Abstract
The identification of immunophenotypic markers with restricted expression has long been a critical issue in diagnostic and therapeutic advances for acute leukemias. We previously developed a monoclonal antibody against a new thymocyte surface antigen, JL1, and showed that JL1 is expressed in the majority of acute leukemia cases. In this study, using multiparameter flow cytometric analyses, we found that JL1 was uniquely expressed in subpopulations of normal bone marrow (BM) cells, implying the association of JL1 with the differentiation and maturation process. Although CD34+ CD10+ lymphoid precursors and some of maturing myeloid cells express JL1, neither CD34+ CD38−/lo nor CD34+ AC133+ noncommitted pluripotent stem cells do. As for the myeloid precursors, CD34+ CD33+ cells do not express JL1. During lymphopoiesis, JL1 on the earliest lymphoid precursors disappear in the CD20+ sIgM+ stage of B-cell development or after CD1a down-regulation in thymocytes. Despite the highly restricted expression of JL1 in normal BM cells, most of the leukemias express JL1 irrespective of their immunophenotypes. These results indicate that JL1 is not only a novel differentiation antigen of hematopoietic cells, but also a leukemia-associated antigen. Therefore, we suggest that JL1 be a candidate molecule in acute leukemia for the diagnosis and immunotherapy that spares the normal BM stem cells.
Leukemia-specific phenotypes, identified by monoclonal antibodies (mAbs) recognizing various cell surface antigens, have long played essential roles in the diagnosis and classification of leukemia. Because the antigens found on certain leukemic cells are also expressed on the surfaces of the normal counterparts in a developmental stage-dependent manner, they can be used as markers for lineage and differentiation. For instance, differentiation antigens such as CD34, CD10 (CALLA), CD13, CD19, CD7, CD20, CD33, and CD13 that are expressed on specific subsets of normal hematopoietic cells have been used as diagnostic markers for leukemic cells. Therefore, the characterization of molecules on the surface of hematopoietic cells is critical for the diagnosis of leukemia as well as for the understanding of hematopoiesis.
Acute leukemia still remains a therapeutic challenge in medical practice even in the age of high cure rates for pediatric leukemia with the advent of intensification of chemotherapy along with hematopoietic stem cell rescue. 1 It is therefore not surprising that alternative strategies, possessing distinct action mechanisms to complement currently used treatment approaches, are needed and immunotherapy has been an appealing candidate. 2-5 In the past trials, mAb-based immunotherapy targeting leukocyte-specific antigens such as CD33, CD45, and CDw52 has been evaluated for the treatment of leukemia. 6-16 Although immunotherapy might be an attractive approach for the treatment of patients with leukemia, all these antibody-based immunotherapies are dependent on limited antigenic properties themselves. As a consequence, it is worth trying to develop mAb(s) that recognize(s) specific antigen(s) with the restricted expression profile.
We previously reported a mAb against a novel human thymocyte differentiation antigen, designated as JL1, 17 which is not expressed on mature T cells. We further showed that the anti-JL1 mAb broadly recognizes various types of acute leukemias of myeloid and B cell origins as well as T cell lineage. 18 This strong co-relationship between JL1 positivity and the diagnosis of leukemia prompted us to investigate the expression pattern of JL1 antigen in normal leukocytes during hematopoiesis in detail. Although JL1 antigen was initially reported not to be expressed in the majority of normal unfractionated bone marrow (BM) cells, if not all, there remained a possibility that a small proportion of BM cells do express JL1, because of the high heterogeneity of mononuclear cells (MNCs) in normal BM. 18 In the present study, to dissect the expression patterns of JL-1 antigen on the leukocytes of different lineage and maturation stages in BM, cord blood (CB), and thymus, we fractionated the MNCs using lineage-specific markers and re-evaluated JL1 expression on their surfaces. We found that JL1 molecules are expressed on some of the precursor cells of lymphoid and myelomonocytic lineages but not on pluripotent stem cells. As most of the leukemias express JL1 antigen on the cell surfaces, anti-JL1 mAb may have the implications for the immunotherapeutic potential for the treatment of leukemia.
Materials and Methods
Cell Preparation
CB cells (n = 7) were obtained at Keimyung University Hospital, and normal (n = 3) or G-CSF-primed (n = 10) BM cells were aspirated from the posterior iliac crests of healthy adult and pediatric transplantation donors with written consents following guidelines approved by the Institutional Review Board for Human Research, Seoul National University Hospital or Catholic University Hospital in conformity with the Helsinki protocols. Recombinant human G-CSF (Filgrastim; Amgen, Thousand Oaks, CA) was administered subcutaneously at a dosage of 10 μg/kg/day for 3 consecutive days. Both BM aspirates and CB were drawn into 10-ml syringes containing 100 U of preservative-free heparin and then diluted with RPMI 1640 (Life Technologies, Inc., Grand Island, NY) supplemented with 100 U/ml penicillin/streptomycin (Life Technologies, Inc.). MNCs were isolated by density-gradient centrifugation on Ficoll-Hypaque (1077 g/cm3; Pharmacia, Uppsala, Sweden). Normal thymic specimens (n = 5) were obtained from children (<3 years old) undergoing corrective cardiac surgery. Thymocyte single-cell suspensions were prepared, washed, and used for immunofluorescence staining.
Antibodies
The murine mAbs, anti-JL1, were produced and purified as previously described. 17,19 Biotinylation of anti-JL1 mAb was performed with biotin hydrazine (Pierce, Rockford, IL) according to the manufacturer’s protocols. 20,21 The directly labeled mAbs were purchased as follows: CD1a (YG19-FITC), CD4 (YG22-PE), CD8 (DN17-FITC), CD10 (D4-3-1-PE), CD16 (GRM-1-PE), CD19 (SJ25-C1-FITC, -PE), CD20 (B-Ly1-PE), CD33 (WM53-PE), CD45 (AP4-FITC), CD56 (ERIC-1-PE), and CD71 (RVS10-PE) from DiNonA Inc. (Suwon, Korea); CD3 (UCHT1-FITC, -PE) from Southern Biotechnology Associates, Inc. (Birmingham, AL); CD34 (HPCA2-FITC, -PE) from Becton Dickinson Immunocytometry Systems (BDIS, San Jose, CA); CD23 (M-L233-PE), CD38 (HIT2-PE), IgD (IA6-2-PE), IgM (G20–127-PE) from PharMingen (San Diego, CA); AC133 (AC133/2-PE) from Miltenyl Biotec (Bergisch Gladbach, Germany). As for the control experiments, cells were incubated with fluorochrome-labeled isotype-matched controls (IgG1-FITC, -PE; Ig2a-FITC, -PE, biotinylated IgG1). For biotinylated antibodies, streptavidin-cychrome (PharMingen) were used as third fluorochrome for the analysis. Especially, JL1lo cells were well visualized, because cychrome-conjugated streptavidin has excellent quantum efficiency. All stained cells were analyzed using FACScalibur (BDIS) flow cytometry.
Immunofluorescence Staining and Flow Cytometric Analysis
Three-color flow cytometric analysis was used for anti-JL1 mAb screening. The 10 6 cells were first incubated with biotinylated mAbs in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% sodium azide for 30 minutes at 4°C. These cells were then washed with PBS and stained with cychrome-conjugated streptavidin. To minimize nonspecific staining in three-color fluorescence-activated cell sorting (FACS) analysis, this step was followed by incubation with unlabeled irrelevant-murine IgG1 (MOPC21; Sigma, St. Louis, MO) or mouse serum (DAKO, Carpinteria, CA). After indirect staining, relevant fluorochrome-labeled mAbs were used. Before analysis, the cell suspension was passed through a 30-μm nylon mesh (Swiss Silk Bolting Mfg. Co., Zurich, Switzerland). Flow cytometric analysis was performed on a FACScalibur (BDIS) equipped with an argon laser tuned with 488 nm. Forward light scattering, orthogonal light scattering, and fluorescence signals (FL-1-FITC, FL-2-PE or -Red613, FL-3-cychrome) were stored in list mode data files. Results were analyzed for at least 10,000 cells (10,000 to 30,000 cells) per test using the CellQuest software program (BDIS).
Immunophenotyping of Leukemia and Statistical Analysis
To analyze JL-1 expression in leukemic cells from the clinical material, BM specimens from the consecutive patients diagnosed as leukemia in the Seoul National University Hospital were used. The classification of the acute leukemia was made according to the French-American-British classification. MNCs were isolated by Ficoll-Hypaque density gradient centrifugation. The immunophenotype of the leukemic cells was determined by the same method described previously. 18 According to the standard criteria, samples were considered positive when the percentage of stained cells exceeded that of the control by at least 20%. Expression of JL1 or CD34 antigen in leukemia cases was given as a percentage of positive cases. For the statistical analysis of correlation, the expressions of JL1 and CD34 antigens were considered as discontinuous variables. All chi-square P values indicated were two-tailed and reported as statistically significant if less than 0.05. The processing and statistical analysis of the data were performed with the software SPSS V6.1.2. (SPSS, INC., Chicago, IL).
Results
JL1 Expression of Lineage-Committed Leukocytes in BM
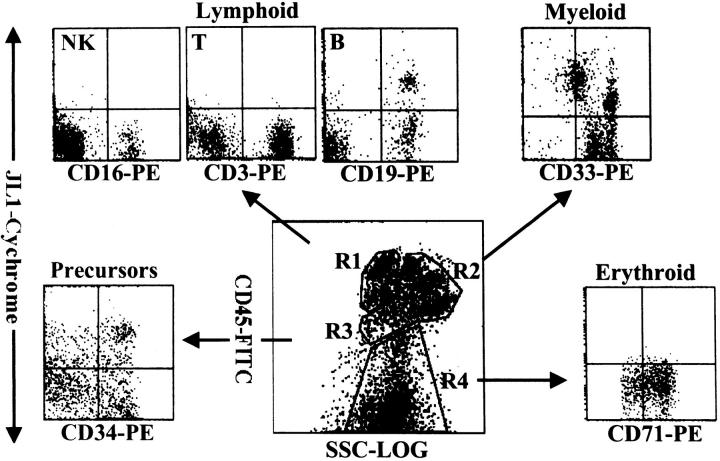
JL1 expression in fresh BM MNCs was investigated using multiparameter FACS analysis with a panel of conjugated mAbs directed against leukocyte surface antigens. Although we previously reported that JL1 antigen was not detectable in unfractionated BM cells, 18 we have reproducibly observed a minimal subpopulation of JL1-expressing cells in subsequent experiments. To confirm the presence of JL1+ subpopulations in BM cells, we first performed FACS analysis with a use of CD45 intensity side-scatter gating and painting and identified JL1+ cells in various leukocyte lineages. 22-25 As shown in Figure 1 ▶ , BM MNCs were able to be separated into four distinct clusters such as lymphoid lineages (R1), myelomonocytic lineages (R2), early precursors (R3), and nucleated red blood cells (R4). The types of corresponding leukocytes in each of the gated regions were confirmed by staining with the respective lineage markers (data not shown). JL1+ cells were detected in CD19+ B cells and CD34+ early precursors, whereas CD3+ T cells, CD16+ NK cells, and nucleated erythrocytes did not express JL1 antigen. The expression pattern of JL1 in the myelomonocytic region was heterogeneous and divided into three subgroups (Figure 3 ▶ , see below). These results were also observed in CB MNCs (data not shown).
Figure 1.
Flow cytometric analysis of JL1 expression of BM leukocytes. MNCs obtained by density-gradient separation were labeled with biotinylated JL1 mAb and SA-cychrome, followed by staining with CD45-FITC in combination with CD3-PE, CD19-PE, CD16-PE, CD33-PE, CD71-PE, or CD34-PE, respectively. Normal BM cells were divided into four normal populations by CD45-side scatter analysis and the expressions of JL1 as well as lineage-specific markers were measured on the corresponding regions. R1, lymphocytes and lymphoblasts; R2, myelomonocytic lineage; R3, early precursors; R4, erythroid cells including nucleated red blood cells.
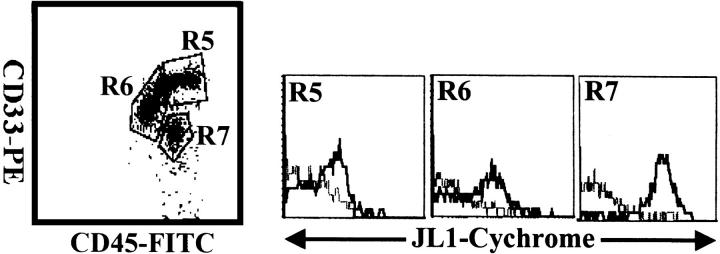
Figure 3.
Three-color flow cytometric analysis of JL1 expression in myeloid population of G-CSF-treated BM MNCs. MNCs obtained by density-gradient separation were labeled with biotinylated JL1 mAb and SA-cychrome, followed by staining with CD45-FITC and CD33-PE. Each lineage cell cluster was painted on the CD45 versus side-scatter plot and JL1 expression was compared. Dashed lines represent isotype control levels for each population. R5, promonocytes and monocytes; R6, promyelocytes, myelocytes, and metamyelocytes; R7, band forms.
JL1 Expression in CD34+ Cells of Human BM and CB
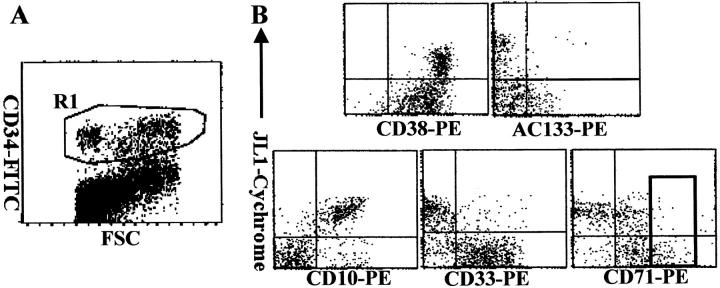
The proportion of JL1+ cells showed a great interdonor variation (5 to 50%) in the early precursor compartment, and was remarkably reduced in CB MNCs (1 to 10%) (data not shown). We correlated JL1 reactivity with the expression of CD34, along with those of AC133, CD38, CD10, CD33, and CD71 to allow identification of noncommitted and various lineage-committed progenitor cells (Figure 2) ▶ . Pluripotent stem cells were defined as AC133+CD34+ or CD38−/dimCD34+ cells in BM or CB, 26-30 and they did not express JL1 molecules at all. In addition, JL1 was not expressed on CD34+CD33+ cells that contain virtually all colony-forming cells such as progenitor cells capable of forming granulocytes, erythrocytes, monocytes, megakaryocytes (CFU-GEMM), CFU-GM, and burst-forming unit erythrocytes (Figure 2B) ▶ . CD34+CD71bright erythroid-committed progenitor cells were also JL1-negative (Figure 2B) ▶ . In contrast, as shown in Figure 2B ▶ , the expression of JL1 antigen was observed on the majority of lymphoid-committed CD10+CD34+ precursors.
Figure 2.
The expression of JL1 molecules on CD34+ BM progenitor cells. BM cells were labeled with biotinylated JL1 mAb and SA-cychrome, followed by staining with CD34-FITC in combination with CD38-PE, AC133-PE, CD10-PE, CD33-PE, or CD71-PE, respectively. CD34+ cells were gated (R1; A) and JL1 expression in R1 gate was compared on each of the progenitor cells (B). A square indicates CD71bright cell population.
The expression profile of the JL1 molecule in CD34+ CB cells was almost same as that in BM cells. However, less CD34+ cells were JL1-positive as compared with that in BM cells (data not shown), and these results are directly attributed to the fact that there are less AC133−CD34+ cells in CB MNCs. Therefore, these data indicate that JL1 expression is restricted to only lymphoid precursor cells among the CD34+ BM or CB MNCs.
JL1 Expression and Myeloid Differentiation in Human BM
Because the cells in the myelomonocytic lineage showed a heterogeneous pattern of JL1 expression, BM cells of myeloid-lineage were enriched by G-CSF administration 31 and they were subdivided on the basis of CD33 and CD45 expression intensities. 32,33 CD33 positivity is strong on promonocytes and monocytes (R5 in Figure 3 ▶ ); median on promyelocytes, myelocytes, and metamyelocytes (R6); and dim on band forms (R7). CD33med promyelocytes, myelocytes, and metamyelocytes showed none or only dim expression of JL1. Most remarkably, the CD33dim band forms expressed JL1 at high levels, whereas mature granulocytes were completely JL1-negative (data not shown). JL1 was also expressed on proportions of CD33bright promonocytes, whereas peripheral monocytes did not express JL1 antigen (data not shown).
JL1 Expression and B-Cell Differentiation in Human BM
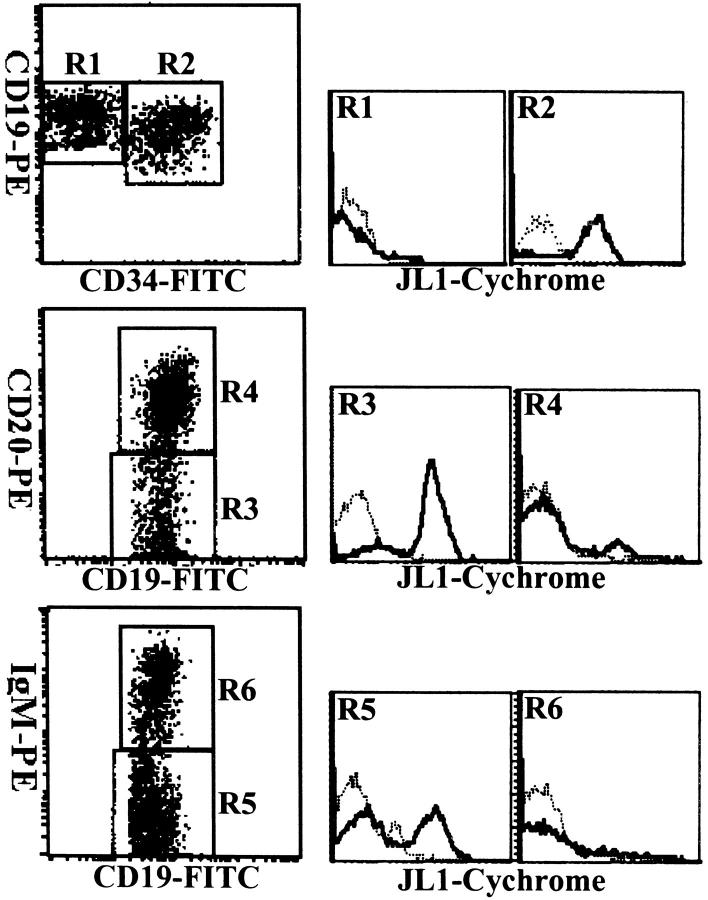
The coordinate expression of the markers such as CD34, CD19, CD20, and CD45RA has been exploited to characterize the developmental pathway of B lymphopoiesis in BM, and the development of B cell precursors could be divided into three major stages. 34,35 To investigate the relationship between the expression of JL1 molecule and B cell development, cells of lymphoid and precursor-rich regions (R1 and R3 gate in Figure 1 ▶ ) were only analyzed, and mAbs against CD19 and surface-IgM (sIgM) were included in the staining protocols.
As shown in Figure 4 ▶ , at least three major stages of B cell differentiation could be identified. The earliest B-lineage cells are characterized by their expression of CD34 and CD19, and these CD34+CD19+ cells showed a high level of JL1 expression. The next stage B cell precursors, characterized by the loss of CD34 expression, weak CD10 expression, and intermediate CD45RA density (CD45RAint) on their surfaces, can be subdivided into two subsets, CD20−/dimsIgM− cells and CD20highsIgM+ cells according to the expression patterns of CD20 and sIgM. The most mature B cell precursors are CD20highsIgM+ cells with CD19+CD10− immunophenotype. Although JL1 antigen was detected on CD20−/dim or sIgM− lymphoblasts, it was no longer observed on CD20high or sIgM+ cells. Therefore, these results revealed that JL1 antigen appeared from the earliest CD34+CD10+ lymphoid precursor cells and disappeared in the CD20highsIgM+ stage of B cell development.
Figure 4.
Three-color cytometric analysis of the JL1 expression of B-lineage cells in BM MNCs. BM cells were labeled with biotinylated JL1 mAb and SA-cychrome, followed by staining with CD34-FITC and CD19-PE, or CD19-FITC in the combination with CD20-PE and IgM-PE, respectively. Dashed lines represent isotype control levels for each of the colored populations.
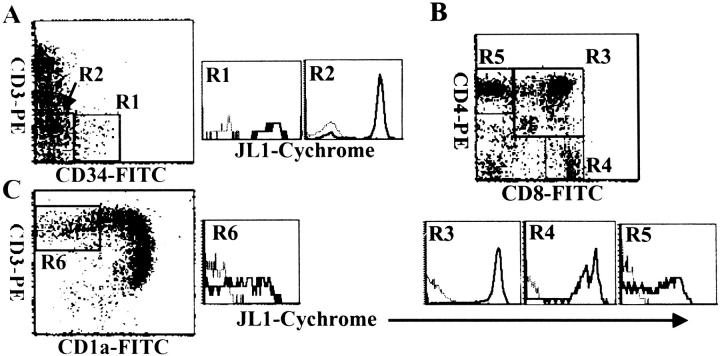
JL1 Expression and T Cell Differentiation in Human Thymus
Because T cell development in human thymus proceeds along the sequential acquisition and loss of the antigens such as CD34, CD1a, CD3, CD4, and CD8, their expression pattern has been used to assess the differentiation pathway of T lymphocytes (Figure 5) ▶ . 36 When JL1 expression was analyzed for T-cell differentiation stage, the expression of JL1 was detected from the earliest CD34+CD1a−CD3− thymic precursor cells to mature single-positive medullary thymocytes through the double-positive CD4+CD8+ thymocytes (Figure 5, A and B) ▶ . Most remarkably, the JL1 expression disappeared after CD1a down-regulation in single-positive CD3high CD4+ or CD3high CD8+ thymocytes (Figure 5C) ▶ . CD1ahigh CD4+ CD8+ CD3−/low immature cortical thymocytes displayed the highest JL1 density on their surfaces among all leukocytes (Figure 5B) ▶ . In addition, a subpopulation of CD3−CD4−CD8− thymocytes did not express JL1 antigen and this subset included virtually all of the CD56+ NK cells (data not shown).
Figure 5.
Three-color flow cytometric analysis of the JL1 expression in human thymocytes. Thymocytes were labeled with biotinylated JL1 mAb and SA-cychrome, followed by staining with CD34-FITC and CD3-PE (A), or CD8-FITC and CD4-PE (B), or CD1a-FITC and CD3-PE (C). Dashed lines represent isotype control levels for each of the colored populations.
JL1 Expression in Leukemia
As summarized in Table 1 ▶ , JL1 antigen was expressed on more than 20% of blast cells in 181 (87.0%) of 208 leukemia cases and was effective for the detection of leukemias regardless of the phenotype. However, in sIg+ non-T-ALL, JL1 positivities were significantly lower than the other subtypes, which is in accordance with the JL1 expression pattern in BM. JL1 positivity was slightly higher than CD34 positivity (76.4%) in all types of leukemia, and this difference is attributed to higher positivity of JL1 antigen in non-T-ALL (P = 0.027). No statistically significant co-relationship was observed between expression of JL1 and that of CD34 in leukemia. Most of the JL1-positive leukemias, including AML, however, expressed CD34 molecules. Furthermore, either JL1 or CD34 was expressed in most of leukemia cases tested, indicating that flow cytometric analysis using both anti-JL1 and anti-CD34 mAbs might be able to detect almost all types of leukemia.
Table 1.
FACS Profiles of Anti-CD34 and Anti-JL1 mAb Immunofluorescence in Leukemia
| Type of leukemia | Percent of JL1+ cases | Percent of CD34+ cases | Percent of CD34+ JL1+ cases |
|---|---|---|---|
| AML | 90.1 (82*/91†) | 83.5 (76/91) | 76.9 (67/91) |
| M0 | 33.3 (1/3) | 100.0 (3/3) | 33.3 (1/3) |
| M1, M2 | 97.4 (38/39) | 92.3 (36/39) | 89.7 (35/39) |
| M3 | 100.0 (7/7) | 42.9 (3/7) | 42.9 (3/7) |
| M4, M5 | 84.4 (27/32) | 81.3 (26/32) | 68.8 (22/32) |
| Others‡ | 90.0 (9/10) | 80.0 (8/10) | 70.0 (7/10) |
| Non-T-ALL | 80.9 (55/68) | 64.7 (44/68) | 61.8 (42/68) |
| CD10+ | 90.6 (48/53) | 73.6 (39/53) | 67.9 (36/53) |
| CD10− | 83.3 (5/6) | 66.7 (4/6) | 66.7 (4/6) |
| sIg+ | 22.2 (2/9) | 11.1 (1/9) | 11.1 (1/9) |
| T-ALL | 87.0 (20/23) | 70.0 (16/23) | 60.9 (14/23) |
| Acute biphenotypic leukemia | 100.0 (13/13) | 84.6 (11/13) | 84.6 (11/13) |
| CML in blast crisis | 84.6 (11/13) | 92.3 (12/13) | 84.6 (11/13) |
| Total | 87.0 (181/208) | 76.4 (159/208) | 71.2 (148/208) |
*No. of positive cases.
†No. of cases tested.
‡Others included M6 (acute erythroleukemia), M7 (acute megakaryocytic leukemia), MDS (myelodysplasic syndromes), etc.
AML (acute myelogenous leukemia); M0 (acute myelogenous leukemia with minimal differentiation); M1 (acute myeloblastic leukemia without maturation); M2 (acute myeloblastic leukemia with maturation); M3 (acute promyelocytic leukemia); M4 (acute myelomonocytic leukemia); M5 (acute monocytic leukemia); non-T-ALL (non-T-cell acute lymphoblastic leukemia); T-ALL (T-cell acute lymphoblastic leukemia); CML (chronic myeloid leukemia).
Discussion
JL1 was discovered during the development of mAbs recognizing human thymocyte-specific antigens. 17 JL1 is detectable on cortical thymocytes but not on peripheral lymphocytes. In earlier studies using unfractionated human BM, subsets of JL1-positive cells were not readily detected. 18 In the present study, we re-examined the level of the expression of JL1 antigen on human leukocytes in BM, CB, and thymus using a multiparameter flow cytometric analysis, to reveal the level of its expression in CD34+ and other lineage-committed precursor cells. Especially, immunostaining was performed in a biotin-streptavidin complex cychrome system to increase the sensitivity of the detection of JL1 antigen. In this study, we provide the evidence that JL1 antigen is a novel differentiation antigen and its expression is restricted to certain subsets of lymphoid and myeloid lineages and not expressed on pluripotent stem cells in BM and CB.
Currently available antigen to identify the pluripotent and progenitor cells in human BM is CD34. 37 The CD34+ population in BM, however, represents heterogeneous cell subsets including erythroid-, lymphoid-, or monomyeloid-committed cells. CD34+ cells, therefore, were subject to further analysis on the basis of AC133 expression. The CD34+ AC133+ population is presently classified as pluripotent cells containing majority of the CFU-GM, a proportion of the CFU-Mix, and a minor population of burst-forming unit erythrocytes, whereas the remaining progenitor cells are included in CD34+ AC133− population. 26 Interestingly, we found that expression of JL1 was completely absent on CD34+ AC133+ cells, whereas one-third of CD34+ AC133− cells were positive for JL1 antigen (Figure 2) ▶ . These data indicate that JL1 antigen is expressed on some progenitor cells but not on pluripotent stem cells or on the earliest stages of myelomonocytic and erythroid lineages. In addition, CD34+ CD38− cells that have been known to be nonlineage-committed progenitors 29 did not express JL1 antigen, confirming that JL1 is not expressed on pluripotent hematopoietic stem cells.
For further analysis of the CD34+ AC133− population to examine the expression patterns of JL1 molecule in terms of other surface markers in detail, the BM blasts were stained with CD34 and lineage-specific markers (Figure 2) ▶ . CD34+CD33+ cells contained all colony-forming cells such as CFU-GEMM, CFU-GM, and burst-forming unit erythrocytes except lymphoid progenitors. The lymphoid- and erythroid-committed progenitor cells are defined with high expression levels of CD10 and CD71, respectively. 26 Whereas JL1 antigen appeared to be expressed on most of CD34+ CD10+ lymphoid precursors, CD34+ CD33+ cells and CD34+ CD71bright erythroid-committed cells were all JL1-negative (Figure 2) ▶ . Therefore, the JL1− fraction of the CD34+ AC133− population consists of CD34+CD33+ cells, and all lymphoid precursors are JL1-positive.
As CD34+ CD10+ population includes CD34+ CD10+ CD19−CD7+ early T-lineage cells, the lymphoid-lineage cells can be further divided into T and B cell lineages in terms of CD19 expression. 38 In B lineage cells, the highest JL1 expression was seen in CD34+ CD19+ cells and a significant reduction of JL1 expression had a inverse relationship with the expression levels of CD20 and sIgM at later stages. Similarly, it was observed that JL1 antigen was expressed from the earliest CD34+CD1a−CD3− thymic precursors to some of the mature single-positive medullary thymocytes during T cell development. Collectively, JL1 is likely to be firstly expressed from common lymphoid progenitors and widely distributed during lymphoid ontogenesis, and to be down-regulated at the time of the final maturation process because JL1 is not expressed on peripheral mature T or B cells. 17 These suggest a possible role of the down-regulation of JL1 molecule in the completion of lymphocyte maturation.
In contrast to its expression in early developmental stages of lymphoid lineage, several interesting patterns of JL1 expression were observed in myelomonocytic lineage cells (Figure 3) ▶ . The CD33dim band forms expressed JL1 at high levels, whereas the CD33med promyelocytes, myelocytes, and metamyelocytes showed none or dim expression (Figure 3) ▶ . CD33high promonocytes and maturing monocytes also showed low levels of JL1 expression. These findings, together with the fact that JL1 is not expressed on mature peripheral monocytes or granulocytes, 17 suggest that JL1 antigen is expressed during the later maturation process in the case of myelomonocytic hematopoiesis.
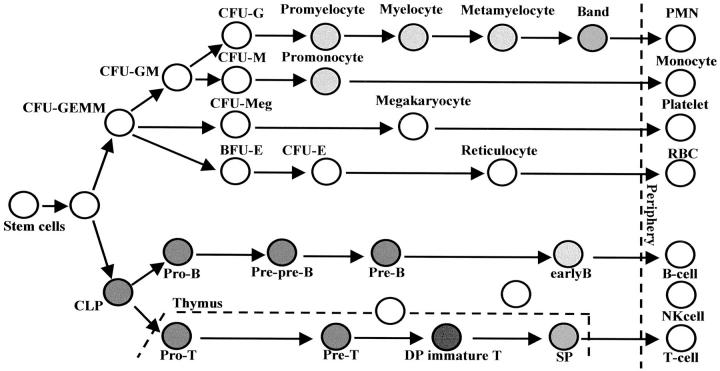
Taken together, these results showed that JL1 antigen is specifically expressed at the earlier stages of B and T cell lineages as well as at the later stage of myelomonocytic cells in BM but not on pluripotent stem cells or not on mature peripheral blood cells (Figure 6) ▶ . Most remarkably, despite the restricted pattern in its tissue distribution, anti-JL1 mAb recognizes various types of acute leukemias (Table 1) ▶ . 18 Therefore, it is likely that JL1 antigen possesses similar characteristics with the CD34 molecule, in that both molecules are expressed within a narrow range of differentiation during hematopoiesis and successfully detect leukemias on the other hand. This finding led us to suggest that JL1 can be applied as an alternative of CD34 in the diagnosis of leukemia, which is currently known to be the most valuable marker antigen in clinical application for the diagnosis and subclassification of leukemia. 37 It is also important to note that JL1 and CD34 antigens were co-expressed in 87.9% of AML. This correlation implies the possibility that this peculiar immunophenotype of AML might be useful for monitoring minimal residual disease after treatment. The current strategy of monitoring minimal residual disease by immunological methods takes advantage of the ectopically co-expressing leukocyte markers on malignant cells, which is not observed in normal BM or PB cells. In this respect, JL1 antigen seems to fit well for this immunophenotypic marker because of the JL1 negativity on normal CD34+CD33+ BM cells. Such phenotypes can be identified by double- or triple-color staining techniques performed with mAbs conjugated to different fluorochromes.
Figure 6.
Expression pattern of JL1 antigen during hematopoiesis. Filled circle represents JL1-expressing populations in BM or thymus, and the degree of its darkness reflects the levels of expression of JL1 molecule.
Restricted expression of CD34 antigen on hematopoietic stem/precursor cells makes the anti-CD34 mAb useful for the quantification and purification of stem/progenitor cells. 37 Anti-CD34 mAb, however, could not be used for immunotherapy of leukemia, because CD34 is expressed on normal vascular endothelial cells as well as on pluripotent stem cells albeit its high expression in leukemia cells. In contrast, JL1 is expressed on various leukemias but not on hematopoietic stem cells or nonhematopoietic tissues. In addition, we recently developed gelonin-conjugated anti-JL1 immunotoxin, which was able to dramatically inhibit the proliferation of in vitro cultured leukemia cells (Bae YM, Shin YK, Park SH, Chung J, Kim SH, Lee I-S, Lee KM, Choi EY, Jung KC, Kim HS, Kim CW, Song HG, manuscript submitted for publication). Therefore, all these findings suggest that JL1 immunotoxin can be useful as a potential candidate for immunotherapy of acute leukemia with negligible damage to normal hematopoietic stem cells.
In this article, we described the expression profile of JL1 antigen on human leukocytes in BM, CB, and thymus in detail. JL1 antigen was expressed in a subpopulation of limited stages during hematopoietic differentiation process, including early lymphoid precursors and maturing myelomonocytic-lineage cells of the late stage. We also demonstrated that JL1 is not expressed on normal hematopoietic pluripotent stem cells. This unique distribution of JL1 antigen further supports our previous suggestion that anti-JL1 mAb might be a good candidate for diagnostic and therapeutic trials of acute leukemia.
Acknowledgments
We thank E. K. Kim (Seoul National University Hospital, Seoul, Korea) and S. K. Chung (Keimyung University Hospital, Taegu, Korea) for their helpful technical support.
Footnotes
Address reprint requests to Seong Hoe Park, Dept. of Pathology, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul 110-799, Korea. E-mail: pshoe@plaza.snu.ac.kr.
Supported by the 1999 BK21 project for Medicine, Dentistry, and Pharmacy, and the 99′ DiNonA Inc. R&D Project, Suwon, Korea.
References
- 1.Compana D, Pui CH: Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood 1995, 85:1416-1434 [PubMed] [Google Scholar]
- 2.Caron PC, Scheinberg DA: Immunotherapy for acute leukemias. Curr Opin Oncol 1994, 6:14-22 [DOI] [PubMed] [Google Scholar]
- 3.Jurcic JG, Caron PC, Scheinberg DA: Monoclonal antibody therapy of leukemia and lymphoma. Adv Pharmacol 1995, 33:287-314 [DOI] [PubMed] [Google Scholar]
- 4.Maloney DG: Advances in immunotherapy of hematologic malignancies. Curr Opin Hematol 1998, 5:237-243 [DOI] [PubMed] [Google Scholar]
- 5.Maloney DG: Advances in the immunotherapy of hematologic malignancies: cellular and humoral approaches. Curr Opin Hematol 1999, 6:222-228 [DOI] [PubMed] [Google Scholar]
- 6.Dillman RO: Antibodies as cytotoxic therapy. J Clin Oncol 1994, 12:1497-1515 [DOI] [PubMed] [Google Scholar]
- 7.Matthews DC: Immmunotherapy in acute myelogenous leukemia and myelodysplastic syndrome. Leukemia 1998, 12(suppl):S33-S36 [PubMed] [Google Scholar]
- 8.Appelbaum FR: Antibody-targeted therapy for myeloid leukemia. Semin Hematol 1999, 36(suppl):S2-S8 [PubMed] [Google Scholar]
- 9.Caron PC, Dumont L, Scheinberg DA: Supersaturating infusional humanized anti-CD33 monoclonal antibody HuM195 in myelogenous leukemia. Clin Cancer Res 1998, 4:1421-1428 [PubMed] [Google Scholar]
- 10.Bernstein ID: Monoclonal antibodies to the myeloid stem cells: therapeutic implications of CMA-676, a humanized anti-CD33 antibody calicheamicin conjugate. Leukemia 2000, 14:474-475 [DOI] [PubMed] [Google Scholar]
- 11.Jurcic JG, DeBlasio T, Dumont L, Yao TJ, Scheinberg DA: Molecular remission induction with retinoic acid and anti-CD33 monoclonal antibody HuM195 in acute promyelocytic leukemia. Clin Cancer Res 2000, 6:372-380 [PubMed] [Google Scholar]
- 12.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA, Hui TE, Martin PJ, Mitchell D, Press OW: Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood 1995, 85:1122-1131 [PubMed] [Google Scholar]
- 13.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA, Hui TE, Martin PJ, Mitchell D, Press OW, Storb R, Bernstein ID: Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood 1999, 94:1237-1247 [PubMed] [Google Scholar]
- 14.Gilleece MH, Dexter TM: Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood 1993, 82:807-812 [PubMed] [Google Scholar]
- 15.Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D, Mellstedt H: Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol 1997, 15:1567-1574 [DOI] [PubMed] [Google Scholar]
- 16.Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H: Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol 1996, 93:151-153 [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Bae YM, Kwon HJ, Kim TJ, Kim J, Lee SJ, Lee SK: JL1, a novel differentiation antigen of human cortical thymocyte. J Exp Med 1993, 178:1447-1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park WS, Bae YM, Chung DH, Kim TJ, Choi EY, Chung JK, Lee MC, Park SY, Park MH, Park SH: A cell surface molecule, JL1; a specific target for diagnosis and treatment of leukemias. Leukemia 1998, 12:1583-1590 [DOI] [PubMed] [Google Scholar]
- 19.Gagnon P: Protein A affinity purification. Gagnon P eds. Purification Tools for Monoclonal Antibodies. 1996, :pp 158-165 Validated Biosystems, Inc, Tucson [Google Scholar]
- 20.Liddell JE, Cryer A: Characterization, purification and labelling. Liddell JE Cryer A eds. A Practical Guide to Monoclonal Antibodies. 1996, :pp 136-137 John Wiley & Sons Ltd, West Sussex [Google Scholar]
- 21.Hermanson GT: Tags and probes. Hermanson GT eds. Bioconjugate Techniques. 1996, :pp 371-400 Academic Press, Inc., San Diego [Google Scholar]
- 22.Civin CI, Loken MR: Cell surface antigens on human marrow cells: dissection of hematopoietic development using monoclonal antibodies and multiparameter flow cytometry. Int J Cell Cloning 1987, 5:267-288 [DOI] [PubMed] [Google Scholar]
- 23.Stelzer GT, Shults KE, Loken MR: CD45 gating for routine flow cytometric analysis of human bone marrow specimens. Ann NY Acad Sci 1993, 677:265-280 [DOI] [PubMed] [Google Scholar]
- 24.Jennings CD, Foon KA: Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood 1997, 90:2863-2892 [PubMed] [Google Scholar]
- 25.Borowitz MJ, Guenther KL, Shults KE, Stelzer GT: Immunophenotyping of acute leukemia by flow cytometric analysis. Use of CD45 and right-angle light scatter to gate on leukemic blasts in three-color analysis. Am J Clin Pathol 1993, 100:534-540 [DOI] [PubMed] [Google Scholar]
- 26.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW: AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90:5002-5012 [PubMed] [Google Scholar]
- 27.Fritsch G, Buchinger P, Printz D: Rapid discrimination of early CD34+ myeloid progenitors using CD45-RA analysis. Blood 1993, 81:2301-2309 [PubMed] [Google Scholar]
- 28.DiGiusto D, Chen S, Combs J, Webb S, Namikawa R, Tsukamoto A, Chen BP, Galy AH: Human fetal bone marrow early progenitors for T, B, and myeloid cells are found exclusively in the population expressing high levels of CD34. Blood 1994, 84:421-432 [PubMed] [Google Scholar]
- 29.Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR: Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38− progenitor cells. Blood 1991, 77:1218-1227 [PubMed] [Google Scholar]
- 30.Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, Terstappen L: The “common stem cell” hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood 1995, 85:2422-2435 [PubMed] [Google Scholar]
- 31.Martinez C, Urbano-Ispizua A, Rozman M, Rovira M, Marin P, Montfort N, Carreras E: Effects of short-term administration of G-CSF (filgrastim) on bone marrow progenitor cells: analysis of serial marrow samples from normal donors. Bone Marrow Transplant 1999, 23:15-19 [DOI] [PubMed] [Google Scholar]
- 32.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF: A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leuk Res 1984, 8:512-534 [DOI] [PubMed] [Google Scholar]
- 33.Andrews RG, Torok-Storb B, Bernstein ID: Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood 1983, 62:124-132 [PubMed] [Google Scholar]
- 34.Loken MR, Shah VO, Dattilio KL, Civin CI: Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood 1987, 70:1316-1324 [PubMed] [Google Scholar]
- 35.Shah VO, Civin CI, Loken MR: Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol 1988, 140:1861-1867 [PubMed] [Google Scholar]
- 36.Terstappen LW, Huang S, Picker LJ: Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood 1992, 79:666-677 [PubMed] [Google Scholar]
- 37.Krause DS, Fackler MJ, Civin CI, May WS: CD34: structure, biology, and clinical utility. Blood 1996, 87:1-13 [PubMed] [Google Scholar]
- 38.Gore SD, Kastan MB, Civin CI: Normal human bone marrow precursors that express terminal deoxynucleotidyl transferase include T-cell precursors and possible lymphoid stem cells. Blood 1991, 77:1681-1690 [PubMed] [Google Scholar]