Abstract
Chondromodulin-I (ChM-I) is a novel cartilage-specific matrix protein. In the growth plates of the long bones, ChM-I was shown to be expressed in mature to upper hypertrophic chondrocytes, and to be deposited in the cartilage matrix. As ChM-I strongly inhibits angiogenesis, cartilage is avascular. Also, ChM-I has bifunctional activity against chondrocyte proliferation. On the other hand, pleomorphic adenomas of the salivary glands frequently have chondroid elements. To elucidate the relationship between chondroid formation and hypovascularity in salivary pleomorphic adenomas, we immunohistochemically examined the expression and localization of ChM-I in 35 cases of this tumor. ChM-I was immunolocalized to the lacunae in the chondroid elements of pleomorphic adenomas (100%). Type II collagen and aggrecan were immunolocalized throughout the matrix around lacuna cells of the chondroid element (100%, 91.7%), and ChM-I was infrequently immunolocalized to the spindle-shaped myoepithelial cells in the myxoid element (37.5%). Fibroblast growth factor-2 was strongly immunolocalized to the lacuna cells in the chondroid element (100%), among the neoplastic myoepithelial cells in the myxoid elements (96.9%), and on the basement membranes around the solid nests of neoplastic myoepithelial cells (71.4%). Although CD34 is a marker of endothelial cells, CD34 was expressed in the endothelial cells in only a few areas around the epithelial elements and in the fibrous element of pleomorphic adenomas. No signals for CD34 were observed in chondroid elements in pleomorphic adenomas (P < 0.001), but a few signals were seen in the myxoid elements (P < 0.05). These findings suggested that lacuna cells and neoplastic myoepithelial cells expressed ChM-I, and that this molecule may play an important role in hypovascularity and chondroid differentiation in pleomorphic adenoma. In conclusion, pleomorphic adenoma expressed ChM-I, which is involved in hypovascularity and chondroid formation in this type of tumor.
Pleomorphic adenoma of the salivary glands is characterized by the so-called “mixed” appearance of epithelial and mesenchymal-like elements. In previous studies, mesenchymal-like elements including chondroid and myxoid elements were shown to be related to neoplastic myoepithelial cells migrating into the stroma. Recently, we demonstrated that bone morphogenetic proteins (BMPs) were associated with chondroid formation in pleomorphic adenoma. 1,2 Also, we reported that co-expression of fibroblast growth factor (FGF)-2 and FGF receptor-1 in the lacuna cells in chondroid elements inhibited ossification of the chondroid elements. 3 Although FGF-2 is a strong angiogenic factor, pleomorphic adenomas are hypovascular tumors and there were not any capillaries in the chondroid elements of this type of tumor.
Chondromodulin-I (ChM-I), a cartilage-specific noncollagenous matrix protein, was extracted and cloned from bovine cartilage. 4 Recently, ChM-I has been reported to be a strong inhibitor of angiogenesis, responsible for the avasucular nature of cartilage. 5,6 In the growth plates of the long bones, ChM-I mRNA was expressed in the proliferating to the upper hypertrophic chondrocytes and its product was deposited in the interterritotrial matrix around the lacunae. 6 The human ChM-I gene was recently cloned. 7
The findings presented here indicated that ChM-I, a strong angio-inhibitor, may be expressed in chondroid elements of salivary pleomorphic adenomas. We examined expression and localization of ChM-I, in comparison with localization of FGF-2 and/or density of CD34-positive endothelial cells, in salivary pleomorphic adenomas using immunohistochemical methods.
Materials and Methods
Antibodies
Anti-ChM-I polyclonal antibody was raised in a rabbit against mature recombinant human ChM-I protein. 7 On Western blotting analysis, this antibody revealed a single diffuse band of 25 kd. Anti-CD34 monoclonal antibody (cat. no. 1185; Immunotech, Marseille, France), anti-type II collagen monoclonal antibody (clone II-4C11; Fuji Yakuhin Kogyo, Toyama, Japan), and anti-FGF-2 polyclonal antibody (22-97-0175; RD Laboratorien, Herrsching bei Munchen, Germany) were purchased from the sources shown. These antibodies were diluted 1:1, 1:500, and 1:1,000, respectively. Specificities of anti-type II antibody and anti-FGF-2 (basic FGF) antiserum were confirmed previously. 1-3 Anti-aggrecan polyclonal antibody, a kind gift from Dr. T. Yada (Institute for Molecular Science of Medicine, Aichi Medical University, Nagoya,Japan), was raised against rat cartilage aggrecan purified from 1-week-old rat tibial cartilage as previously described. 8 This antiserum against rat aggrecan recognized mouse and human aggrecan core protein on enzyme-linked immunosorbent assay and Western blotting analysis and cross-reacted with human aggrecan.
Tissues
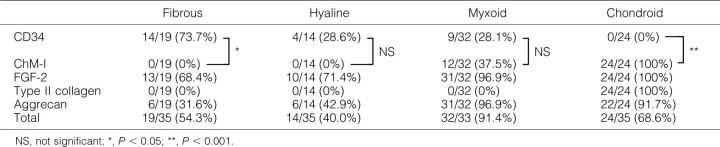
Thirty-five pleomorphic adenoma cases were chosen from the pathology files of the Japanese Red Cross Medical Center, Tokyo, Japan, from the period 1986 to 1998. The tubulo-glandular structures and mesenchymal-like stromas of pleomorphic adenomas are summarized in Table 1 ▶ . Twenty specimens included normal salivary gland tissues. Two neonatal vertebral tissue, one enchondroma tissue, two placenta tissue, and two tracheal cartilage specimens were used as controls. These specimens were fixed in 10% buffered formalin, routinely processed, and embedded in paraffin wax. Sections 3-μm thick were then prepared.
Table 1.
Cases of Pleomorphic Adenoma
| Tubulo-glandular structures | Mesenchymal-like structures | ||||
|---|---|---|---|---|---|
| Inner cells | Outer cells* | Fibrous | Hyaline | Myxoid | Chondroid |
| 30/35 | 35/35 | 19/35 | 14/35 | 32/35 | 24/35 |
| 85.7% | 100% | 54.3% | 40.0% | 91.4% | 68.6% |
*, Outer cells including the solid nests of neoplastic myoepithelial cells.
Immunohistochemistry
Paraffin-embedded sections were deparaffinized in xylene and rehydrated. For ChM-I staining, deparaffinized sections were first pretreated with 0.1% hyaluronidase (H6254; Sigma Chemical Co., St. Louis MO) in acetate buffer, pH 5.0, for 45 minutes at 37°C, followed by two washes with phosphate-buffered saline (PBS), and then they were treated with 0.4% pepsin (P-6887; Sigma Chemical Co.) in 1.0 N HCl solution for 45 minutes at 37°C. For FGF-2 and aggrecan staining, deparaffinized sections were first treated with 0.1% hyaluronidase in acetate buffer, pH 5.0, at 37°C for 30 or 60 minutes, respectively. For type II collagen staining, deparaffinized sections were pretreated with 0.1% trypsin (0152–13; DIFCO Laboratories, Detroit, MI) in PBS for 30 minutes at 37°C. All sections were washed in PBS, and incubated for 30 minutes in 0.3% H2O2 in methanol to inactivate endogenous peroxidases. After incubation for 1 hour at room temperature, sections were washed twice in PBS and incubated with biotinylated anti-rabbit or anti-mouse immunoglobulin antibody (DAKO Japan Co., Kyoto, Japan). After two additional washes, they were incubated in peroxidase-conjugated streptavidin (P397; DAKO Japan Co.) followed by two washes in PBS. Bound peroxidase was developed with 0.02% 3,3′-diaminobenzidine in 0.1 mol/Tris buffer, pH 7.6, in 0.005% H2O2 for 5 minutes, and counterstaining was performed with 5% methyl green. As negative controls, the primary antibodies were replaced with normal mouse or rabbit serum, PBS, or were preabsorbed with recombinant mature human ChM-I protein.
The density of CD34 expression was evaluated as follows: −, no signal; +/−, 1 to 2 capillaries seen in an area of 0.5 mm 2 using a micrometer with a ×200 objective magnification; +, 3 to 5 capillaries; ++, 6 to 10 capillaries; +++, >11 capillaries. For statistical analysis, we used one factor analysis of variance (analysis of variance) and the chi-square test. P values <0.05 were considered statistically significant.
Results
ChM-I, FGF-2, Type II Collagen and Aggrecan Expression in Human Cartilage
Signals for type II collagen were seen throughout the matrix of the tracheal cartilage and cartilaginous matrix of the vertebral tissue (Figure 1A) ▶ . Signals for aggrecan were observed in the matrix of the tracheal cartilage and especially in the matrix of mature to hypertrophic cartilage of the vertebral tissue (Figure 1B) ▶ , whereas weak signals were seen throughout the cartilaginous matrix. Signals for FGF-2 were seen in the proliferating to mature chondrocytes of the vertebral tissues, and in the chondrocytes of the tracheal cartilage (Figure 1C) ▶ . Also, signals for FGF-2 were seen on the osteoclast-like multinucleated giant cells near the new bone. Strong signals for ChM-I were observed on the interterritorial regions around the lacunae of the cartilaginous tissues, especially in the areas around the mature to hypertrophic chondrocytes, and were also seen throughout the cartilaginous matrix in the vertebral tissues (Figure 1D) ▶ . Also, signals for ChM-I were seen in the cytoplasm of the chondrocytes of the tracheal cartilage (data not shown). However, no signals for ChM-I, type II collagen, or aggrecan were seen in the normal salivary gland tissue, whereas numerous CD34-positive capillaries were observed in the stroma of the normal salivary glands (data not shown).
Figure 1.
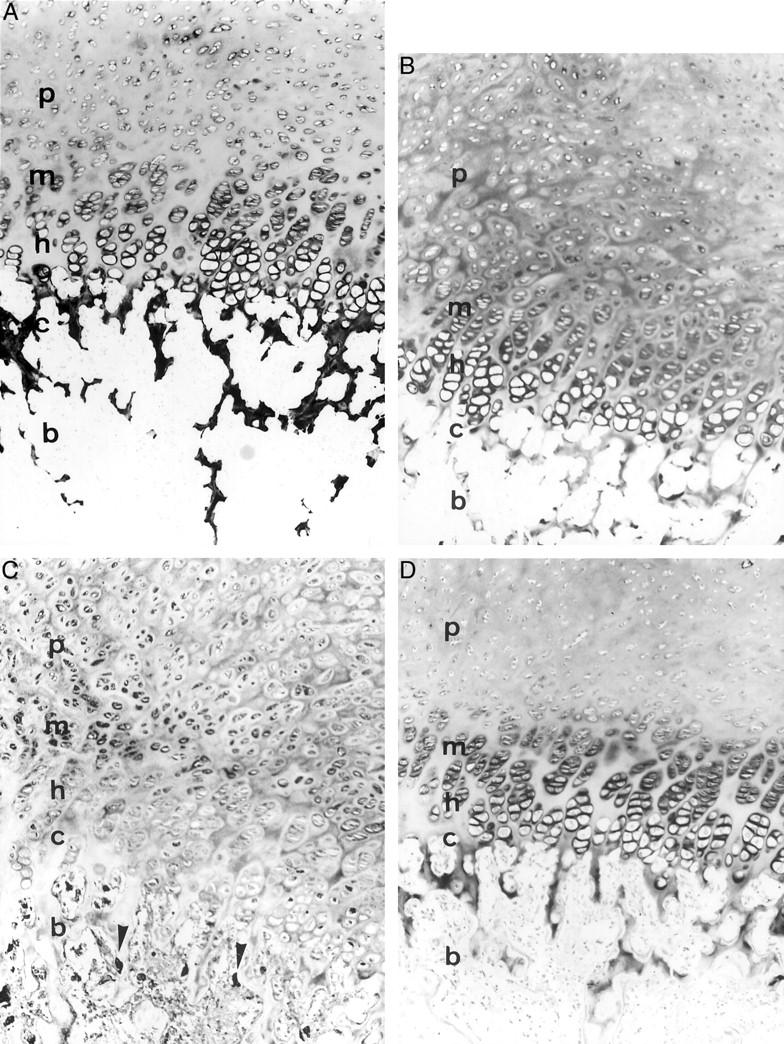
Results of control studies for each antibody using endochondral ossification tissues of the neonatal vertebra. p, proliferating cartilage; m, mature cartilage; h, hypertrophic cartilage; c, calcifying cartilage; b, bone. A: Diffuse type II collagen immunoreactivity in the matrix of the vertebral cartilage. Intense immunoreactivity was seen around the lacunae of the mature to hypertrophic cartilage. Weak immunoreactivity was seen around the proliferating chondrocytes. Although intense signals for type II collagen were observed on the interterritorial matrix of the calcifying cartilage, no signals were seen in the bone tissue. B: Aggrecan immunoreactivity in the matrix of the mature to hypertrophic cartilage of the vertebral cartilage. Note that diffuse signals for aggrecan were seen throughout the cartilaginous matrix. C: Strong FGF-2 immunoreactivity in the proliferating to mature chondrocytes of the vertebral cartilage. Note that signals for FGF-2 were seen in the cytoplasm of the osteoclasts (arrowheads). D: Strong ChM-I immunoreactivity on the interterritorial matrix around the lacunae of the mature to hypertrophic cartilage of the vertebral cartilage tissue. Weak ChM-I immunoreactivity was seen throughout the cartilage matrix. Original magnifications, ×100.
CD34 Expression in Salivary Pleomorphic Adenomas
CD34 is a marker of endothelial cells. The profiles of the 35 cases of pleomorphic adenoma examined in this study are shown in Table 2 ▶ . All cases showed typical histological characteristics. Four stromal types were observed: fibrous (54.3%), hyaline (40.0%), myxoid (91.4%), and chondroid (68.6%). Fibrous elements showed spindle-shaped neoplastic myoepithelial cells with bundles of collagen fibers. Hyaline elements revealed hypocellular areas with dense eosin staining. Myxoid elements showed a pale hematoxylin-positive matrix with stellate or spindle-shaped cells. Chondroid elements showed hyaline cartilage-like areas with lacuna formation, in which lacuna cells were observed.
Table 2.
Density of CD34 Positivity in Pleomorphic Adenoma
| Fibrous | Hyaline | Myxoid | Chondroid | |
|---|---|---|---|---|
| − | 4/19 (21.1%) | 5/14 (35.7%) | 14/32 (43.7%) | 24/24 (100%) |
| +− | 1/19 (5.3%) | 5/14 (35.7%) | 9/32 (28.1%) | 0/24 (0%) |
| Subtotal | 5/19 (26.3%)* | 10/14 (71.4%)* | 23/32 (71.9%)* | 24/24 (100%)** |
| + | 4/19 (21.1%) | 1/14 (7.1%) | 6/32 (18.8%) | 0/24 (0%) |
| ++ | 4/19 (21.1%) | 3/14 (2.1%) | 3/32 (9.4%) | 0/24 (0%) |
| +++ | 6/19 (31.6%) | 0/14 (0%) | 0/32 (0%) | 0/24 (0%) |
| Subtotal | 14/19 (73.7%)* | 4/14 (28.6%)* | 9/32 (28.1%)* | 0/24 (0%)** |
−, 0: +−; 1–2: +; 3–5: ++; 6–10: +++; >11 CD34 -positive endothelial cells per areas of 0.5-mm2.
NS, not significant; *, P < 0.05; **, P < 0.001.
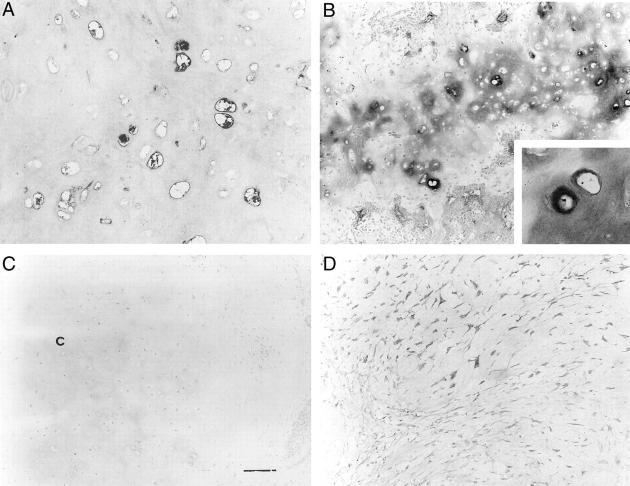
Signals for CD34 were seen in only a few areas of pleomorphic adenomas, ie, capillaries in the fibrous, myxoid and/or hyaline stroma, and the areas around epithelial components, but CD34 expression was never observed in the chondroid component (P < 0.001) (Figure 2, A–C) ▶ . We evaluated the expression and density of CD34-positive capillaries (Table 2) ▶ . CD34-positive capillaries were frequently seen in the fibrous stroma, but they were infrequently observed in the hyaline and myxoid stromas (P < 0.05). CD34 positivity was significantly different between the chondroid stroma and other stromas. On the other hand, pleomorphic adenomas showed numerous CD34-positive capillaries in the fibrous capsules (Figure 2D) ▶ .
Figure 2.
A: A few CD34-positive capillaries were seen in the fibrous area of pleomorphic adenoma. B: Few CD34-positive capillaries were seen in the myxoid area of pleomorphic adenoma. Scale bar, 120 μm. C: No immunoreactivity for CD34 was seen in the chondroid area of pleomorphic adenoma. Scale bar, 120 μm. D: CD34 immunoreactivity was seen in the fibrous capsules of pleomorphic adenoma. f, fibrous capsule; t, tumor; n, normal salivary gland. Note that many CD34-immunoreactive capillaries were seen in the normal salivary gland. Original magnifications, ×120.
FGF-2 and ChM-I Expression in the Salivary Pleomorphic Adenomas
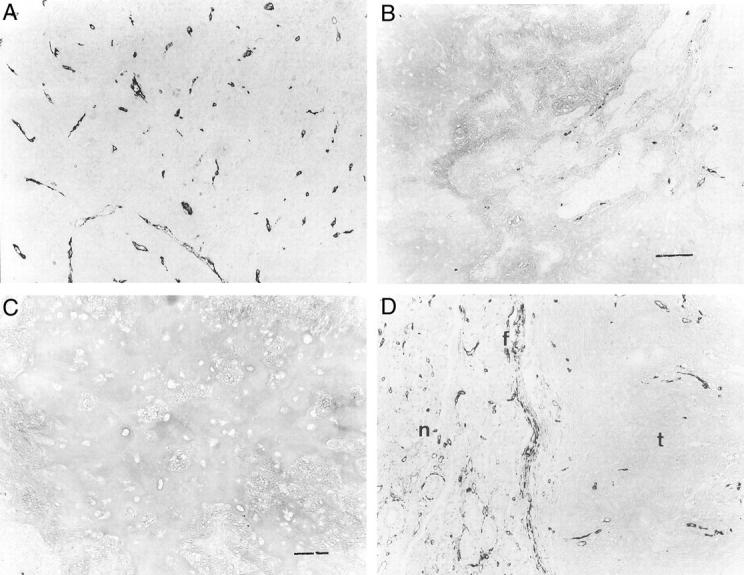
Signals for FGF-2 were seen in the lacuna cells in the chondroid elements and also on the basement membrane regions of the solid nests of the neoplastic myoepithelial cells or around the spindle-shaped or stellate cells in the myxoid elements (Figure 3A) ▶ . These results were compatible with those of our previous study. 3 FGF-2 positivity, however, was not significantly different between myxoid/chondroid stromas and other stromas (Table 3) ▶ .
Figure 3.
A: Strong FGF-2 immunoreactivity was seen in the lacuna cells of the chondroid area of pleomorphic adenoma. Note that FGF-2 immunoreactivity was seen in the pericellular spaces of the lacuna cells. B: Diffuse ChM-I immunoreactivity was seen in the interterritorial spaces around lacunae of the chondroid area of pleomorphic adenoma. Weak signals were seen throughout the chondroid matrix. Inset: Strong immunoreactivity for ChM-I was seen in the interterritorial regions of the lacuna cells. C: No immunoreactivity for ChM-I was seen after pre-absorption of the antibody with recombinant human ChM-I protein. c, chondroid area. Scale bar, 100 μm. D: Moderate ChM-I immunoreactivity was seen in the spindle-shaped neoplastic myoepithelial cells of myxoid area of pleomorphic adenoma. Note that weak immunoreactivity was seen in the myxoid matrix of pleomorphic adenoma. Original magnifications: ×200 (A and D), ×100 (B and C), and ×256 (inset in B).
Table 3.
Positivity of Stromas of Pleomorphic Adenomas
Strong signals for ChM-I were observed on the interterritorial spaces around the lacunae, whereas weak signals were seen in the chondroid matrix (Figure 3B ▶ and Table 4 ▶ ). Immunoreactivity for ChM-I was observed in the neoplastic myoepithelial cells of the myxoid elements (Figure 3D) ▶ . In the chondroid areas, FGF-2 was immunolocalized to the surface and cytoplasm of the lacuna cells, whereas ChM-I was immunolocalized to the interterritorial spaces around the lacunae. Pre-absorption of the antibody with recombinant human ChM-I protein completely abolished signals for ChM-I, and no staining was seen in negative controls (Figure 3C) ▶ .
Table 4.
Intensity of Stromas of Pleomorphic Adenomas
| Fibrous | Hyaline | Myxoid | Chondroid | |
|---|---|---|---|---|
| CD 34 | +++ | + | + | − |
| ChM-I | − | − | ++ | +++ |
| FGF-2 | ++ | ++ | +++ | +++ |
| Type II collagen | − | − | − | +++ |
| Aggrecan | + | + | +++ | +++ |
−, No staining; +, weak staining; ++, moderate staining; +++, strong staining.
Type II Collagen and Aggrecan Expression in the Salivary Pleomorphic Adenomas
Strong signals for type II collagen were seen throughout the chondroid matrix, but none were seen in other mesenchymal-like elements or epithelial elements (Figure 4A) ▶ . On the other hand, strong signals for aggrecan were observed consistently in the matrix of the chondroid elements (Figure 4B) ▶ . Such signals were also seen in small myxoid areas within or near the epithelial sheets, and occasionally in the intercellular spaces of the solid nests of the neoplastic myoepithelial cells (Figure 4, C and D) ▶ . These results were similar to those reported previously. 8 Aggrecan positivity was significantly different between myxoid/chondroid stromas and other stromas (P < 0.001) (Tables 3 and 4) ▶ ▶ . No staining was observed in negative controls.
Figure 4.
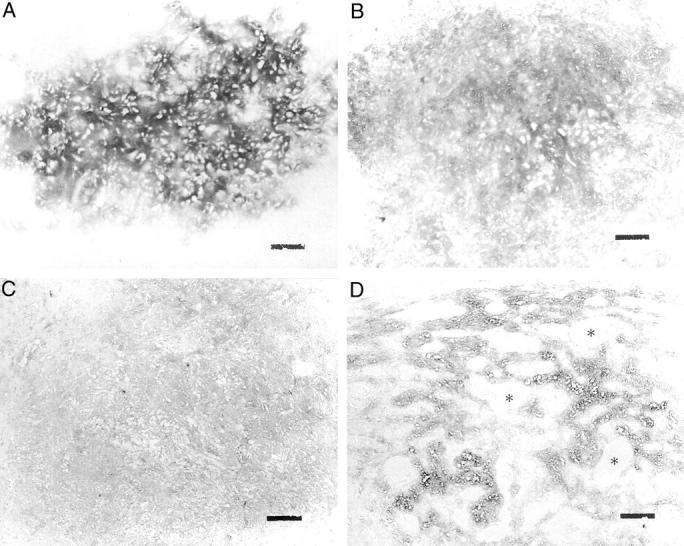
A: Diffuse type II collagen immunoreactivity was seen in the chondroid matrix of pleomorphic adenoma. B: Diffuse aggrecan immunoreactivity was seen in the chondroid matrix of pleomorphic adenoma. C: Diffuse aggrecan immunoreactivity was seen in the myxoid area. D: Moderate aggrecan immunoreactivity was seen in the interspaces among the tubulo-glandular structures. Asterisks, tubulo-glandular structures. Scale bars, 100 μm. Original magnifications, ×100.
Discussion
Pleomorphic adenoma of the salivary glands, the most frequent type of benign tumor, is considered an epithelial tumor. However, pleomorphic adenoma shows various histological features, including myxoid and chondroid characteristics. 9,10 We reported previously that chondroid areas of pleomorphic adenoma expressed type II collagen protein and mRNA, and lacuna cells were similar to authentic chondrocytes in phenotype. 1 The present study also showed the deposition of type II collagen and aggrecan in the stroma of the chondroid areas. Aggrecan is a cartilage-specific large aggregating chondroitin sulfate proteoglycan, and our results were similar to those reported previously, 8 ie, aggrecan was deposited in the chondroid matrix of pleomorphic adenoma. The BMP-2 produced by neoplastic myoepithelial cells may induce chondroid tissue by a paracrine mechanism, 1 and the BMP-6 produced by lacuna cells may maintain chondroid tissue by an autocrine mechanism in pleomorphic adenoma. 2 Thus, the co-expression of FGF-2 and FGF receptor-1 may inhibit osteogenesis in the chondroid areas. 3 BMP-2 and FGF-2 are expressed in neoplastic myoepithelial cells of myxoid areas of pleomorphic adenomas, which may be related to the differentiation of neoplastic myoepithelial cells and mesenchymal-like tissue formation including fibrous, hyaline, myxoid, and chondroid tissues.
On the other hand, although FGF-2 is a strong angiogenic factor, 11-13 pleomorphic adenoma is a hypovascular tumor as indicated by the CD34 expression and density of endothelial cells in this type of tumor. We expected there to be some strong angioinhibitors in pleomorphic adenoma, and a good candidate was ChM-I, a 25-kd glycoprotein purified from bovine epiphyseal cartilage, 4 that was recently identified as a novel endothelial cell growth inhibitor. 5,6 Cartilage is generally avascular and exhibits resistance to vascular invasion because of an intrinsic angiogenesis inhibition, which is because of the effects of ChM-I. ChM-I was reported to inhibit DNA synthesis and tube morphogenesis of cultured vascular endothelial cells in vitro, 14 but this molecule stimulated DNA synthesis and proteoglycan synthesis in cultured growth plate chondrocytes in vitro. 4,12 ChM-I is a glycoprotein of 121 amino acid residues and is encoded as the C-terminal portion of a large precursor (335 amino acids). 4 Mature ChM-I is assumed to be secreted from chondrocytes after proteolytic cleavage by a precursor-endoprotease, furin. 15,16 In situ hybridization analysis indicated that ChM-I mRNA was expressed in the proliferating to upper hypertrophic chondrocytes in the growth plate of the long bones, but the expression of the gene was reduced in the lower hypertrophic and calcified zone, allowing vascular invasion. 6 Immunohistochemical examination clearly indicated that the localization of ChM-I protein completely overlapped the area of its gene expression reported previously. 6 ChM-I was accumulated in the interterritorial spaces around the lacunae of the cartilage matrix as well as on the cartilage matrix itself. In contrast, FGF-2 protein was confined to the cell surface or pericellular space in the territorial regions. Thus, the distribution of ChM-I protein in cartilage was clearly distinct from that of FGF-2. Immunostained FGF-2 is tightly associated with the cell surface, whereas ChM-I diffuses away from the cells, explaining the avascular nature of the tumor in the chondroid areas despite the presence of both angiogenic and anti-angiogenic factors. The unique localization pattern may explain how ChM-I exerts its anti-angiogenic effect on cartilage.
Recently, we reported that BMP-6 was expressed in the lacuna cells of the chondroid areas in pleomorphic adenoma. 2 In the present study, we also demonstrated that aggrecan was deposited in the chondroid matrix in this type of tumor. The phenotype of lacuna cells in chondroid areas was similar to that of mature to upper hypertrophic chondrocytes with regard to expression of aggrecan and BMP-6. 2,17 On the other hand, FGF-2 was expressed in the proliferating to mature chondrocytes of the areas of endochondral ossification, whereas the lacuna cells in chondroid areas showed strong expression of FGF-2. Our results showed that, similarly to aggrecan, ChM-I was localized to the interterritorial spaces around the lacunae in the chondroid matrix, which is an avascular region in pleomorphic adenoma. These findings suggested that the phenotype of chondroid areas of pleomorphic adenoma was an authentic mixed cartilaginous phenotype, but the phenotype of the lacuna cells in chondroid areas may be similar to that of the mature to upper hypertrophic chondrocytes. Type X collagen is expressed in the lower hypertrophic chondrocytes and therefore the expression of this molecule in pleomorphic adenomas is presently under investigation in our laboratory.
In conclusion, pleomorphic adenomas of the salivary glands expressed ChM-I protein in the chondroid areas, and ChM-I plays an important role in determining the avascular features of the chondroid area of this type of tumor, although the ubiquitous angiogenic growth factor FGF-2 was localized to lacuna cells of chondroid areas. Thus, with regard to expression of ChM-I protein, pleomorphic adenoma should be classified as a hypovascular tumor.
Acknowledgments
We thank S. Skiguchi, K. Wakamatsu, S. Hashimoto, A. Niizeki, and Y. Aoki; the staff of the Department of Pathology, Japanese Red Cross Medical Center, Tokyo, Japan, for their technical assistance; Mr. Takashi Yoshizawa, the Photo Center, Tokyo Medical and Dental University, Tokyo, Japan, for microphotography; and Drs. Toshikazu Yada and Koji Kimata, Institute for Molecular Science of Medicine, Aichi Medical University, Nagoya, Japan, for generously providing specific antibody against aggrecan.
Footnotes
Address reprint requests to Kimihide Kusafuka D.D.S., Ph.D, Department of Pathology, Japanese Red Cross Medical Center, 4-1-22 Hiroo, Shibuya-ku, Tokyo 150-8935, Japan.
References
- 1.Kusafuka K, Yamaguchi A, Kayano T, Fujiwara M, Takemura T: Expression of bone morphogenetic proteins in salivary pleomorphic adenomas. Virchows Arch 1998, 432:247-253 [DOI] [PubMed] [Google Scholar]
- 2.Kusafuka K, Yamaguchi A, Kayano T, Takemura T: Immunohistochemical localization of the bone morphogenetic protein-6 in salivary pleomorphic adenomas. Pathol Int 1999, 49:1023-1027 [DOI] [PubMed] [Google Scholar]
- 3.Kusafuka K, Yamaguchi A, Kayano T, Takemura T: Immunohistochemical localization of fibroblast growth factors (FGFs) and FGF receptor-1 in human normal salivary glands and pleomorphic adenomas. J Oral Pathol Med 1998, 27:287-292 [DOI] [PubMed] [Google Scholar]
- 4.Hiraki Y, Tanaka H, Inoue H, Kondo J, Kamizono A, Suzuki F: Molecular cloning of a new class of cartilage-specific matrix, chondromodulin-I which stimulates growth of cultured chondrocytes. Biochem Biophys Res Commun 1991, 175:971-977 [DOI] [PubMed] [Google Scholar]
- 5.Hiraki Y, Kono T, Sato M, Shukunami C, Kondo J: Inhibition of DNA synthesis and tube morphogenesis of cultured vascular endothelial cells by chondromodulin-I. FEBS Lett 1997, 425:321-324 [DOI] [PubMed] [Google Scholar]
- 6.Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, Iijima S, Suzuki F, Kondo J: Identification of chondromodulin I as a novel endothelial cell growth inhibitor; purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem 1997, 272:32419-32426 [DOI] [PubMed] [Google Scholar]
- 7.Hiraki Y, Mitsui K, Endo N, Takahashi K, Hayami T, Inoue H, Shukunami C, Tokunaga K, Kono T, Yamada M, Takahashi HE, Kondo J: Molecular cloning of human chondromodulin-I, a cartilage-derived growth modulating factor, and its expression in Chinese hamster ovary cells. Eur J Biochem 1999, 260:869-878 [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Takata T, Ogawa I, Yada T, Kimata K, Nikai H: Immunohistochemical evaluation of the small and large proteoglycans in pleomorphic adenoma of the salivary glands. J Oral Pathol Med 1999, 28:37-42 [DOI] [PubMed] [Google Scholar]
- 9.Waldron CA: Mixed tumor (pleomorphic adenoma) and myoepithelioma. Ellis GL Auclair PL Gnepp R eds. Surgical Pathology of the Salivary Glands. 1991, :pp 165-182 WB Saunders, Philadelphia [Google Scholar]
- 10.Dardick I, Van Nstand AWP, Jeans MTD, Rippstein P, Edward V: Pleomorphic adenoma. II Ultrastructural organization of “stromal regions.” Hum Pathol 1983, 14:798-809 [DOI] [PubMed] [Google Scholar]
- 11.Folkman J, Klagsburn M: Angiogenic factors. Science 1967, 235:442-447 [DOI] [PubMed] [Google Scholar]
- 12.Schulze-Osthoff K, Risau W, Vollmer E, Sorg C: In situ detection of basic fibroblast growth factor by highly specific antibody. Am J Pathol 1990, 37:85-92 [PMC free article] [PubMed] [Google Scholar]
- 13.Cordon-Cardo C, Vlodavsky I, Haimovitz-Friedman A, Hicklin D, Fuks Z: Expression of basic fibroblast growth factor in normal human tissues. Lab Invest 1990, 63:832-884 [PubMed] [Google Scholar]
- 14.Mori Y, Hiraki Y, Shukunami C, Kakudo S, Shiokawa, Kagoshima M, Man H, Hakeda Y, Kurokawa T, Suzuki F,Kumegawa M: Stimulation of osteoblast proliferation by the cartilage-derived growth promoting factors chondromodulin-I and -II. FEBS Lett 1997, 406:310–314 [DOI] [PubMed]
- 15.Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakayama K: Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem 1991, 266:12127\. [PubMed] [Google Scholar]
- 16.Hatsuzawa K, Hosaka M, Nakagawa T, Nagase M, Shoda A, Murakami K, Nakayama K: Structure and expression of mouse furin, a yeast Kex2-related protease. J Biol Chem 1990, 265:22075-22078 [PubMed] [Google Scholar]
- 17.Luo W, Guo C, Zheng J, Chen TL, Wang PY, Vertel BM, Tanzer ML: Aggrecan from start to finish. J Bone Miner Metab 2000, 18:51-56 [DOI] [PubMed] [Google Scholar]