Abstract
Several clinical observations and experimental studies indicate that pituitary hormones, including growth hormone, play a role in the development of human breast cancer. We analyzed 48 human breast carcinomas using reverse transcription polymerase chain reaction, immunohistochemistry, and Western blotting techniques to assess growth hormone receptor expression. In 17 of these cases, adjacent normal breast tissue was similarly analyzed. These analyses revealed that growth hormone receptor (GHR) is expressed in human breast cancer and appears to be up-regulated compared to adjacent normal breast tissue. GHR expression correlated inversely with tumor grade and MIB-1 index. Progesterone receptor expression correlated positively with GHR expression. These findings, along with our observation of GHR expression in breast cancer stromal cells and previous reports of local production of growth hormone in breast carcinoma, suggest that GHR-mediated signaling pathways are involved in the development of human breast cancer, possibly via autocrine or paracrine mechanisms.
Breast cancer is by far the most common malignancy in women, affecting one in eight in the United States and Western Europe. An increased risk of breast cancer is associated with early menarche, late menopause, and nulliparity. These observations suggest that ovarian hormones play an important role not only in normal breast development, but in the development of breast cancer and its progression. Pituitary hormones are also essential for normal breast development. Moreover, some observations suggest they are also involved in the development of breast cancer. Administration of growth hormone (GH) to aging primates induces a marked increase in mammary gland size and epithelial proliferation index. 1 Conditions with increased GH levels, such as acromegaly, are associated with an increased risk of malignancy, including breast cancer. 2-4 In the treatment of advanced breast cancer, hypophysectomy has beneficial, ovary-independent effects. 5 Breast cancer cell lines grow in response to both prolactin (PRL) and GH administration, and are inhibited by PRL and GH antagonists. 6-8 GH receptor (GHR) expression in human breast cancer and breast cancer cell lines has previously been detected using reverse transcription polymerase chain reaction (RT-PCR), in situ hybridization, and immunohistochemistry. 9-11 However, quantitative analyses for levels of GHR protein expression using Western blot techniques have not been performed. We studied 48 human breast carcinomas as well as adjacent normal mammary tissue using RT-PCR, Western blot, and immunohistochemistry. Our results indicate that GHR expression is up-regulated in breast cancer and suggest a role for GHR signaling in this disease.
Materials and Methods
The study included 48 breast carcinomas (47 primary tumors and one lymph node metastasis) from 47 patients who had surgery at the Sahlgrenska University Hospital in Göteborg, Sweden. Tumor size, histological type, and tumor grade (Bloom-Richardson-Elston score 12 ), as well as axillary lymph node status (positive versus negative) were recorded in all cases. Material was snap-frozen in liquid nitrogen for RT-PCR (36 cases) and Western blotting (28 cases). Fresh frozen material of adjacent normal mammary tissue was obtained in 17 cases. Immunohistochemical analyses were performed in 47 cases; in all cases, the selected histological sections included carcinoma as well as adjacent normal breast tissue.
Immunohistochemistry
All immunostains were performed according to standardized protocols using the TechMate Horizon immunostainer (DAKO, Copenhagen, Denmark). For detection of GHR, the mouse mAb 263 (AGEN Biomedical, Brisbane, Australia) was used at a dilution of 1:500 with application of tyramide signal amplification system (NEN Life Science Products, Boston, MA). All primary tumors were also analyzed for estrogen receptor (ER; clone 105, DAKO), progesterone receptor (PR; clone PgR 636, DAKO), MIB-1 (Immunotech, Marseille, France), and p53 (D0–7, DAKO). The immunoreactions for mAb 263 were graded as negative (0), weakly positive (1), moderately positive (2), or strongly positive (3). For ER, PR, MIB-1, and p53, the estimated percentage of positive tumor cell nuclei was recorded.
RT-PCR
Preparation of RNA was performed. 13 cDNA was synthesized from 0.5 μg RNA with 5 U avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) and 0.5 μg oligo-(dt) primer (Promega). For the PCR reaction, the following primers were used: 5′-GCTGCTGTTGACCTTGGC-3′ (sense) and 5′-ACCTCATCTGTCCAGTGG-3′ (antisense) (Scandinavian Gene Synthesis, Köping, Sweden) located in exons 2 and 4, respectively. These primers will amplify a 201-bp fragment corresponding to nucleotides 58–258 of the human GHR cDNA. If the GHR lacks the nucleotides corresponding to exon 3, the amplified fragment will be 135 bp. PCR was performed following a standard protocol. Samples were amplified for 30 cycles at an annealing temperature of 55°C. Specificity of the PCR products was verified by specific cleavage with the restriction enzyme Bsp 1286 I (Promega), which cleaves the GHR in exon 3, rendering two fragments 119 and 82 bp in size when PCR products containing exon 3 are digested, whereas PCR products without exon 3 are left intact. Specificity of PCR products was also confirmed by Southern hybridization (not shown).
Western Blotting
The antibody used, GHR06, was a mouse monoclonal raised against amino acids 396–407 of the extracellular part of human GHR. It recognizes the human GHR protein by Western blotting, by immunoprecipitation, and by fluorescence-activated cell sorting (G Norstedt, unpublished data).
Soluble tissues were prepared by homogenization in PE buffer (10 mmol/L potassium phosphate buffer, pH 6.8 and 1 mmol/L EDTA) containing 6 mg/ml 3-(3-cholamidopropyl)dimethyl-ammonio 1-propane sulfate (CHAPS), aprotinin (200 kallikrein inhibitory units per milliliter), leupeptin (10 μg/ml), pepstatin (10 μg/ml), and Pefabloc (1 mg/ml; Boehringer Mannheim, Mannheim, Germany). After sonication and centrifugation, supernatants were collected and protein concentrations were determined by the Bio-Rad method. Supernatants were stored at −70°C until analysis. The samples were diluted in SDS sample buffer and denatured before loading on a SDS-polyacrylamide gel (8% Tris-glycine; NOVEX, San Diego, CA). Fifty micrograms of total protein were loaded into each lane. A prestained standard (SeeBlue, NOVEX) was used as weight marker. After electrophoresis, the proteins were transferred to a polyvinyldifluoride (PVDF) membrane (Amersham, Buckinghamshire, UK) using a standard electroblotting system, followed by incubation with GHR06, diluted 1:1000. Immunoreactive protein was visualized by chemiluminescence using an ALP-conjugated secondary antibody (goat-anti-mouse, SIGMA), diluted 1:30000 and CDP Star (Tropix, Bedford, MA) as substrate. Membranes were exposed to ECL film (Amersham) for 10 seconds to 3 minutes and developed in a Curix 60 developing machine (AGFA). Autoradiograms were scanned and the bands corresponding to GHR protein were analyze by densitometry. Quantitative analyses were performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Statistical Analyses
Correlation between GHR expression as determined both by mAb 263 immunostaining and by Western blot analysis, and patient age, tumor size, tumor grade, tumor type, axillary lymph node status, estrogen receptor expression, progesterone receptor expression, MIB-1 immunoreactivity, and p53 immunoreactivity was evaluated using Fisher’s permutation test. Differences in GHR expression between tumors and adjacent normal mammary tissue as determined by Western blot analysis were evaluated using a paired t-test. P values <0.05 were considered significant.
Results
The patient and tumor characteristics are summarized in Table 1 ▶ . The 47 patients ranged in age from 34 to 90 years (median, 59 years). Tumors ranged from 15 to 110 mm in greatest dimension. The vast majority of tumors were invasive ductal carcinomas (n = 41); the remainder were invasive lobular carcinomas (n = 3), atypical medullary carcinomas (n = 2), and invasive tubular carcinoma (n = 1). Axillary lymph nodes were removed in 42 patients; 22 patients had metastases and 20 did not.
Table 1.
Summary of Clinical Data of 48 Carcinomas and RT-PCR, Immunohistochemical, and Western Blot Analyses
| Case no. | Age (years) | Tumor size (mm) | Tumor type | BRE (3–9) | Axillary lymph node status | GHR detection | |||
|---|---|---|---|---|---|---|---|---|---|
| RT-PCR | Western blotting (arbitrary units) | IHC (0–3) | |||||||
| GHR | Exon 3 | ||||||||
| 1 | 83 | 43 | IDC | 7 | pos. | + | yes | 1.05 | 3 |
| 2 | 50 | 26 | IDC | ND | neg. | + | yes | 0.47 | 1 |
| 3 | 51 | 25 | IDC | 6 | neg. | + | yes | ND | 3 |
| 4 | 62 | 34 | IDC | 7 | pos. | + | yes | ND | 1 |
| 5 | 79 | 90 | IDC | 7 | pos. | + | yes | ND | 3 |
| 6 | 59 | 40 | IDC | 8 | pos. | + | yes | 0.53 | 3 |
| 7 | 60 | 42 | IDC | 7 | pos. | + | yes | 1.12 | 3 |
| 8 | 68 | 29 | IDC | 6 | neg. | + | yes | 0.78 | 3 |
| 9 | 53 | 30 | IDC | 7 | pos. | + | yes | 0.56 | 3 |
| 10 | 39 | 25 | IDC | 8 | neg. | + | yes | 0.84 | 2 |
| 11 | 43 | 65 | IDC | 5 | neg. | + | yes | 0.91 | 3 |
| 12 | 62 | 60 | IDC | 7 | pos. | + | yes | 0.30 | 2 |
| 13 | 34 | 28 | IDC | 8 | neg. | + | yes | 0.63 | 3 |
| 14 | 88 | 30 | IDC | 7 | ND | + | yes | 0.58 | 1 |
| 15 | 71 | 25 | IDC | 6 | neg. | + | yes | 0.84 | 3 |
| 16 | 37 | 20 | IDC | 7 | ND | + | yes | ND | 1 |
| 17 | 37 | 110 | IDC | 6 | pos. | + | yes | ND | 3 |
| 18 | 56 | 40 | IDC | 8 | neg. | + | yes | 0.30 | 2 |
| 19 | 83 | 28 | IDC | 9 | ND | + | yes | 0.70 | 3 |
| 20 | 48 | 33 | IDC | 7 | neg. | + | no | ND | 3 |
| 21 | 34 | 40 | IDC | 9 | neg. | + | no | 0.47 | 2 |
| 22 | 42 | 30 | IDC | 6 | neg. | + | no | 0.77 | 3 |
| 23 | 39 | 30 | IDC | 7 | pos. | + | no | 1.31 | 3 |
| 24 | 49 | 25 | IDC | 7 | pos. | + | both | 0.80 | 2 |
| 25 | 46 | 55 | IDC | 9 | pos. | + | both | 1.00 | 1 |
| 26 | 33 | 40 | IDC | 9 | neg. | + | both | 0.97 | 2 |
| 27 | 44 | 40 | IDC | 9 | pos. | + | both | 0.52 | ND |
| 28 | 84 | 30 | IDC | 6 | pos. | + | both | 0.73 | 2 |
| 29 | 67 | 23 | IDC | 7 | neg. | + | both | 0.64 | 3 |
| 30 | 86 | 40 | IDC | 7 | ND | + | both | 1.27 | 3 |
| 31 | 71 | 30 | IDC | 6 | ND | ND | ND | 3 | |
| 32 | 80 | 40 | IDC | 7 | pos. | ND | ND | 3 | |
| 33 | 84 | 80 | IDC | 9 | neg. | ND | ND | 2 | |
| 34 | 63 | 20 | IDC | 8 | neg. | ND | ND | 3 | |
| 35 | 72 | 60 | IDC | 9 | neg. | ND | ND | 1 | |
| 36 | 43 | 25 | IDC | 9 | pos. | ND | ND | 2 | |
| 37 | 50 | 15 | IDC | 9 | neg. | ND | ND | 0 | |
| 38 | 47 | 40 | IDC | 9 | pos. | ND | ND | 0 | |
| 39 | 64 | 43 | IDC | 9 | pos. | ND | ND | 1 | |
| 40 | 48 | 23 | IDC | 7 | pos. | ND | ND | 3 | |
| 41 | 49 | ND | IDC | 9 | pos. | ND | ND | 3 | |
| 42 | 90 | 110 | ILC | 5 | pos. | + | no | 0.52 | 3 |
| 43 | 73 | 22 | ILC | 8 | pos. | + | both | 0.28 | 1 |
| 44 | 73 | 35 | ILC | 5 | neg. | ND | ND | 3 | |
| 45 | 90 | ND | ILC, Ln M | ND | pos. | + | no | ND | 1 |
| 46 | 66 | 60 | AMC | 9 | neg. | + | no | 0.22 | 2 |
| 47 | 48 | 45 | AMC | 8 | neg. | + | yes | 1.23 | 3 |
| 48 | 81 | 30 | ITC | 4 | pos. | + | yes | ND | 2 |
IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; AMC, atypical medullary carcinoma; ITC, invasive tubular carcinoma; Ln M, lymph node metastasis; BRE, Bloom-Richardson-Elston score; IHC, immunohistochemistry; pos., positive; neg., negative; ND, not determined.
Immunohistochemistry
The GHR (mAb 263) immunostaining results are summarized in Table 1 ▶ . A variable degree of cytoplasmic staining of tumor cells was seen in all but two of the 47 analyzed tumors (Figure 1, a–c) ▶ . Weak focal nuclear staining was observed in a few instances. Weak or moderate cytoplasmic staining also occurred in some stromal cells (Figure 1b) ▶ . Adjacent normal breast tissue was negative (Figure 1d) ▶ , or weakly positive, sharply contrasting with mostly strongly positive tumors. A significant inverse correlation was found between GHR expression and tumor grade (P < 0.05).
Figure 1.
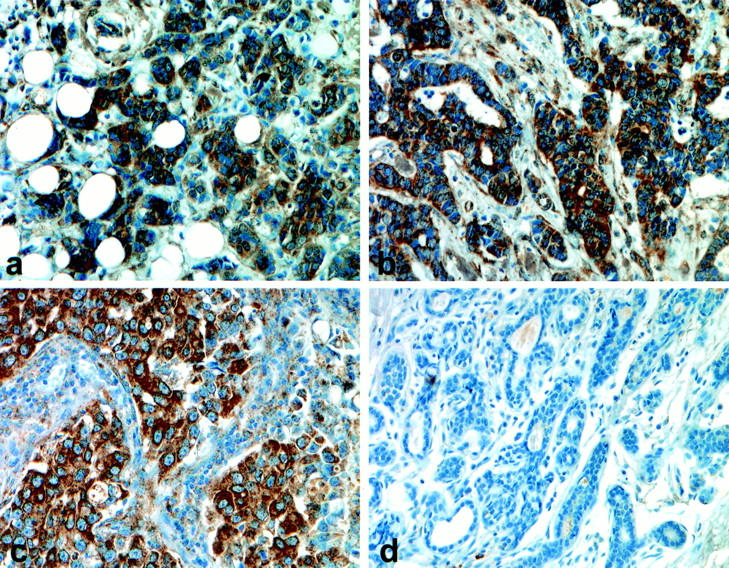
GHR (mAb 263) immunostaining of invasive ductal carcinomas of the breast (a and b) and an atypical medullary carcinoma (c). Strong cytoplasmic immunostaining of the epithelial component and weak staining of some stromal cells are seen (b and c). Adjacent normal breast tissue is negative (d) or weakly positive.
Estrogen and progesterone receptor positivity, defined as >10% positive tumor cell nuclei, was observed in 26/44 and 18/46 tumors, respectively. MIB-1 positivity was ≤10% in 17/46 tumors, >10% and <50% in 18/46, and ≥50% in 11/46 tumors. p53 immunostaining >20% was seen in 14/47 tumors. There was a positive correlation between GHR expression and progesterone receptor expression (P < 0.01), and an inverse correlation between GHR expression and level of MIB-1 immunoreactivity (P < 0.05).
RT-PCR
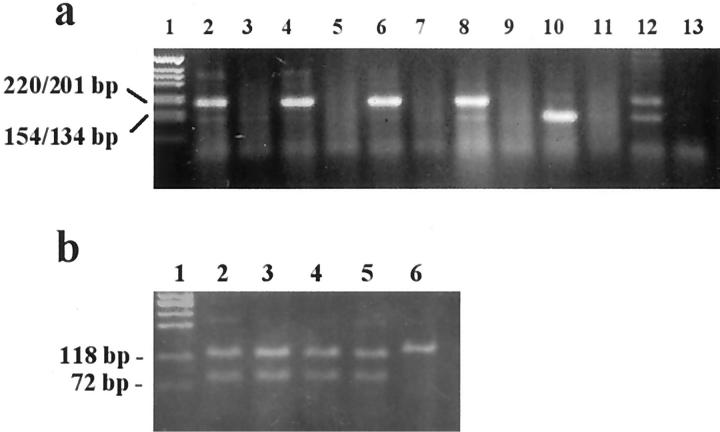
Amplified fragments of the expected sizes (201 or 135 bp) were detected in all 36 tumor samples examined (Figure 2a ▶ and Table 1 ▶ ). To verify their identity, the PCR products were digested by the restriction enzyme Bsp 1286 I (Figure 2b) ▶ . Twenty-one of 36 tumors expressed the GHR form containing exon 3, seven tumors expressed the GHR form without exon 3, and eight tumors expressed both forms. In 17 cases, GHR mRNA expression was also assessed in adjacent normal mammary tissue. GHR mRNA was detected in all normal mammary tissues and was always of the same form as the corresponding tumor.
Figure 2.
a: RT-PCR of GHR expression in six breast cancer cases. Lanes 2, 4, 6, 8, 10, and 12 represent RT-PCR of tumor tissues. Lanes 3, 5, 7, 9, 11, and 13 represent no template controls. Lane 1 shows a DNA marker (1 kb, GIBCO BRL). The full-length GHR renders a fragment of 201 bp (lanes 2, 4, 6, and 8). The GHR without exon 3 renders a fragment of 135 bp (lane 10). Lane 12 represents a case in which both forms are expressed. b: Digestion of the PCR fragment with Bsp 1286 I. Cleavage occurs in the third exon, resulting in two fragments that are 119 and 82 bp in size (lanes 2–5) or, in the case of exon 3 deletion, leaving the fragment undigested (lane 6). Lane 1 is the DNA marker X174 HaeIII.
Western Blotting
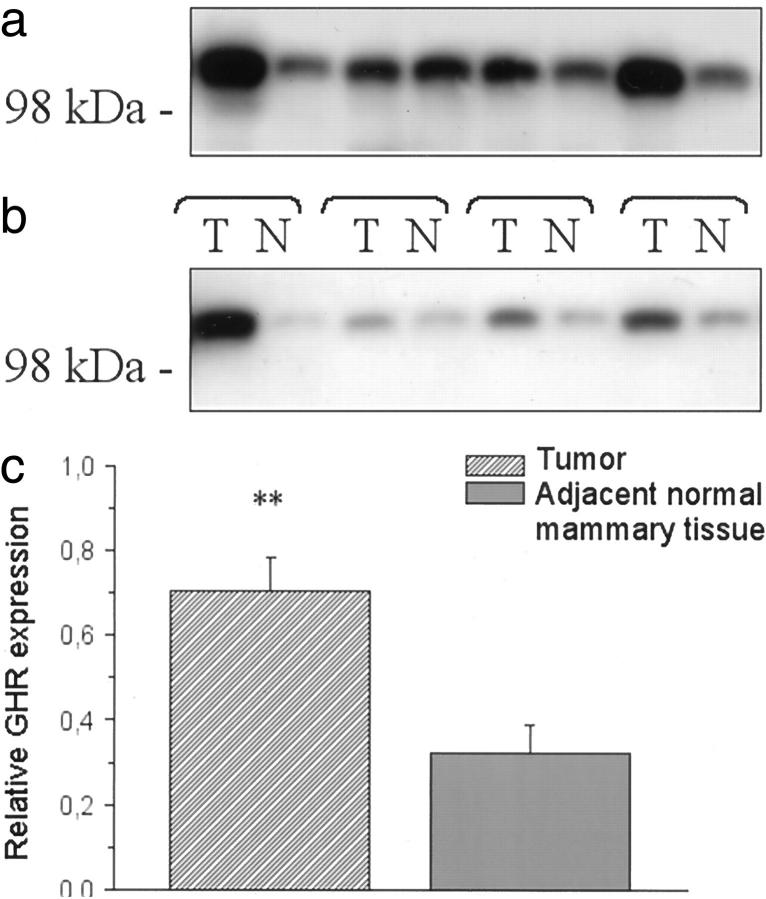
GHR protein was detected in all 28 tumors analyzed. The amount of GHR varied between different tumors (Figure 3a ▶ and Table 1 ▶ ). The relative protein levels ranged between 0.22 and 1.31 arbitrary units (median, 0.71). In adjacent normal breast tissue (n = 17), the relative protein levels ranged between 0.09 and 0.75 arbitrary units (median, 0.26). Comparison of GHR expression levels in 16 tumors and adjacent normal mammary tissues (Figure 3b) ▶ revealed significantly higher levels of GHR in tumors (P < 0.01, paired t-test; Figure 3c ▶ ). No significant correlation was found between GHR expression levels using Western blotting and any of the clinical, morphological, or immunophenotypic parameters recorded. However, there was a positive correlation between expression levels of GHR detected by Western blotting and the intensity of GHR immunoreactivity (P < 0.05).
Figure 3.
Western blots of GHR in human breast cancer tissues. a: Representative blot of GHR expression in eight breast cancer cases. b: Representative blot of GHR expression in tumor tissues and adjacent normal breast tissues. c: Relative GHR expression levels in breast cancer tissues are compared to relative GHR expression levels of adjacent normal mammary tissues (n = 16). Mean levels are lower in normal mammary tissues compared to cancer tissues (P < 0.01).
Discussion
The RT-PCR, Western blotting, and immunohistochemistry results clearly demonstrate that GHR is expressed in human breast cancer. These findings agree with previous reports of GHR detected by RT-PCR in surgically removed breast cancers and breast cancer cell lines, 9 or by using immunohistochemical 10,11 and in situ hybridization techniques. 11 Moreover, our immunohistochemical and Western blotting analyses demonstrated greater GHR expression in tumors than normal breast tissues. Thus, though adjacent normal breast tissues showed no or only weak immunoreactivity for GHR, all but a few carcinomas showed strong or moderate diffuse cytoplasmic staining. Also, the semiquantitative Western blotting results showed significantly increased GHR expression in tumors compared to adjacent normal breast tissues.
The predominantly cytoplasmic GHR immunoreactivity found in our study supports previous observations. 14-16 Weak nuclear staining was occasionally seen; this has also been previously reported 17 and is of interest since nuclear translocation of GHR may be induced by GH stimulation. 18 Another interesting observation was the detection of GHR in stromal cells of the breast carcinomas. The stromal compartment of the normal mammary gland is suggested to be the site of action for GH during normal mammary development in rodents. 19,20 GH, possibly via local insulin-like growth factor (IGF)-I production, acts synergistically with estradiol during normal mammary gland development in rodents 21,22 and primates. 23
In this study of human breast cancer, there was an inverse correlation between GHR expression as determined by immunohistochemistry and proliferative activity (MIB-1 immunostaining) as well as tumor grade, whereas there was a positive correlation between GHR expression and progesterone receptor expression. These findings, as well as the previous detection of GHR in benign epithelial proliferations of the breast, all suggest that increased GHR expression does not correspond to aggressive biological behavior per se. Additional points of interest are the detection of GHR expression by RT-PCR and Western blotting, and strong immunoreactivity for GHR in both stromal and epithelial cells of a benign phyllodes tumor not included in this series.
Despite the significant correlation between GHR expression levels detected by Western blotting and immunostaining intensity, a significant correlation between progesterone receptor expression, tumor grade, and proliferative activity (MIB-1) was found only with immunostaining intensity. This apparent discrepancy could be explained by the relatively small number of cases analyzed using Western blotting.
The detection of an additional GHR cDNA lacking exon 3 24 (in this series, seen as the sole form in 7/36 tumors; 21/36 tumors expressed the full-length form; 8/36 expressed both forms) was originally believed to be due to an alternative splicing event. Subsequent studies suggested tissue-specific and individual-specific expression patterns. 25,26 The expression of GHR cDNA without exon 3, however, has recently been shown to be the result of a deletion of this part of the GHR gene, which, in turn, is due to a recombination of two retro-elements flanking exon 3. 27 Thus, our detection of the same GHR cDNA in tumors and normal breast tissues from the same individual supports these findings. Interestingly, the invasive ductal carcinomas of the breast with the exon 3-deleted GHR occurred in patients who were significantly younger than those who had full-length GHR (mean age 41 vs. 59 years). However, analysis of a larger series is required to draw any conclusions.
This study indicates that GH and GHR play a role in human breast cancer, but the exact mechanisms involved remain unclear. Autocrine/paracrine mechanisms have been suggested based on the detection of local GH production in normal breast tissue and carcinoma, 28 and transfection studies of GH expression plasmids in MCF-7 breast cancer cells in which increased growth response was recorded with autocrine/paracrine stimulation compared to exogenous GH administration. 8 Because GH-mediated stimulation of IGF-I production occurs in normal breast development, a similar phenomenon could occur in the development of breast cancer.
GH is closely related to PRL; although both hormones have specific receptors, GH also activates PRL receptors (PRLR) in primates. 29 Thus, in many studies of GH action, it is not possible to discriminate between GHR- and PRLR-mediated effects. Tumor-promoting effects have been shown to be mediated via PRLR and not GHR in mice. 30 The situation in human breast cancer, however, is probably more complex. Both GHR and PRLR are expressed in most human breast carcinomas 11,31 with higher expression levels of PRLR and GHR in breast cancers compared to normal breast tissues 32 (and this study). Local production of PRL, as well as GH, has also been demonstrated in breast carcinomas. 28,33 More potent mitogenic signals are suggested to be a result of PRLR activation rather than GHR activation. 7
In conclusion, this study, which provides evidence of GHR expression and up-regulation in human breast cancer, indicates a role for GHR signaling in human breast cancer. To further understand the mechanisms involved, additional studies are necessary, including analyses of benign breast lesions and precancerous conditions.
Acknowledgments
We thank Gunnar Norstedt for providing the GHR antibody GHR06.
Footnotes
Address reprint requests to Maria Gebre-Medhin at her current address: Department of Physiology, Lund University, Sölvegatan 19, SE 223 62 Lund, Sweden. E-mail: Maria.gebre-medhin@medic.gu.se.
Supported by grants from the Swedish Cancer Society, the Ingabritt and Arne Lundberg Foundation, the Assar Gabrielsson Foundation, the Syskonen Svensson Foundation, the Wilhelm and Martina Lundgren Foundation, and the Göteborg Medical Society.
References
- 1.Ng ST, Zhou J, Adesanya OO, Wang J, LeRoith D, Bondy CA: Growth hormone treatment induces mammary gland hyperplasia in aging primates. Nat Med 1997, 3:1141-1144 [DOI] [PubMed] [Google Scholar]
- 2.Nabarro JD: Acromegaly. Clin Endocrinol (Oxf) 1987, 26:481-512 [DOI] [PubMed] [Google Scholar]
- 3.Melmed S, Ho K, Klibanski A, Reichlin S, Thorner M: Clinical review 75: recent advances in pathogenesis, diagnosis, and management of acromegaly. J Clin Endocrinol Metab 1995, 80:3395-3402 [DOI] [PubMed] [Google Scholar]
- 4.Colao A, Merola B, Ferone D, Lombardi G: Acromegaly. J Clin Endocrinol Metab 1997, 82:2777-2781 [DOI] [PubMed] [Google Scholar]
- 5.VanGilder JC, Goldenberg IS: Hypophysectomy in metastatic breast cancer. Arch Surg 1975, 110:293-295 [DOI] [PubMed] [Google Scholar]
- 6.Shiu RP, Paterson JA: Alteration of cell shape, adhesion, and lipid accumulation in human breast cancer cells (T-47D) by human prolactin and growth hormone. Cancer Res 1984, 44:1178-1186 [PubMed] [Google Scholar]
- 7.Fuh G, Wells JA: Prolactin receptor antagonists that inhibit the growth of breast cancer cell lines. J Biol Chem 1995, 270:13133-13137 [DOI] [PubMed] [Google Scholar]
- 8.Kaulsay KK, Mertani HC, Törnell J, Morel G, Lee KO, Lobie PE: Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp Cell Res 1999, 250:35-50 [DOI] [PubMed] [Google Scholar]
- 9.Decouvelaere C, Peyrat JP, Bonneterre J, Djiane J, Jammes H: Presence of the two growth hormone receptor messenger RNA isoforms in human breast cancer. Cell Growth Differ 1995, 6:477-483 [PubMed] [Google Scholar]
- 10.Lincoln DT, Sinowatz F, Temmim-Baker L, Baker HI, Kolle S, Waters MJ: Growth hormone receptor expression in the nucleus and cytoplasm of normal and neoplastic cells. Histochem Cell Biol 1998, 109:141-159 [DOI] [PubMed] [Google Scholar]
- 11.Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G: Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer 1998, 79:202-211 [DOI] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO: Assessment of histological grade. Elston CW Ellis IO eds. The Breast, 1998, vol. 13.:365-384 Churchill Livingstone, Edinburgh [Google Scholar]
- 13.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 14.Lesniak MA, Roth J: Regulation of receptor concentration by homologous hormone: effect of human growth hormone on its receptor in IM-9 lymphocytes. J Biol Chem 1976, 251:3720-3729 [PubMed] [Google Scholar]
- 15.Kover K, Hung CH, Moore WV: The characteristics of hGH binding to the liver macrophages. Horm Metab Res 1986, 18:26-30 [DOI] [PubMed] [Google Scholar]
- 16.Lincoln DT, Sinowatz F, Gabius S, Gabius HJ, Temmim L, Baker H, Mathew TC, Waters MJ: Subpopulations of stromal cells from long-term human bone marrow cultures: ontogeny of progenitor cells and expression of growth hormone receptors. Anat Histol Embryol 1997, 26:11-28 [DOI] [PubMed] [Google Scholar]
- 17.Waters MJ, Rowlinson SW, Gobius KS, Lobie PE, Garcia-Aragon J, Muscat GE, Basitras S, Zhang C, Young W, Barnard R: Biochemistry and cellular distribution of the GH receptor. Growth Hormone and Somatomedins during Life Span.> Edited by Müller EE. Heidelberg. Springer 1993, pp 26–43
- 18.Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G: Nuclear translocation and anchorage of the growth hormone receptor. J Biol Chem 1994, 269:31735-31746 [PubMed] [Google Scholar]
- 19.Walden PD, Ruan W, Feldman M, Kleinberg DL: Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 1998, 139:659-662 [DOI] [PubMed] [Google Scholar]
- 20.Ilkbahar YN, Thordarson G, Camarillo IG, Talamantes F: Differential expression of the growth hormone receptor and growth hormone-binding protein in epithelia and stroma of the mouse mammary gland at various physiological stages. J Endocrinol 1999, 161:77-87 [DOI] [PubMed] [Google Scholar]
- 21.Ruan W, Catanese V, Wieczorek R, Feldman M, Kleinberg DL: Estradiol enhances the stimulatory effect of insulin-like growth factor- I (IGF-I) on mammary development and growth hormone-induced IGF-I messenger ribonucleic acid. Endocrinology 1995, 136:1296-1302 [DOI] [PubMed] [Google Scholar]
- 22.Ruan W, Kleinberg DL: Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 1999, 140:5075-5081 [DOI] [PubMed] [Google Scholar]
- 23.Kleinberg DL, Niemann W, Flamm E, Cooper P, Babitsky G, Valensi Q: Primate mammary development: effects of hypophysectomy, prolactin inhibition, and growth hormone administration. J Clin Invest 1985, 75:1943-1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbanek M, MacLeod JN, Cooke NE, Liebhaber SA: Expression of a human growth hormone (hGH) receptor isoform is predicted by tissue-specific alternative splicing of exon 3 of the hGH receptor gene transcript. Mol Endocrinol 1992, 6:279-287 [DOI] [PubMed] [Google Scholar]
- 25.Sobrier ML, Duquesnoy P, Duriez B, Amselem S, Goossens M: Expression and binding properties of two isoforms of the human growth hormone receptor. FEBS Lett 1993, 319:16-20 [DOI] [PubMed] [Google Scholar]
- 26.Wickelgren RB, Landin KL, Ohlsson C, Carlsson LM: Expression of exon 3-retaining and exon 3-excluding isoforms of the human growth hormone-receptor is regulated in an interindividual, rather than a tissue-specific, manner. J Clin Endocrinol Metab 1995, 80:2154-2157 [DOI] [PubMed] [Google Scholar]
- 27.Pantel J, Machinis K, Sobrier ML, Duquesnoy P, Goossens M, Amselem S: Species-specific alternative splice mimicry at the growth hormone receptor locus revealed by the lineage of retroelements during primate evolution. J Biol Chem 2000, 275:18664-18669 [DOI] [PubMed] [Google Scholar]
- 28.Mol JA, Henzen-Logmans SC, Hageman P, Misdorp W, Blankenstein MA, Rijnberk A: Expression of the gene encoding growth hormone in the human mammary gland. J Clin Endocrinol Metab 1995, 80:3094-3096 [DOI] [PubMed] [Google Scholar]
- 29.Kleinberg DL, Todd J: Evidence that human growth hormone is a potent lactogen in primates. J Clin Endocrinol Metab 1980, 51:1009-1013 [DOI] [PubMed] [Google Scholar]
- 30.Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Törnell J: Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest 1997, 100:2744-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV: Expression of prolactin and its receptor in human breast carcinoma. Endocrinology 1997, 138:5555-5560 [DOI] [PubMed] [Google Scholar]
- 32.Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicoias A, Trivin C, Postel-Vinay MC, Kuttenn F, Kelly PA: Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab 1998, 83:667-674 [DOI] [PubMed] [Google Scholar]
- 33.Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE: Expression of prolactin and prolactin receptor in human breast carcinoma: evidence for an autocrine/paracrine loop. Am J Pathol 1995, 146:695-705 [PMC free article] [PubMed] [Google Scholar]