Abstract
Using a cDNA microarray, we compared the expression of approximately 8000 genes between two unique, clonally related T cell lines derived from different stages of a progressive T cell lymphoma involving skin. A total of 180 genes was found to be differentially expressed at the RNA level by a factor of fivefold or greater. Compared with the cells from the earlier, clinically indolent stage of the lymphoma, 56 genes were up-regulated, whereas 124 genes were down-regulated in the cells from the advanced, clinically aggressive stage lymphoma. The functions of approximately 65% of these genes are currently unknown. The 22 genes with a known function that were up-regulated in the advanced lymphoma cells included several genes involved in promotion of cell proliferation and survival as well as drug resistance. The 42 functionally characterized genes that were down-regulated in the advanced lymphoma cells included negative regulators of cell activation and cell cycle, and mediators of cell adhesion, apoptosis, and genome integrity. The differential expression identified by the cDNA microarray analysis was confirmed for selected genes by reverse transcription-polymerase chain reaction and Northern blotting. The identified differences in gene expression may be related to the differences in behavior between the early and advanced stages of the T cell lymphoma and point to directions for further investigations into mechanisms of lymphoma progression.
Cutaneous T cell lymphoma is the most common lymphoproliferative disorder involving skin. It usually is an indolent, low-grade tumor at the time of presentation. Over time, the CTCL often undergoes transformation to an aggressive, usually fatal high-grade large cell lymphoma. 1 Although dysregulation of p53 and p16INK4a tumor suppressor genes has been implicated in the lymphoma progression, 2,3 the exact molecular mechanism underlying the large cell transformation of cutaneous T cell lymphoma as well as other lymphoid malignancies remains poorly defined.
Recently, complementary DNA (cDNA) microarrays have been used to identify physiologically and pathologically relevant gene expression patterns in a variety of organisms including humans. 4-12 This new technology is based on the fluorescence in situ hybridization in which two different cDNA populations (each labeled with either red or green fluorochrome) are hybridized simultaneously with a microarray containing thousands of deposited cDNA fragments. The ratio of fluorescence intensity (red/green) represents the ratio of concentrations of mRNA molecules that hybridize to each of the cDNAs spotted on the array. In contrast to the traditional molecular techniques that focus on one to a few genes at a time, cDNA microarrays allow gene expression patterns to be analyzed on a genomic scale.
We have previously established two clonally related T cell lymphoma cell lines from a patient with a progressive cutaneous T cell lymphoproliferative disorder. PB1 cell line was established from a relatively early, indolent stage lymphoma, and 2A cell line was derived from the same lymphoma at a later, histologically high-grade, and clinically aggressive stage. 13-15 The cell lines are representative of the primary tumors and retain the morphological, immunophenotypic, and genotypic features of the original lymphoma. In the present study, we compared the gene expression profiles between these two cell lines using a cDNA microarray to investigate molecular changes related to lymphoma progression.
Materials and Methods
Cell Lines
PB1, 2A, and 2B T cell lymphoma cell lines, established from a single patient with a progressive cutaneous T cell lymphoproliferative disorder, have been described in detail previously. 13-15 In brief, the PB1 cell line was obtained at a relatively early stage of the lymphoma from neoplastic T cells circulating in the peripheral blood. The 2A and 2B cell lines were established 2 years later at a clinically aggressive lymphoma stage from two separate skin nodules, which contained high-grade, anaplastic large T cell lymphoma. The common clonal origin of these three T cell lines was demonstrated by cytogenetic and T cell receptor gene rearrangement studies, which were identical to those, found in the patient’s lymphoma tissues. The cell lines were essentially identical to fresh biopsy specimens in regard to morphology, immunophenotype and genotype and retained in culture features of the original lymphoma cells. Sez4 cell line was derived from a patient with Sezary syndrome and also bears close morphological, immunophenotypic, and genotypic resemblance to the original tumor. 16 JB6, SUDHL-1, SUP-M2 and KARPAS299 cell lines were derived from four different NPM/ALK-positive large T/null-cell lymphomas. 17,18 HUT102 and C10MJ cell lines represent HTLV-1-related acute T cell lymphoma/leukemia. 16 L428 and HS445 cell lines were obtained from patients with Hodgkin lymphoma. 19 The exact nature of the HS445 line is uncertain. Although derived from patient with Hodgkin lymphoma, this line may represent an Epstein-Barr virus (EBV)-transformed lymphoblastoid B cell line. 20 20A represents an EBV-transformed low-grade B cell lymphoma cell line and BCBL cell line was derived from an EBV-positive large B cell lymphoma. 21 The MOLT4 cell line represents an acute T cell lymphoblastic leukemia. 20 All cell lines were maintained at 37°C in RPMI1640 supplemented with 10% heat-inactivated fetal calf serum.
cDNA Clones
The 9703 human cDNA clones used in these experiments were obtained from Research Genetics (Huntsville, AL) as bacterial colonies in 96-well microtiter plates. 9 Approximately 8000 distinct Unigene clusters (representing nominally unique genes) were represented in this set of clones.
To date, the identities of approximately 3000 clones have been confirmed by us by re-analysis of the clone DNA sequence. Genes labeled with an asterisk (see Tables 2 and 3 ▶ ▶ ) represent clones whose identities were confirmed by the re-sequencing.
Table 2.
Genes Up-Regulated in Advanced Stage 2A as Compared to Earlier Stage Pb1 Lymphoma Cells
| Genes | Fold increase |
|---|---|
| Group A (genes involved in cell signaling) | |
| Interleukin-6* | 20 |
| Midkine | 6 |
| Platelet-derived growth factor beta chain | 6 |
| Interleukin-1 receptor, type II* | 24 |
| Erythropoietin receptor | 22 |
| Cell surface glycoprotein A15/Talla* | 19 |
| Grancalcin* | 6 |
| Tyrosine-protein kinase CAK* | 9 |
| Transcription factor BTF3* | 6 |
| Transcription factor SATB1* | 5 |
| Zinc finger protein IA-1 | 8 |
| Group B (genes involved in drug resistance) | |
| Bleomycin hydrolase gene* | 13 |
| Na/taurocholate cotransporting polypeptide | 23 |
| Group C (miscellaneous) | |
| Cytochrome c oxidase subunit Vb | 29 |
| Glucosamine-6-phosphate isomerase | 6 |
| Hemoglobin epsilon | 11 |
| CD83 | 6 |
| Myelin basic protein* | 5 |
| Alpha-1-antitrypsin* | 50 |
| Tissue plasminogen activator | 28 |
| Profilin II | 7 |
| Heparin cofactor II | 10 |
*Identity of the clones as defined by the provider was additionally confirmed by us by re-sequencing
Table 3.
Genes Down-Regulated in Advanced Stage 2A Compared to Earlier Stage PB1 Lymphoma Cells
| Genes | Fold decrease |
|---|---|
| Group A (putative inhibitiors of cell signaling) | |
| Protein tyrosine phosphatase 1C* | 8 |
| Protein tyrosine phosphatase gamma* | 5 |
| 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase gamma 2 | 11 |
| Protein tyrosine phosphatase (tissue type: foreskin) | 17 |
| cAMP-dependent 3′,5′-cyclic phosphodiesterase 4* | 5 |
| CD45* | 10 |
| Group B (mediators of cell adhesion) | |
| Fibronectin receptor beta subunit* | 5 |
| Laminin gamma-1 chain* | 9 |
| Leukocyte adhesion glycoprotein LFA-1 alpha chain* | 13 |
| Vascular cell adhesion protein-1 | 14 |
| Platelet endothelial cell adhesion molecule | 11 |
| Cadherin 5 | 6 |
| Thrombin receptor* | 15 |
| Testican | 26 |
| Group C (genes involved in DNA repair and cell apoptosis) | |
| Ataxia-telangiectaxia group-D-associated protein* | 23 |
| D4-GTPase dissociation inhibitor protein* | 63 |
| Annexin I | 7 |
| TNF-related apoptosis inducing ligand TRAIL* | 9 |
| Granzyme H* | 11 |
| Guanine nucleotide regulatory protein (nep1)* | 9 |
| Cathepsin L* | 6 |
| Glutaredoxin* | 10 |
| Group D (negative regulator of cell cycle progression) | |
| P16 (cyclin-dependent kinases regulatory subunit 1) | 5 |
| Group E (miscellaneous) | |
| T-cell receptor alpha chain* | 20 |
| Natural Killer cell G7 protein* | 12 |
| Human (2′-5′) oligo A synthetase E | 7 |
| Thromboxane A synthase* | 6 |
| Complement factor H* | 6 |
| Tonsillar lymphocyte LD78 beta protein* | 8 |
| Macrophage capping protein | 8 |
| Low-density lipoprotein receptor | 9 |
| Mac-2 binding protein* | 9 |
| Ankyrin G | 9 |
| Drebrin E | 10 |
| FLI-1 oncogene* | 10 |
| Beta-2 adrenergic receptor* | 13 |
| Interferon-induced guanylate-binding protein-1 | 17 |
| Antileukoproteinase-1* | 16 |
| Glia-derived nexin* | 20 |
| Guanine nucleotide-binding protein alpha- 16 subunit* | 19 |
| Placental calcium-binding protein | 24 |
*Identify of the clones as defined by the provider was additionally confirmed by us by resequencing.
Production of cDNA Microarrays
The arrays used in this study were produced at Synteni Inc. (now Incyte Genomics, Palo Alto, CA) as part of a collaborative effort. 9,10 Each insert was amplified from a bacterial colony by sampling one microliter of bacterial media and performing polymerase chain reaction (PCR) amplification of the insert using consensus primers for the three plasmids represented in the clone set (5′-TTGTAAAACGACGGCCAGTG-3′, 5′-CACACAGGAAACAGCTATG-3′). Each 100-μl PCR product was purified by gel exclusion, concentrated, and resuspended in 10 μl of 3× SSC buffer. The PCR products were then printed on treated glass microscope slides using a robot with four printing tips. Detailed protocols for assembling and operating a microarray printer, and printing and for experimental application of DNA microarrays are available at http://cmgm.stanford.edu/pbrown.
Preparation and Hybridization of Fluorescent Labeled cDNA
For each comparative array hybridization, labeled cDNA was synthesized by reverse transcription from test cell mRNA in the presence of Cy5-dUTP, and from the reference mRNA with Cy3-dUTP, using the Superscript II reverse-transcription kit (Gibco-BRL) as described previously. 9 In brief, mRNA was mixed with an anchored oligo-dT (d-20T-d(AGC)) primer, heated to 70°C for 10 minutes, and cooled on ice. To this sample were added an unlabeled nucleotide pool (dATP, dCTP, dGTP, and dTTP), either Cy3 or Cy5 conjugated dUTP (Amersham), 5× first-strand buffer, 0.1 mol/L DTT, and 400 U of Superscript II reverse transcriptase. After a 2-hour incubation at 42°C, the RNA was degraded by addition of 1 N NaOH, and incubation at 70°C for 10 minutes. The mixture was neutralized by adding 1 N HCL, and the volume increased by adding TE buffer. Cot1 human DNA (Gibco-BRL) was added, and the probe was purified by centrifugation in a Centricon-30 microconcentrator (Amicon). The two separate fluorochrome-labeled probes were combined and concentrated. PolyA RNA (Sigma) and tRNA (Gibco-BRL) were added, and the volume was adjusted with distilled water. For final probe preparation, 20× SSC (1.5 mol/L NaCl, 150 mmol/L sodium citrate, pH 8.0) and 10% SDS were added. The probes were denatured by heating for 2 minutes at 100°C, incubated at 37°C for 20 to 30 minutes, and placed on the array under a 22 mm × 22 mm glass coverslip. The slides were incubated at 65°C for 14 to 18 hours in a custom slide chamber with humidity maintained by a small reservoir of 3X SSC. Arrays were washed by submersion and agitation for 2 to 5 minutes in 2× SSC with 0.1% SDS, followed by 1× SSC, and then 0.1× SSC. The arrays were spun dry by centrifugation for 2 minutes in a slide-rack in a Beckman GS-6 tabletop centrifuge in Microplus carriers at 650 RPM for 2 minutes.
Array Quantitation and Data Processing
After hybridization, arrays were scanned using a laser-scanning microscope described at http://cmgm.stanford.edu/pbrown. Separate images were acquired for Cy3 and Cy5. Data reduction was performed with the program ScanAlyze (Michael Eisen; available at http://rana.stanford.edu/software). Each spot was defined by manual positioning a grid of circles over the array image. For each fluorescent image, the average pixel intensity within each circle was determined, and a local background was computed for each spot equal to the median pixel intensity in a square of 40 pixels in width and height centered on the spot center, excluding all pixels within any defined spots. Net signal was determined by subtraction of this local background from the average intensity for each spot. Spots deemed unsuitable for accurate quantitation because of array artifacts were manually flagged and excluded from further analysis. Data files generated by ScanAlyze were entered into a custom database that maintains web accessible files. Signal intensities between the two fluorescent images were normalized by applying a uniform scale factor to all intensities measured for the Cy5 channel. The normalization factor was chosen so that the mean log(Cy3/Cy5) for a subset of spots that achieved a minimum quality parameter (approximately 6000 spots) was 0. This effectively defined the signal-intensity-weighted “average” spot on each array to have a Cy3/Cy5 ratio of 1.0. A gene is considered to be differentially expressed when the difference in fluorescent intensity between the two fluorochromes is more than fivefold. Except for expressed sequence tags, genes that are differentially expressed are further divided into smaller groups based on their functions reported in the literature, such as promotion or inhibition of cell signal transduction, drug resistance, cell adhesion, proliferation, DNA repair, cell cycle progression, and apoptosis.
Reverse Transcription (RT)-PCR and Northern Blotting
Total RNAs were isolated from all cell lines using the standard TRIzol reagent protocol. The first-strand cDNAs were synthesized using a Superscript II-RNase H− reverse transcriptase kit, and were used as templates for PCR with gene-specific primer sets as listed in Table 1 ▶ . The PCR products were separated on a 2% agarose gel followed by ethidium bromide staining. Northern blots were performed using the standard protocols. 20 Briefly, total RNAs were electrophoresed on 1.5% agarose gel containing 50% formaldehyde, transferred to a nitrocellulose membrane and hybridized with P32-labeled DNA probes using a random-primer labeling kit. Autoradiographs were quantitatively analyzed using a Molecular Dynamics Densitometer and Imagequant Version 3.22 software (Molecular Dynamics, Sunnyvale, CA).
Table 1.
Primers Used for PCR Amplification of the Selected Genes
| Gene | Primer | Sequence |
|---|---|---|
| AT | Forward | 5′TGACGGGGTCACCCACACTGTGCCCATCTA3′ |
| Reverse | 5′CTAGAAGCATTGCGGTGGACGATGGAGGG3′ | |
| BH | Forward | 5′CATCTAGGAAAGACAGTGAT3′ |
| Reverse | 5′CGATTGAGGTGTATGGAGAGA3′ | |
| Na/TCT | Forward | 5′TGGGATTTGGGTATTTGAGTA3′ |
| Reverse | 5′CTTTCTCCAGCATTTCCAGTA3′ | |
| D4-GDI | Forward | 5′TTGTTCTCTTGTGTCGTTTAC3′ |
| Reverse | 5′ATCTTTTCCCACCCTGTCACT3′ |
AT, alpha-actin; BH, bleomycin hydrolase gene; Na/TCT, Na/taurocholate cotransporting polypeptide; D4-GDI, D4-GTPase dissociation inhibitor protein.
Results and Discussion
Cell proliferation, differentiation, apoptosis, migration, and interactions are controlled by tightly regulated programs of differential gene expression. Disturbances in the gene expression profiles occur in both tumor initiation and progression. 22 The standard techniques of molecular biology have been successfully used to identify an increasing number of genes involved in oncogenesis. However, these methods are highly focused, targeting one or a few genes at a time, and do not provide insight into global gene expression. With the advent of cDNA microarrays and similar technologies, we are now able to study simultaneously thousands of genes and to explore on a genomic scale their expression patterns in cells under physiological and pathological conditions. 4-12
In this study, we investigated the expression of almost ten thousand genes in two clonally-related T cell lymphoma cell lines, PB1 and 2A, 13-15 using a cDNA microarray. Because these two cell lines were derived from the same T cell lymphoma at different stages of tumor progression (indolent versus aggressive, respectively), we concentrated on the differences in gene expression to identify genes that might be involved in the transition of lymphoma to a more malignant phenotype. Transcripts of 56 genes were found to be at least fivefold more abundant in the 2A cell line derived from the advanced stage lymphoma as compared to PB1 cell line from the earlier stage lymphoma. Twenty-two of these genes have known functions (Table 2) ▶ , whereas the others are represented by expressed sequence tags whose function remains to be determined. Among those with known functions, approximately half are involved in signal transduction pathways that promote cell proliferation and survival, including genes coding for cytokines, growth factors and their receptors, cytoplasmic calcium-binding protein, protein kinases, and transcription factors (Group A in Table 2 ▶ ). Many of these genes have been found to be strongly expressed in a variety of carcinomas and lymphomas. 22-25 Two genes that may play a role in drug resistance showed increased expression in the 2A cells (Group B). Ferrando et al 26 showed that one of these genes, encoding bleomycin hydrolase (Figure 1) ▶ , was expressed at an elevated level in head and neck carcinomas when compared to an adjacent normal mucosa. They also observed that bleomycin hydrolase expression is low or undetectable in Hodgkin’s disease, which contains mostly normal reactive cells, but is high in Burkitt’s lymphomas. These results are consistent with a proposed role for human bleomycin hydrolase in resistance of some tumors to bleomycin chemotherapy. Interestingly, our finding suggests that in this case the expression of bleomycin hydrolase may have been intrinsic to tumor progression rather than secondary to chemotherapy because there is no record of the patient’s exposure to bleomycin. The role, if any, of the genes in the third, rather heterogeneous group C in lymphoma progression remains to be determined.
Figure 1.
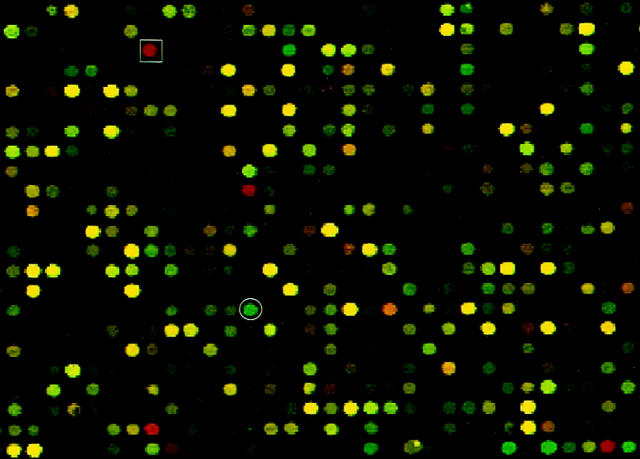
The expression of bleomycin hydrolase gene (red spot in square box) and D4-GTPase dissociation inhibitor protein gene (green spot in circle) is up- and down-regulated, respectively, in the 2A cell line derived from an advanced stage of cutaneous T cell lymphoma as compared to the PB-1 cell line derived from an early stage of the same lymphoma. 13-15 The figure shows a computer-generated image of the representative area of the cDNA microarray hybridized with the cell line-derived cDNA labeled with two different fluorochromes.
The expression of 124 genes was diminished in the 2A cells as compared to PB1. More than two-third of the genes were represented only by expressed sequence tags. Based on their reported functions, the remaining genes can be divided into five broad categories (Table 3) ▶ . Genes in Group A encode protein phosphatases and related proteins, which can act to down-modulate signal transduction pathways that promote cell growth. For example, protein tyrosine phosphatase 1C interacts with Grb2 adaptor protein and modulates the effect of the Ras-signaling transduction pathway. 22,27,28 Therefore, diminished expression of the phosphatase and other molecules from this category, may confer an increased proliferative activity on malignant cells. Indeed a high proliferative rate characteristic of advanced, high-grade lymphomas was seen in the lymphoma from which the 2A cell line was derived. Genes in Group B are cell adhesion molecules involved in cell-cell and cell-matrix interactions. Down-regulation of the adhesion molecules may be related to the high propensity of advanced tumors to metastasize. 29,30 The fact that the patient’s lymphoma was confined to skin and peripheral blood at the time when the PB-1 cell line was established, and displayed a wide-spread involvement of internal organs shortly after the 2A cell line was obtained, 13 is in agreement with such a possibility. Furthermore, Gregory et al 31 demonstrated that the decreased expression of the adhesion molecules LFA-3 and ICAM-1 by lymphoma cells interferes with virus-specific T cell surveillance against Epstein-Barr virus-positive Burkitt’s lymphoma. This observation suggests an additional role for cell adhesion molecules as targets for tumor immunosurveillance. Genes in group C consist of those involved in the regulation of DNA damage repair, genome stability and apoptotic cell death. For example, patients with mutations of Ataxia-Telangiectasia (AT) gene are prone to develop hematopoietic and non-hematopoietic malignancies. 32 Defects in the AT gene are believed to affect DNA repair leading to accumulation of additional genetic changes that may be directly involved in malignant cell transformation. Another gene in this group, D4-GTPase dissociation inhibitor protein (D4-GDI; Figure 1 ▶ ), belongs to the Rho-related family of small GTP-binding proteins, which play an important role in a wide range of cellular functions including actin polymerization, cell cycle progression and apoptosis. D4-GDI is preferentially expressed at a very high level in hematopoietic cells, 33 and it is specifically degraded during apoptosis. 34,35 Another gene relatively underexpressed in the 2A cells, P16INK4a, represents a negative regulator of cell cycle progression (Group D). P16INK4a is one of cyclin-dependent kinase regulators and inhibits the G1/S cell cycle progression. It has also been shown to prevent cellular transformation by H-ras. 36,37 Loss of P16INK4a expression in non-Hodgkin’s lymphomas including cutaneous T cell lymphoma, is frequently associated with tumor progression. 3,38 Our results provide additional evidence that P16INK4a may play a role in the malignant progression of cutaneous T cell lymphoma. The last group of the genes, designated E, contains a variety of genes whose possible impact, if any, on tumor progression is uncertain. Interestingly, there is a marked decrease in expression of T cell receptor α chain seen also on the protein level by flow cytometry (MA Wasik, unpublished data). This decrease may reflect the diminished dependence on receptor-mediated stimuli in the more advanced lymphoma. 14,15,20
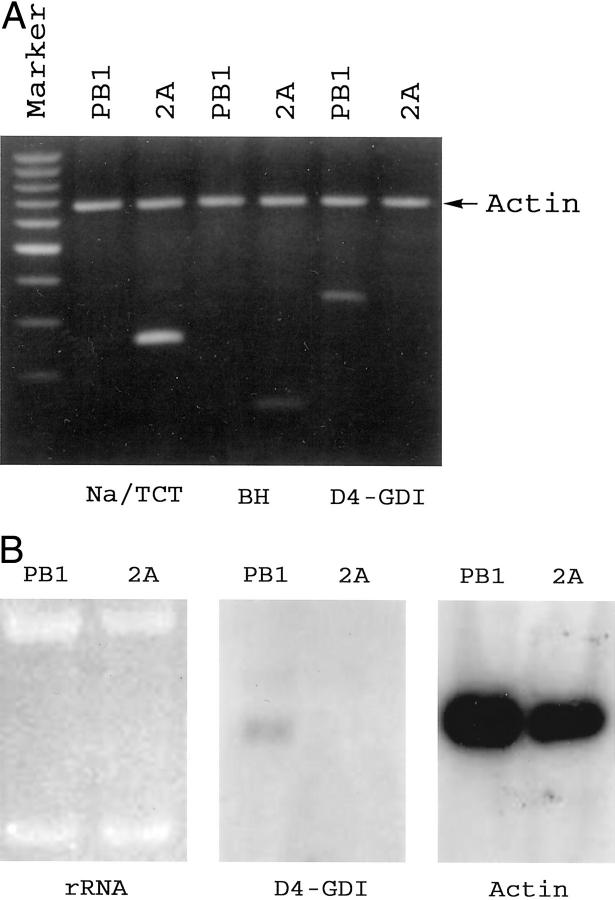
To confirm and validate the results obtained by cDNA microarray, we analyzed expression of selected, differentially expressed genes by conventional molecular methods. RT-PCR analysis (Figure 2A) ▶ , verified the diminished expression of D4-GDI and increased expression of the bleomycin hydrolase gene and the sodium/taurocholate cotransporting polypeptide gene in 2A cells in relation to PB1 cells. The relatively decreased expression of D4-GDI in 2A cells was additionally confirmed by Northern blot analysis (Figure 2B) ▶ and the ratio of D4-GDI mRNA between the two cell lines as determined by a densitometer analysis was similar to that detected by cDNA microarray.
Figure 2.
Confirmation of the cDNA microarray-identified differential expression of selected genes between the less and more advanced stages of cutaneous T cell lymphoma represented by PB1 and 2A cells, respectively. A: RT-PCR. D4-GDI, D4-GTPase dissociation inhibitor protein; Na/TCT, Na/taurocholate cotransporting polypeptide; BH, bleomycin hydrolase gene. The DNA bands reflecting expression of these genes are highlighted by white arrowheads. Analysis of actin expression served as a positive control. The marker is a synthetic 100-bp DNA ladder. B: Northern blot analysis of D4-GDI gene expression. Equal amounts of intact total RNA from the two cell lines was loaded as indicated by the intensity and integrity of the ribosomal RNA (rRNA) bands on the ethidium bromide-stained agarose gel. Detection of actin expression served as a positive control. rRNA, ribosomal RNA.
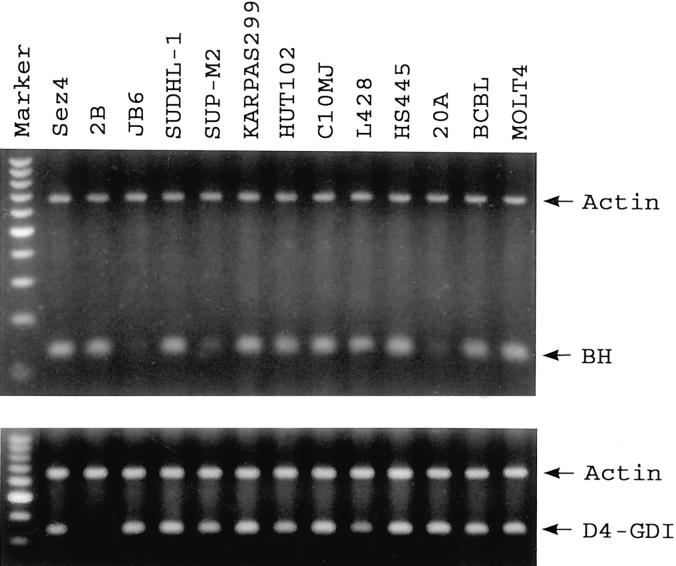
We further investigated the expression pattern of bleomycin hydrolase gene and the D4-GDI gene in a variety of lymphoma cell lines. As shown in Figure 3 ▶ , the majority of the lymphoma cell lines, which usually represent highly transformed malignant cells, overexpressed bleomycin hydrolase gene at a level similar to that seen in the 2A and 2B cell lines derived from the advanced lymphoma. The down-regulation of D4-GDI gene expression, however, was almost completely specific to 2A and 2B cell lines suggesting that the role this gene might play in malignant progression is likely to be restricted to specific types of T cell lymphoma.
Figure 3.
RT-PCR analysis of the BH and D4-GDI gene expression in cell lines representing various types of malignant lymphoma. See Materials and Methods for the line description. Analysis of actin expression served as a positive control. The marker comprises a 100-bp DNA ladder.
In summary, by using an 8000 gene cDNA microarray we have identified functionally-related groups of genes that are differentially expressed in two T cell lines derived from different stages of a cutaneous T cell lymphoma. Genes involved in cell proliferation, cell survival and resistance to drugs were among the genes we found to be more highly expressed in the advanced, progressed stage of the lymphoma. Conversely, negative regulators of cell signaling and cell cycle, promoters of cell adhesion and apoptosis and genes involved in DNA repair were among the genes with lower level of expression in the advanced lymphoma stage. These results correlate well the progressive changes that occurred in the biological features and clinical behavior of the lymphoma and provide new leads for investigation into the molecular pathomechanisms of lymphoma progression. Such investigation should be performed with primary patient samples to define the frequency of genetic changes identified by our analysis as well as identify additional changes. A better understanding of pathogenesis and progression of cutaneous T cell lymphoma and other malignancies should result in refined diagnosis and novel therapies targeting the aberrantly expressed genes.
Footnotes
Address reprint requests to Mariusz A. Wasik, Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center, Founders 7.106, 3400 Spruce Street, Philadelphia, PA 19104. E-mail: wasik@mail.med.upenn.edu.
Supported in part by National Cancer Institute grant CA89194 to MAW, National Human Genome Research Institute grant HG00450 to P. O. B. and the Howard Hughes Medical Institute. M. A. W. is a recipient of the Shannon Award from the National Cancer Institute and P. O. B. is an associate investigator of the Howard Hughes Medical Institute.
S. L.’s current address: Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30322.
References
- 1.Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R: Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood 1998, 92:1150-1159 [PubMed] [Google Scholar]
- 2.Li G, Chooback L, Wolfe JT, Rook AH, Felix CA, Lessin SR, Salhany KE: Overexpression of p53 protein in cutaneous T cell lymphoma: relationship to large cell transformation and disease progression. J Invest Dermatol 1998, 110:767-770 [DOI] [PubMed] [Google Scholar]
- 3.Navas IC, Ortiz-Romero PL, Villuendas R, Martinez P, Garcia C, Gomez E, Rodriguez JL, Garcia D, Vanaclocha F, Iglesias L, Piris MA, Algara P: P16(INK4a) gene alterations are frequent in lesions of mycosis fungoides. Am J Pathol 2000, 156:1565-1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schena M, Shalon D, Davis RW, Brown PO: Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270:467-470 [DOI] [PubMed] [Google Scholar]
- 5.DeRisi J, Penland L, Brown PO, Bittner ML, Meltzer PS, Ray M, Chen Y, Su YA, Trent JM: Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet 1996, 14:457-460 [DOI] [PubMed] [Google Scholar]
- 6.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JCF, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO: The transcriptional program in the response of human fibroblasts to serum. Science 1999, 283:83-87 [DOI] [PubMed] [Google Scholar]
- 7.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES: Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 1999, 286:531-537 [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Staudt LM: Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403:503-511 [DOI] [PubMed] [Google Scholar]
- 9.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO: Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 2000, 24:227-235 [DOI] [PubMed] [Google Scholar]
- 10.Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, Scudiero DA, Eisen MB, Sausville EA, Pommier Y, Botstein D, Brown PO, Weinstein JN: A gene expression database for the molecular pharmacology of cancer. Nat Genet 2000, 24:236-244 [DOI] [PubMed] [Google Scholar]
- 11.Rusiniak ME, Yu M, Ross DT, Tolhurst EC, Slack J: Identification of B94 (TNFAIP2) as a potential retinoic acid target gene in acute promyelocytic leukemia. Cancer Res 2000, 60:1824-1829 [PubMed] [Google Scholar]
- 12.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Aksien LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein: molecular portraits of human breast tumours. Nature 2000, 406:747–752 [DOI] [PubMed]
- 13.Davis TH, Morton CC, Miller-Cassman R, Balk SP, Kadin ME: Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med 1992, 326:1115-1121 [DOI] [PubMed] [Google Scholar]
- 14.Wasik MA, Seldin DC, Butmarc JR, Gertz R, Marti R, Maslinski W, Kadin ME: Analysis of IL-2, IL-4, and their receptors in clonally-related cell lines derived from a patient with a progressive cutaneous T-cell lymphoproliferative disorder. Leuk Lymphoma 1996, 23:125-136 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Nowak I, Vonderheid EC, Rook AH, Kadin ME, Nowell PC, Shaw LM, Wasik MA: Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc Natl Acad Sci USA 1996, 93:9148-9153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Lee B, Korecka M, Li G, Weyland C, Eck S, Gessain A, Arima N, Lessin SR, Shaw LM, Luger S, Kamoun M, Wasik MA: Differences in phosphorylation of the IL-2R associated Jak/STAT proteins between HTLV-1(+), IL-2 independent and IL-2-dependent cell lines and unculturead leukemic cells from patients with adult T-cell lymphoma/leukemia. Leuk Res 1999, 23:373-384 [DOI] [PubMed] [Google Scholar]
- 17.Dirks WG, Zaborski M, Jager K, Challier C, Shiota M, Quentmeier H, Drexler HG: The (2;5)(p23;q35) translocation in cell lines derived from malignant lymphomas: absence of t(2;5) in Hodgkin-analogous cell lines. Leukemia 1996, 10:142-149 [PubMed] [Google Scholar]
- 18.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT: Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263:1281-1284 [DOI] [PubMed] [Google Scholar]
- 19.Drexler HG: Recent results on the biology of Hodgkin and Reed-Sternberg cells. II. Continuous cell lines. Leuk Lymphoma 1993, 9:1-25 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Raghunath PN, Vonderheid E, Ødum N, Wasik MA: Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter. Am J Pathol 2000, 157:1137-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, Silberstein LE, Nowell PC, Schuler W, Shaw LM, Wasik MA: The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA 2000, 97:4285-4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA: The hallmark of cancer. Cell 2000, 100:57-70 [DOI] [PubMed] [Google Scholar]
- 23.Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T, Muramatsu H, Kadomatsu K, Muramatsu T: Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer 2000, 83:701-706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, Watanabe K, Murari M, Isogai C, Kinoshita T, Nagai H, Ohashi H, Nagasaka T, Kadomatsu K, Muramatsu H, Muramatsu T, Saito H, Mori N, Murate T: Midkine expression in Reed-Sternberg cells of Hodgkin’s disease. Leuk Lymphoma 2000, 37:415-424 [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki H, Seon BK: Molecular nature of a cell membrane antigen specific for human T-cell acute lymphoblastic leukemia. Cancer Res 1987, 47:4283-4286 [PubMed] [Google Scholar]
- 26.Ferrando AA, Velasco G, Campo E, Lopez-Otin C: Cloning and expression analysis of human bleomycin hydrolase, a cysteine proteinase involved in chemotherapy resistance. Cancer Res 1996, 56:1746-1750 [PubMed] [Google Scholar]
- 27.Greer SF, Justement LB: CD45 regulated tyrosine phosphorylation of CD22 and its association with the protein tyrosine phosphatase SHP-1. J Immunol 1999, 162:5278-5286 [PubMed] [Google Scholar]
- 28.Kon-Kozlowski M, Pani G, Pawson T, Siminovitch KA: The tyrosine phosphatase PTP1C associates with Vav, Grb2, and mSos1 in hematopoietic cells. J Biol Chem 1996, 271:3856-3862 [DOI] [PubMed] [Google Scholar]
- 29.Stroeken PJ, van Rijthoven EA, Boer E, Geerts D, Roos E: Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene 2000, 19:1232-1238 [DOI] [PubMed] [Google Scholar]
- 30.Terol MJ, Lopez-Guillermo A, Bosch F, Villamor N, Cid MC, Campo E, Montserrat E: Expression of beta-integrin adhesion molecules in non-Hodgkin’s lymphoma: correlation with clinical and evolutive features. J Clin Oncol 1999, 17:1869-1875 [DOI] [PubMed] [Google Scholar]
- 31.Gregory CD, Murray RJ, Edwards CF, Rickinson AB: Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt’s lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med 1988, 167:1811-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidemann K, Henze G, Beck JD, Sauerbrey A, Kuhl J, Mann G, Reiter A: Non-Hodgkin’s lymphoma in pediatric patients with chromosomal breakage syndromes (AT and NBS): experience from the BFM trials. Ann Oncol 2000, 11(suppl 1):141-145 [PubMed] [Google Scholar]
- 33.Scherle P, Behrens T, Staudt LM: Ly-GDI, a GDP-dissociation inhibitor of the Rho GTP-binding protein, is expressed preferentially in lymphocytes. Proc Natl Acad Sci USA 1993, 90:7568-7572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essmann F, Wieder T, Otto A, Muller EC, Dorken B, Daniel PT: GDP dissociation inhibitor D4-GDI (Rho-GDI2), but not the homologous GDI 1, is cleaved by caspase-3 during drug-induced apoptosis. Biochem J 2000, 345 Pt3:777-783 [PMC free article] [PubMed] [Google Scholar]
- 35.Na S, Chuang TH, Cunningham A, Turi TG, Hanke JH, Bokoch GM, Danley DE: D4-GDI, a substrate of CPP32, is proteolyzed during Fas-induced apoptosis. J Biol Chem 1996, 271:11209-11213 [DOI] [PubMed] [Google Scholar]
- 36.Serrano M, Hannon GJ, Beach D: A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366:704-707 [DOI] [PubMed] [Google Scholar]
- 37.Serrano M, Gomez-Lahoz E, DePinho RA, Beach D, Bar-Sagi D: Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science 1995, 267:249-252 [DOI] [PubMed] [Google Scholar]
- 38.Villuendas R, Sanchez-Beato M, Martinez JC, Saez AI, Martinez-Delgado B, Garcia JF, Mateo MS, Sanchez-Verde L, Benitez J, Martinez P, Piris MA: Loss of p16/INK4A protein expression in non-Hodgkin’s lymphomas is a frequent finding associated with tumor progression. Am J Pathol 1998, 153:887-897 [DOI] [PMC free article] [PubMed] [Google Scholar]