4 ml of 35% (wt/vol) sucrose and 4 ml of 5% (wt/vol) sucrose, both in MNE buffer, and subsequently centrifuging at 175,000 × g and 4°C for 20 h in an SW40 swinging bucket rotor (Beckman–Spinco). Starting from the top of the gradient, 12 × 1-ml gradient fractions were collected. A 13th fraction was obtained by sonicating the pellet present at the bottom of the gradient in 1 ml of MNE containing 1% Triton X-100.
SDS/PAGE and Western Blot Analysis.
The equivalent of 20–50 μg of total protein was generally separated by SDS/PAGE on 10% minigels and analyzed by Western blotting as described (21). First, the following antibodies were diluted as indicated below in washing buffer with BSA (150 mM NaCl/10 mM Tris⋅HCl, pH 8/10 mg/ml BSA/0.05% Tween-20): anti-human iNOS, 1:40,000; anti-cav-1, 1:10,000; anti-actin, 1:5,000; and anti-PKCα, 1:5,000. After washing, membranes were incubated for 1 h at room temperature with horseradish peroxidase-coupled second antibodies diluted 1:10,000 in washing buffer with BSA. After washing, specifically bound antibodies were detected by enhanced chemiluminescence (Amersham Pharmacia).
Northern Blot Analysis.
Total RNA was extracted in the presence of guanidinium thiocyanate according to the manufacturer's instructions (Qiagen, Hilden, Germany), and iNOS mRNA levels were detected by Northern blot analysis by using a human hepatocyte iNOS cDNA probe as described (24).
Construction and Purification of Glutathione S-Transferase (GST)–Cav-1 Fusion Proteins.
Full-length cDNA encoding dog cav-1 was obtained by reverse transcription–PCR and cloned into the BamHI/KpnI sites of pGEX2T modified as described (25). Cav-1 cDNA sequences encoding residues 1–31, which are present in cav-1α but not cav-1β (26), 1–101, which contains the scaffolding domain corresponding to amino acids 82–101 (27), 1–134, which covers the putative membrane-spanning domain (amino acids 102 to 134), or 1–178, which corresponds to the entire protein (full-length), were generated by PCR amplification of the full-length sequence. Primers were designed to incorporate BamHI or KpnI restriction sites for subcloning into the modified pGEX2T vector. All constructs were confirmed by DNA sequencing in both directions. GST–cav-1 fusion proteins were expressed in Escherichia coli (XL1B or BL-21) and purified as described (28).
Interaction of iNOS with GST–cav-1 Fusion Proteins.
GST or GST–cav-1 fusion proteins prebound to glutathione-agarose beads were washed three times in buffer containing 50 mM Tris⋅HCl (pH 7.4) and 20% (vol/vol) glycerol. Cytokine-stimulated HT29 cytosolic fraction (200 μg protein) was precleared for 1 h at 4°C with GST beads and incubated for 1 h with the different fusion proteins. After a centrifugation at 20,000 × g for 2 min, beads were washed three times in 0.5 ml of 50 mM Hepes, pH 7.5/400 mM NaCl/1 mM EDTA/0.1% Triton X-100. Bound proteins were eluted in Laemmli buffer and analyzed by Western blot analysis.
NOS Activity in Vivo and in Vitro.
Activity of iNOS was determined in intact cells by measuring nitrite accumulation in the culture medium (24) and in the cell extracts by measuring [3H]arginine conversion to [3H]citrulline (29) as described.
Results
Increased Basal Cav-1 Expression Reduced iNOS Protein and Activity Levels in HT29 and DLD1 Cells.
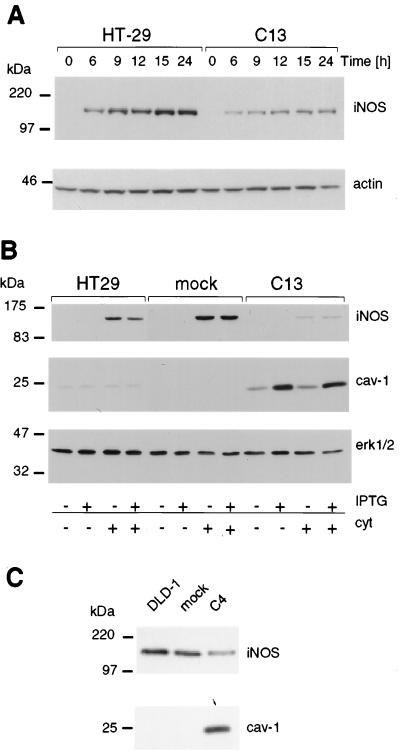
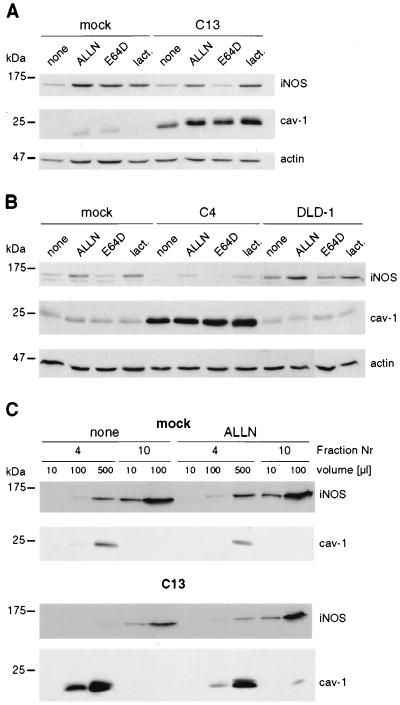
Colon carcinoma cells generally express very low levels of cav-1 (21). To investigate whether cav-1 modulates iNOS in cells, HT29- (C13 and C16 cells) and DLD1-derived (C4 cells) lines expressing exogenous cav-1 (21) were studied. In the absence of cytokines, iNOS was not expressed in HT29 cells (Fig. 1 A and B); however, after the addition of cytokines, iNOS protein increased, reaching a maximum within 15 h, and then remained at that level for at least an additional 9 h (Fig. 1A). Analysis by Western blotting revealed a striking difference in iNOS protein levels in the presence of exogenous cav-1 (Fig. 1 A and B). In C13 cells, the amount of iNOS protein was dramatically reduced as compared with mock-transfected cells or parental HT29 cells. Down-regulation of iNOS protein was already apparent 6 h after onset of cytokine stimulation (Fig. 1A). Basal levels of cav-1 expression, observed in the transfected line (C13) in the absence of IPTG, were already sufficient for this effect (Fig. 1B). Similar results were observed in the cav-1-expressing HT29 clone C16 (data not shown). Increased presence of cav-1 in transfected DLD1 cells reduced iNOS protein levels (Fig. 1C) in a manner comparable to that seen in HT29 cells.
Figure 1.
Levels of iNOS protein were reduced on ectopic expression of cav-1 in HT29 and DLD1 cells. iNOS and cav-1 protein levels were investigated by Western blot analysis. (A) The kinetics of cytokine-induced iNOS protein expression in HT29 and cav-1-expressing C13 cells is shown. Reduced levels of iNOS expression were apparent as early as 6 h after cytokine stimulation and remained low throughout the experiment up to 24 h after induction. Actin was used as control for protein loading. (B) Analysis of parental, mock-, and cav-1-transfected (C13) HT29 cells in the presence (+) or absence (−) of cytokines (cyt) and/or IPTG (150 μM). Extracellular signal-regulated kinase 1/2 (erk1/2) was used as control for protein loading in each lane. (C) iNOS and cav-1 protein levels were determined in DLD1-, mock-, and cav-1-transfected C4 cells after stimulation of the cells with cytokines. As above for HT29 cells, iNOS protein levels were substantially lower in cells ectopically expressing cav-1. Migration positions of molecular-mass marker proteins are indicated to the left of the individual panels.
Activity of iNOS was significantly (P < 0.05) reduced in transfected HT29 (C13 and C16) cells with increased basal levels of cav-1 protein, as compared with either nontransfected or mock-transfected clones (Table 1). In C13 and C16 cells, NOS activity measured by the arginine-to-citrulline conversion assay was 5-fold lower than in parental and mock-transfected HT29 cells. A somewhat lower 2-fold difference was detected for the same cells by measuring nitrite accumulation in the medium. Likewise, ectopic expression of cav-1 in DLD 1 cells reduced iNOS activity from 4.1 ± 0.7 nmol NO2−/mg of protein to 2.9 ± 0.7 (P < 0.05), as assessed in the nitrite-accumulation assay. Thus, increased basal cav-1 levels obtained after transfection reduced iNOS protein levels and activity to a similar extent in different colon carcinoma cell lines.
Table 1.
Ectopic expression of cav-1 reduced iNOS activity in HT29 cells
| Cell line | Activity in vitro | Activity in situ |
|---|---|---|
| HT29 | 33.6 ± 13.8 | 8.2 ± 1.2 |
| Mock | 25.2 ± 7.5 | 7.6 ± 1.4 |
| C13 | 6.5 ± 1.5* | 4.5 ± 0.8* |
| C16 | 5.4 ± 3.3* | 4.5 ± 1.4* |
iNOS activity was assessed in vitro by measuring [3H]arginine conversion to [3H]citrulline (picomoles citrulline per minute per milligram of protein) or in situ by measuring nitrite accumulation in the conditioned medium (nanomoles nitrite per milligram of protein) of parental cells (HT29), mock-transfected cells, or cav-1-transfected (C13 and C16) cells stimulated during 15 h by IL-1β, IL-6, and IFNγ. Mean ± SEM; n = 3–5; *, P < 0.05, t test vs. HT29 or mock.
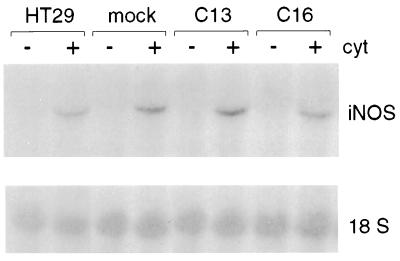
Cav-1 Did Not Prevent Cytokine-Induced iNOS mRNA Levels.
To analyze whether increased cav-1 levels modulated cytokine-induced transcriptional events, iNOS mRNA levels were examined 15 h after cytokine stimulation of HT29 cells. No difference in iNOS mRNA levels was detected in clones C13 and C16, as compared with mock-transfected or nontransfected HT29 lines (Fig. 2). Thus, although we cannot exclude that cav-1 may decrease iNOS transcription and at the same time increase iNOS mRNA half-life (or vice versa), resulting in no significant net change in iNOS steady-state levels, these observations suggested that cav-1 did not regulate iNOS protein levels in HT29 cells by a transcriptional mechanism.
Figure 2.
Cav-1 expression did not alter iNOS mRNA levels induced by cytokines. iNOS mRNA levels were investigated by Northern blot analysis in parental, mock-, and cav-1-transfected (C13 and C16) HT29 cells in the presence (+) or absence (−) of cytokine (cyt) stimulation (15 h). No significant difference in iNOS mRNA levels was observed between the different cell lines. Labeling of the 18S RNA band is shown as a control for loading in each lane.
A Small Amount of iNOS Is Present in Detergent-Insoluble Membrane Fractions.
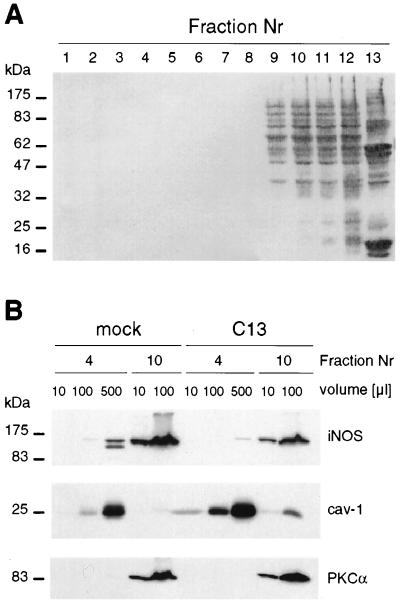
Cav-1 is considered a marker protein for small invaginations of the plasma membrane referred to as caveolae. Hallmarks of these membrane microdomains are their distinct protein and lipid composition as well as detergent insolubility (14). To investigate the distribution of cav-1 and iNOS in cells after stimulation of the cells with cytokines, a fractionation procedure permitting isolation of detergent (Triton X-100)-insoluble, low-density membrane microdomains or rafts (23) was used. As expected (Fig. 3A), most cellular proteins were recovered in high-density fractions (fractions 8–12) containing proteins of the plasma membrane and cytosol, whereas cav-1 was enriched in the light fraction (Fig. 3B, fraction 4) together with exogenously provided 14C-labeled cholesterol (data not shown).
Figure 3.
Cofractionation of cav-1 and iNOS in detergent-insoluble fractions. Mock- or cav-1-transfected (C13) HT29 cells were stimulated during 15 h with cytokines, lysed in 1% Triton X-100, and fractionated on a sucrose gradient. Samples from each fraction (1–12) of the sucrose gradient (plus pellet; fraction 13) were characterized by Western blot analysis. (A) Total protein distribution in a representative gradient is shown after Ponceau Red S staining (10 μl was loaded for each fraction). (B) Western blot analysis of iNOS and cav-1 expression. The abundance of iNOS in the light fraction was determined by comparing increasing volumes (10, 100, or 500 μl) of fraction 4 and fraction 10 (10 or 100 μl). PKCα was used as a control protein that was absent from cav-1-containing fractions. Nr, number.
iNOS was predominantly recovered in fractions 9–12 for both mock- and cav-1-transfected C13 cells (data not shown). However, when increasing volumes of gradient fractions 4 and 10 were compared by Western blotting analysis (Fig. 3B), a small but significant amount of iNOS was detected together with cav-1 in detergent-insoluble membrane fractions of mock- and cav-1-transfected cells. In the mock-transfected cells, a second protein of lower molecular mass was recognized by the anti-iNOS antibody. A similar pattern was observed in C13 cells after longer autoradiography exposure (data not shown). Because the anti-iNOS antibody is directed against the seven COOH-terminal amino acids of human iNOS (30), these results indicated that a 17- to 20-kDa fragment was being cleaved from the NH2 terminus of iNOS and that such processing of iNOS occurred exclusively in the light fraction.
In cav-1-transfected HT29 cells, basal cav-1 levels were roughly 10-fold higher in fraction 4 than endogenous levels detected in mock-transfected HT29 cells. Coincident with a general reduction in iNOS protein levels, this protein became almost undetectable in the light fraction of cav-1-transfected C13 cells, even when large sample volumes were analyzed. In contrast to iNOS, PKCα was not detected in fraction 4 under any conditions analyzed (Fig. 3B). These results support the following three conclusions: (i) ectopically expressed cav-1 protein was recovered in the same low-density detergent-insoluble fractions as the endogenous protein; (ii) iNOS that cofractionated with endogenous cav-1 was degraded at the NH2 terminus; and (iii) loss of iNOS protein on ectopic expression of cav-1 was most apparent in detergent-insoluble sucrose gradient fractions.
Direct Interaction of iNOS with Cav-1.
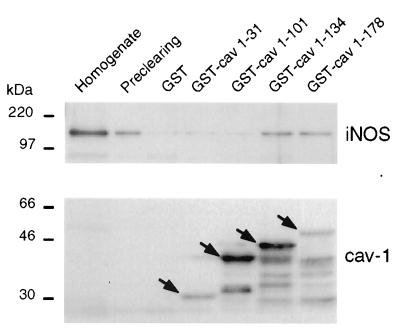
Cav-1 is known to interact directly with eNOS and nNOS (17–20). To determine whether a similar interaction occurs with iNOS, segments of cav-1 including residues 1–31, 1–101, 1–134, or 1–178 (full-length) were expressed as GST fusion proteins in E. coli and purified by affinity chromatography on glutathione-agarose. GST or GST–cav-1 fusion proteins bound to glutathione-agarose beads were used for in vitro binding assays with cytosol from cytokine-stimulated HT29 cells (Fig. 4). iNOS bound specifically to the GST–cav-1 fusion proteins containing segments 1–134 and 1–178, whereas negligible amounts of iNOS bound to GST alone, GST–caveolin (1–31), or GST–caveolin () (Fig. 4).
Figure 4.
In vitro binding of GST–cav-1 fusion proteins to iNOS. Cytosolic fractions (200 μg of protein) of cytokine-induced HT29 cells were precleared with GST-agarose beads, and incubated with immobilized GST or GST–cav-1 fusion proteins. After washing, bound proteins were eluted in Laemmli buffer and analyzed by Western blotting. Bound iNOS was detected in eluates from GST–caveolin (), and GST–caveolin () beads, but not from beads with immobilized GST, GST–caveolin (1–31), or GST–caveolin (). As a control, GST–cav-1 fusion proteins were visualized by a cav-1-specific antibody revealing that similar amounts of each fusion protein were immobilized in each case. Cav-1 fusion proteins of the expected size are indicated with arrows.
Protease Inhibitors Increase iNOS Levels in the Presence of Cav-1.
To identify the proteases involved in cav-1-induced iNOS down-regulation, several inhibitors were added 15 h after cytokine stimulation. Presence of either the calpain and proteasome inhibitor ALLN (31) or the proteasome inhibitor lactacystin (32) increased iNOS levels observed after stimulation of both HT29 (Fig. 5A) and DLD1 (Fig. 5B) cells. Addition of (2S,3S) E64D, a specific calpain inhibitor (33), had no effect (Fig. 5 A and B). Similarly, z-VAD (50 μM), a generic caspase inhibitor, did not increase iNOS protein levels (data not shown). Furthermore, the inhibitor ALLN blocked iNOS degradation observed in the light fraction of mock- and cav-1-transfected HT29 cells (Fig. 5C). Thus, direct interactions between cav-1 and iNOS in HT29 cells are most likely to occur in the detergent-insoluble membrane fraction, involve only a small fraction of iNOS molecules at any given time point, and promote iNOS degradation.
Figure 5.
Cav-1-induced degradation of iNOS was mediated by the proteasome in detergent-insoluble fractions. Cells were stimulated for 15 h with cytokines before the addition of inhibitors for another 9 h. Degradation of iNOS protein was prevented by treatment of the cells with ALLN (10 μM) or lactacystin (lact.; 10 μM) but not E64D (50 μM) in both HT29 (A) and DLD1 (B) cells, independent of whether the parental, mock-, or cav-1-transfected (C13 or C4) cells were treated. (C) Mock- or cav-1-transfected (C13) HT29 cells were stimulated with cytokines, treated with ALLN, and fractionated on sucrose gradients as described.
Discussion
Cav-1 has been described as an inhibitor of various signaling pathways, including those leading to NO production (27). Indeed, ectopic expression of cav-1 in HT29 and DLD1 colon carcinoma cells reduced iNOS activity (Table 1). Unexpectedly, however, this decrease correlated with lower iNOS protein levels (Fig. 1), whereas mRNA levels were not altered (Fig. 2). In addition, pulse–chase experiments, analyzing [35S]methionine-labeled iNOS in immunoprecipitates revealed that the half-life of the iNOS protein was reduced by ≈30%, from 7 to 5 h in the HT29 cav-1-transfected clone C13, as compared with mock-transfected HT29 cells (data not shown). Taken together, these results argue that the observed cav-1-dependent reduction of iNOS protein levels involved a posttranslational mechanism.
Degradation of iNOS protein was detected in the detergent-insoluble light fractions (Figs. 3 and 5). A small amount of iNOS (≈1%) cofractionated on sucrose gradients in the presence of Triton X-100 with endogenous cav-1 of HT29 cells. As predicted based on the presence of a highly conserved caveolin-binding motif in all NOS sequences analyzed (data not shown), iNOS and cav-1 were found to interact directly in vitro (Fig. 4). These results suggest that the interaction detected in vitro between cav-1 and iNOS (Fig. 4) is most likely to occur in detergent-insoluble membrane fractions of HT29 cells. Only a small fraction of the total iNOS protein was detected in detergent-insoluble fractions at any given time point (Fig. 4). Assuming iNOS association with the detergent-insoluble fraction is highly dynamic, this small amount may suffice to account for the observed reduction in overall iNOS protein levels.
In the experiments described here, iNOS binding activity of different cav-1 domains was investigated by using cytosolic cell fractions, whereas previously purified eNOS (18, 34) and nNOS (34) were characterized in this respect. iNOS binding was detected (Fig. 4) for GST–cav () and cav (), but not for cav () containing the scaffolding domain (amino acids 82–101), because that region could potentially have been occupied by other proteins present in carcinoma cell extracts. On the other hand, these results confirm reports showing that cav-1 associates with other proteins via sequence elements lying outside the scaffolding domain. For instance, eNOS binds to the fusion proteins GST–cav () and GST–cav () as well as cav () (20), and steroid receptor coactivator activity is inhibited more efficiently by the COOH-terminal domain of cav-1 than by the canonical scaffolding domain (34).
Cav-1-induced down-regulation of iNOS was caused by enhanced iNOS proteolysis by a proteasome-dependent pathway, because the inhibitors ALLN and lactacystin, but not the calpain inhibitor E64D, reversed the effect of cav-1 in both HT29 and DLD1 cells (Fig. 5). Proteasome-mediated iNOS degradation was already apparent in untransfected cells. To what extent cav-1 presence in general regulates iNOS turnover remains unclear. However, CaCo-2 colon cancer cells, which essentially lack cav-1 (35), reportedly express iNOS even in the absence of cytokines (24). As a consequence, cav-1 would seem to play a very significant role in determining basal iNOS protein levels in colon carcinoma cells. In this context, it is also important to note that basal cav-1 levels in the transfected colon carcinoma cells analyzed in this study were not higher than the levels detected in normal colon epithelium obtained from patients (21), indicating that regulation of iNOS observed was not effected by the presence of physiologically irrelevant cav-1 levels.
The proteasome system generally is held responsible for the degradation of cytosolic proteins (36). More recently, it has been implicated in ligand-induced degradation of growth hormone (37), Met tyrosine kinase (38), or epidermal growth factor (39) receptors. As for these receptors, iNOS turnover seems to be controlled by the proteasome in a membrane fraction. More specifically for HT29 cells, iNOS degradation was observed exclusively in detergent-resistant membrane fractions, was promoted by the presence of cav-1, and was inhibited by either ALLN or lactacystin (Fig. 5). Thus, the results presented here, to the best of our knowledge, identify a previously unknown and unanticipated role for cav-1 in promoting iNOS degradation via the proteasome pathway in detergent-insoluble membrane fractions. Future experiments will reveal whether cav-1 regulates other proteins involved in cellular signaling and transformation in a similar fashion.
Ectopic expression of cav-1 in HT29 and DLD1 cells was recently shown to suppress tumor formation of both these cell lines in nude mice (21). Because endogenously produced NO accelerates tumor growth (40, 41), down-regulation of cav-1 as observed in human colon tumors (21) is expected to promote uncontrolled iNOS activity, genotoxic damage, and tumor development in humans.
Acknowledgments
We are grateful to Olivier Staub for helpful discussion and suggestions, and to Jacques Mauël, Daniel Legler, and Lisette Leyton for critical reading of the manuscript. The work described here is dedicated to Mrs. O. E. Quest, who died of cancer in the course of this project. This work was supported by Swiss National Science Foundation Grants SNSF 3100–050888 (to A.F.G.Q.), SNSF 3100-050713.97 (to C.B.), and SNSF 3100-49662 (to E.F.B.), Swiss Cancer League Grants SCL 636-2-1998 (to A.F.G.Q.); the Sandoz Foundation; and the Chilean National Science Foundation Fondo Nacional de Investigación Científica y Tecnológica Grant 1990893 (to A.F.G.Q.).
Abbreviations
- ALLN
N-acetyl-Leu-Leu-Norleucinal
- cav-1
caveolin-1
- NOS
NO synthase
- eNOS
endothelial NOS
- iNOS
inducible NOS
- nNOS
neuronal NOS
- GST
glutathione S-transferase
- IPTG
isopropyl β d-thiogalactoside
- PKC
protein kinase C
- E64D
(2S,3S)-trans-epoxysuccinyl-l-leucylamido-3-methyl-butane ethyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250406797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250406797
References
- 1.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Griffith O W, Stuehr D J. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C. J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C, Xie Q W. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 5.Marletta M A. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 6.Forstermann U, Gath I, Schwarz P, Closs E I, Kleinert H. Biochem Pharmacol. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen L L, Miles D W, Happerfield L, Bobrow L G, Knowles R G, Moncada S. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobbs C S, Brenman J E, Aldape K D, Bredt D S, Israel M A. Cancer Res. 1995;55:727–730. [PubMed] [Google Scholar]
- 9.Ambs S, Merriam W G, Bennett W P, Felley-Bosco E, Ogunfusika M O, Oser S M, Klein S, Shields P G, Billiar T R, Harris C C. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 10.Souici A C, Mirkovitch J, Hausel P, Keefer L K, Felley-Bosco E. Carcinogenesis. 2000;21:281–287. doi: 10.1093/carcin/21.2.281. [DOI] [PubMed] [Google Scholar]
- 11.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Cardena G, Oh P, Liu J, Schnitzer J E, Sessa W C. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaul P W, Smart E J, Robinson L J, German Z, Yuhanna I S, Ying Y, Anderson R G, Michel T. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R G. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Cardena G, Fan R, Stern D F, Liu J, Sessa W C. J Biol Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 16.Feron O, Belhassen L, Kobzik L, Smith T W, Kelly R A, Michel T. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo V, McIntosh D P, Oh P, Schnitzer J E. J Biol Chem. 1998;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Cardena G, Martasek P, Masters B S, Skidd P M, Couet J, Li S, Lisanti M P, Sessa W C. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 19.Michel J B, Feron O, Sacks D, Michel T. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 20.Ju H, Zou R, Venema V J, Venema R C. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 21.Bender F C, Reymond M A, Bron C, Quest A F G. Cancer Res. 2000;60:5870–5878. [PubMed] [Google Scholar]
- 22.Sherman P A, Laubach V E, Reep B R, Wood E R. Biochemistry. 1993;32:11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- 23.Sargiacomo M, Sudol M, Tang Z, Lisanti M P. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vecchini F, Pringault E, Billiar T R, Geller D A, Hausel P, Felley-Bosco E. Cell Growth Differ. 1997;8:261–268. [PubMed] [Google Scholar]
- 25.Hunn M, Quest A F G. FEBS Lett. 1997;400:226–232. doi: 10.1016/s0014-5793(96)01395-6. [DOI] [PubMed] [Google Scholar]
- 26.Scherer P E, Tang Z, Chun M, Sargiacomo M, Lodish H F, Lisanti M P. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto T, Schlegel A, Scherer P E, Lisanti M P. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 28.Quest A F, Bardes E S, Xie W Q, Willott E, Borchardt R A, Bell R M. Methods Enzymol. 1995;252:153–167. doi: 10.1016/0076-6879(95)52018-x. [DOI] [PubMed] [Google Scholar]
- 29.Felley-Bosco E, Ambs S, Lowenstein C J, Keefer L K, Harris C C. Am J Respir Cell Mol Biol. 1994;11:159–164. doi: 10.1165/ajrcmb.11.2.7519434. [DOI] [PubMed] [Google Scholar]
- 30.Singer I, Kawka D W, Scott S, Weidner J R, Mumford R A, Riehl T E, Stenson W F. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 31.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 32.Fenteany G, Standaert R F, Lane W S, Choi S, Corey E J, Schreiber S L. Science. 1995;268:726–731. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 33.McGowan E B, Becker E, Detwiler T C. Biochem Biophys Res Commun. 1989;158:432–435. doi: 10.1016/s0006-291x(89)80065-8. [DOI] [PubMed] [Google Scholar]
- 34.Venema V J, Ju H, Zou R, Venema R C. J Biol Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 35.Mirre C, Monlauzeur L, Garcia M, Delgrossi M H, Le Bivic A. Am J Physiol. 1996;271:C887–C894. doi: 10.1152/ajpcell.1996.271.3.C887. [DOI] [PubMed] [Google Scholar]
- 36.Ciechanover A. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 37.van Kerkhof P, Govers R, Alves dos Santos C M, Strous G J. J Biol Chem. 2000;275:1575–1580. doi: 10.1074/jbc.275.3.1575. [DOI] [PubMed] [Google Scholar]
- 38.Jeffers M, Taylor G A, Weidner K M, Omura S, Vande Woude G F. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galcheva-Gargova Z, Theroux S J, Davis R J. Oncogene. 1995;11:2649–2655. [PubMed] [Google Scholar]
- 40.Jenkins D C, Charles I G, Thomsen L L, Moss D W, Holmes L S, Baylis S A, Rhodes P, Westmore K, Emson P C, Moncada S. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ambs S, Merriam W G, Ogunfusika M O, Bennett W P, Ishibe N, Hussain S P, Tzeng E E, Geller D A, Billiar T R, Harris C C. Nat Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]