Abstract
Apoptosis-inducing factor (AIF) is a novel mediator in apoptosis. AIF is a flavoprotein that is normally confined to the mitochondrial intermembrane space, yet translocates to the nucleus in several in vitro models of apoptosis. To investigate the role of AIF in the apoptotic process in vivo, we induced retinal detachment (RD) by subretinal injection of sodium hyaluronate, either in Brown Norway rats or in C3H mice. Apoptotic DNA fragmentation, as determined by terminal nick-end labeling, was most prominent 3 days after RD. The subcellular localization of AIF was examined by immunohistochemistry and immunoelectron microscopy. In normal photoreceptor cells, AIF was present in the mitochondrion-rich inner segment. However, AIF was found in the nucleus after RD. Photoreceptor apoptosis developed similarly in C3H control mice, and in mice bearing the gld or lpr mutations, indicating that cell death occurs independently from the CD95/CD95 ligand system. Both the mitochondrio-nuclear transition of AIF localization and the nuclear DNA fragmentation were inhibited by subretinal application of brain-derived neurotrophic factor. To our knowledge, this is the first description of AIF relocalization occurring in a clinically relevant, in vivo model of apoptosis.
Apoptosis plays a critical role in biological processes such as embryogenesis, immunogenesis, and carcinogenesis. 1,2 Recently it has become widely accepted that mitochondria play a key role in the control of apoptotic cell death. 3-5 Early during the apoptotic process, mitochondria can release several apoptogenic proteins including cytochrome c; procaspase-2, -3, and -9; as well as apoptosis-inducing factor (AIF), through the outer mitochondrial membrane into the cytosol. The relocalization of such apoptogenic proteins occurs when apoptotic signals including pro-apoptotic members of the Bcl-2 family induce opening of the permeability transition pore and/or induce the formation of protein-permeant conduits in the outer mitochondrial membrane. Loss of mitochondrial membrane integrity may also affect the mitochondrial electron transport, induce an enhanced generation of reactive oxygen species, and compromise the generation of ATP. 4,6-8
AIF is a novel, caspase-independent apoptogenic factor. In the absence of apoptotic signals, AIF is normally confined to the mitochondrial intermembrane space. 9-11 However, when apoptosis is induced in HeLa cells, Rat-1, or Jurkat cells in vitro, AIF translocates to the cytosol and to the nucleus. 9,10,12,13 When recombinant AIF is injected into the cytoplasm of intact cells or added to purified nuclei from HeLa cells, it causes the nucleus to undergo peripheral chromatin condensation and triggers large-scale DNA degradation to fragments of ∼50 kbp. 9 AIF also induces purified mitochondria to release cytochrome c and caspase-9, suggesting that AIF, once released from mitochondria, accelerates membrane permeabilization in a positive feedforward loop. 9 Microinjection of AIF into the cytoplasm of the intact cells induced a loss of the mitochondrial transmembrane potential and the exposure of phosphatidylserine on the surface of the plasma membrane. 9 All these changes occur in the presence of saturating doses of the wide-ranging caspase inhibitor Z-VAD.fmk, indicating that AIF acts in a caspase-independent manner.
AIF is strongly conserved among mammalian species (>95% amino acid identity between mouse and human) and bears a highly significant homology with flavoprotein oxidoreductases from all eukaryotic and prokaryotic kingdoms in its C-terminal portion. 10 Based on these findings, it is reasonable to speculate that AIF may be a phylogenetically old mediator participating in various aspects of the apoptotic process. However, all studies supporting this hypothesis have been performed in vitro. The role of AIF in apoptosis in vivo, especially in mammalian pathology, remains unknown.
Retinal photoreceptors are neuroectodermal cells essential for vision. 14 A specific part of these polar cells, the ellipsoid, within the inner segment, is packed with regularly arranged mitochondria. Photoreceptors degenerate on traumatic or spontaneous retinal detachment (RD), which is one of the common causes of legal blindness in the young adult. Cell loss is reported to be because of apoptosis rather than because of necrosis. 15-19 Given the fact that RD usually occurs without inflammation or destructive ischemia, it provides a suitable context for studying the morphological changes involved in apoptosis. In this study, we assessed the possible role of AIF in photoreceptor apoptosis induced by experimental RD. Our data provide the first description of a subcellular relocalization of AIF in vivo.
Materials and Methods
Detection of Apoptosis
DNA Nick-End Labeling by the TUNEL Method
Apoptotic photoreceptor degeneration was detected by TdT-dUTP terminal nick-end labeling (TUNEL). Four-μm-thick sections were made from samples fixed in 4% paraformaldehyde and embedded in paraffin. TUNEL staining was performed with the ApopTag fluorescein direct in situ apoptosis detection kit (Intergen Company, New York, NY) according to the manufacturer’s protocols. The sections were co-stained with propidium iodide (Molecular Probes, Eugene, OR), allowing observation of the cell nuclei by a fluorescence microscope (Olympus, Tokyo, Japan). Because the number of photoreceptors in each slide varied depending on the cutting angle, the number of apoptotic photoreceptors also varied. To avoid this sampling artifact, the rate of apoptotic photoreceptors was calculated using the following formula: apoptotic photoreceptor ratio (%) = total number of TUNEL-positive photoreceptors/total number of photoreceptors in the section. Ten sections for each eye specimen were randomly selected and observed by masked observers (six eyes for each time point).
AIF and Glial Fibrillary Acidic Protein (GFAP) Immunohistochemistry
Samples were fixed in 4% paraformaldehyde, embedded in paraffin, deparaffinized in xylene, rehydrated in ethanol, and washed in phosphate-buffered saline (PBS), as described above. A 1:100 dilution of anti-AIF rabbit serum was produced by a previously described method 9 and incubated at 4°C overnight. A nonimmune serum and a pre-absorbed antiserum (with 1 μg/μl recombinant AIF) were used as negative controls. Cy5-labeled secondary antibody (Zymed Laboratories, San Francisco, CA) was used at a dilution of 1:200 for 20 minutes. The sections were co-stained by TUNEL and observed with a fluorescence microscope. Furthermore, to clarify the localization of AIF in photoreceptors or Müller cells, a double immunostaining was made for Müller cell marker, GFAP (1:100 dilution, Santa Cruz Biotechnology, CA) and Cy5-labeled secondary antibody (KPL, Gaithersburg, MD).
Cytochrome c Immunohistochemistry
Four-μm-thick sections were made from samples fixed in 4% paraformaldehyde and embedded in paraffin. Anti-cytochrome c antibody (PharMingen, St. Louis, MO) was used at a 1:200 dilution and the sections were incubated at 4°C overnight. Cy5-labeled secondary antibody (KPL, Gaithersburg, MD) was used at a dilution of 1:200 for 20 minutes and the sections were observed with a fluorescence microscope.
Electron Microscopy and Immunoelectron Microscopy
The eyes were enucleated and the posterior segments were fixed in 1% glutaraldehyde and 1% paraformaldehyde in PBS. The detached retinas were removed and postfixed in veronal acetate buffer osmium tetroxide (2%), dehydrated in ethanol and water, and embedded in Epon. Ultrathin sections were cut from blocks and mounted on copper grids. For immunoelectron microscopy, the eyes were fixed in 1% paraformaldehyde in PBS, and the detached retinas were rinsed with PBS, incubated in NH4Cl, and embedded in London Resin white blocks (London Resin, London, UK). 20 Primary antibody for AIF was used at a 1:150 dilution, and the sections were incubated at 4°C overnight. Anti-rabbit antibody conjugated with 10 nm gold particles (British BioCell, Cardiff, UK) was used as a secondary antibody at a dilution of 1:30 for 90 minutes. The specimens were observed with a JEM 100CX electron microscope (JEOL, Tokyo, Japan). A nonimmune serum was used as a negative control.
Experimental RD
All procedures conformed to the standards set forth in the Association for Research in Vision and Ophthalmology’s statement for the Use of Animals in Ophthalmic and Vision Research. Brown Norway rats (Kyudo, Fukuoka, Japan), postnatal 8 weeks, were studied as follows.
The rats were anesthetized with an intraperitoneal injection of pentobarbital and their pupils were dilated with topical 1% tropicamide and 2.5% phenylephrine hydrochloride. The retinas were detached using a subretinal injection of 1% sodium hyaluronate (Pharmacia, Uppsala, Sweden). An anterior chamber puncture was performed from the corneal limbus to lower the intraocular pressure. Sclera was penetrated at the ocular nasal equator with a 30G needle, and then the needle was slowly advanced into the vitreous space. Soft retina was focally detached from comparatively hard retinal pigment epithelium as the needle moved. Then the needle was pulled and advanced into the subretinal space beneath the focally detached retina. Sodium hyaluronate (0.05 ml) was injected gently to enlarge the RD. Because the volume of the vitreous space of the rat eye is relatively small, the injection of 0.05 ml of sodium hyaluronate reproducibly produced similar RDs (half of the retina, Figure 1 ▶ ). Biomicroscopy (Kowa, Tokyo) demonstrated that the same portion of the retina remained detached for more than 28 days after treatment. Detached retinas that reattached spontaneously were excluded from further study. Eyes with anterior chamber puncture, scleral incision, and no injection of sodium hyaluronate were used as controls. The rats were sacrificed at 6 and 12 hours and on days 1, 3, 5, 7, 14, and 28 after treatment and their eyes were harvested for the following studies. To evaluate the change of outer nuclear layer thickness after RD, the thickness of outer nuclear layer in histological sections (10 sections each day) was measured and analyzed using analysis software (MacScope; Mitani, Fukui, Japan). The results were expressed as the percentage of nondetached control retina.
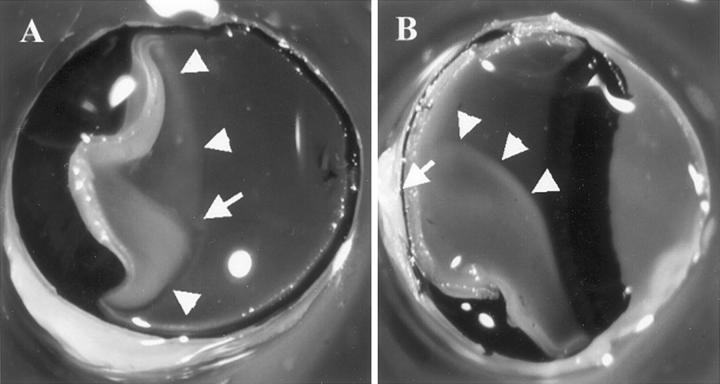
Figure 1.
Rat eyes with RD. The eyes were fixed in 1% glutaraldehyde and 1% paraformaldehyde in PBS, cut in half, and the lens removed. Note the grayish detached retina (arrowheads). The arrow indicates the optic nerve head. A: A frontal section observed from the anterior side. B: A sagittal section.
CD95/CD-95 Ligand System, Downstream Caspases, and AIF in RD
To investigate the role of CD95/CD-95 ligand system in apoptosis, RDs were created in CD95/CD-95 ligand gene mutation mice, C3H-lpr and C3H-gld mice (SLC, Shizuoka, Japan), postnatal age 8 weeks using the method just described. C3H-lpr mice, a model of lymphoproliferation with CD95 gene mutation, and C3H-gld mice, a model of generalized lymphoproliferative disease with CD95 ligand gene mutation have been shown to lack functional CD95 and CD95 ligand. 21-27 Almost the entire retina was detached in these animals and remained detached. Our preliminary study showed that C3H wild-type mice showed a similar apoptotic time course as Brown Norway rats did, and apoptosis was evaluated on a representative day after detachment (day 3). Furthermore, to investigate the participation of caspases, RD was created in rats with sodium hyaluronate containing the wide-ranging caspase inhibitor, Z-VAD.fmk (25 ng per eye).
Neurotrophic Factor and AIF in RD
It is known that the outer layers of the retina containing the photoreceptor cells are supplied by diffusion from the choriocapillaris. To investigate whether apoptosis in RD and AIF depend on nutrition-dependent pathways, RDs were created with sodium hyaluronate containing a representative neurotrophic factor, brain-derived neurotrophic factor (BDNF, 2.5 ng per eye). BDNF and trkB expression have been found in ocular tissue, 28,29 and BDNF became a particularly interesting candidate for trophic activity, especially in the retinal pigment epithelium (RPE), because of its inducibility, 30 and its ability of photoreceptor-protection from degeneration. 31,32
Results
Apoptosis in Experimental RD
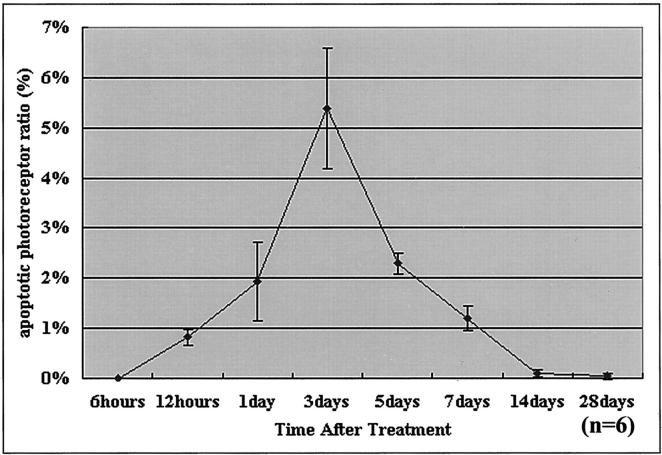
After injection of sodium hyaluronate into the subretinal space of Brown Norway rats, RD was subjected to a macroscopic and histological follow-up for 28 days. In macroscopic terms, the surface of the detached area remained unchanged during this period (Figure 1) ▶ . Histopathological examination revealed that the detached retinas degenerated gradually throughout time, progressively losing photoreceptors. No inflammatory cell infiltration was noted. Whereas control retinas do not label with TUNEL, on RD, TUNEL-positive apoptotic cells appeared in the photoreceptors of the outer retinal layer starting 12 hours after RD, reached a maximum on day 3, and then gradually diminished (Figure 2 ▶ and Figure 3 ▶ ). Simultaneously, the outer nuclear layer thickness of the retina, representing the photoreceptor layer decreased gradually to 88% of the control retina on day 3, 60% on day 7, and 32% on day 28.
Figure 2.
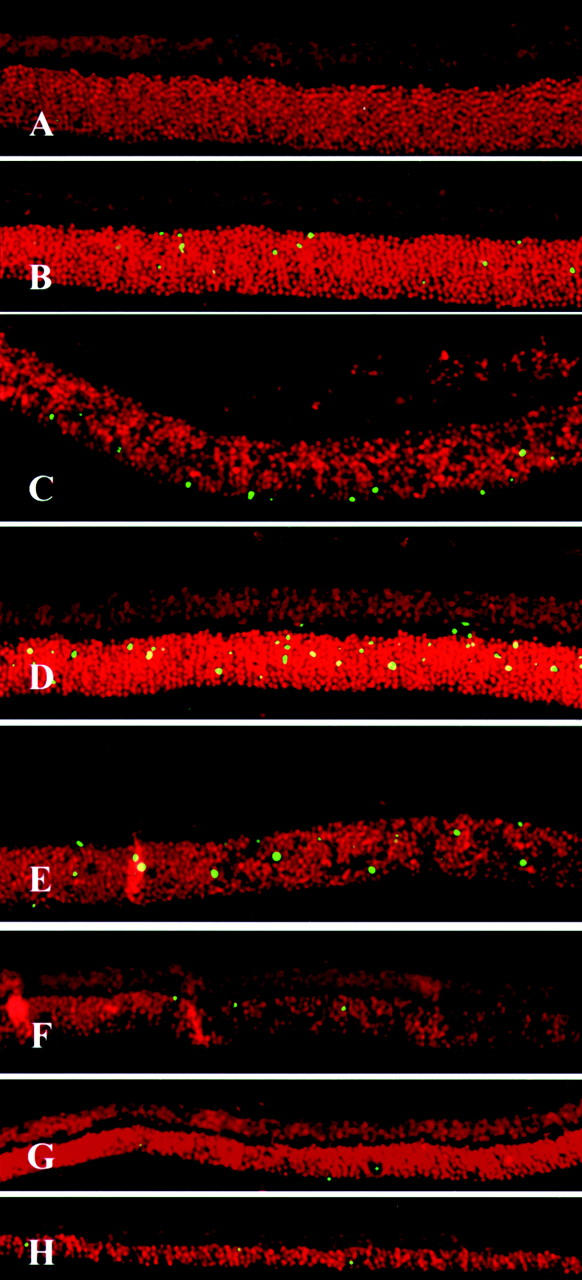
Fluorescent micrographs by TUNEL of sections from experimental RD from 6 hours to 28 days after treatment. Each section was stained with TdT-dUTP TUNEL and propidium iodide (see Materials and Methods). Note the TUNEL-positive cells in the photoreceptor layer (green). The highest frequency of TUNEL-positive photoreceptors is observed 3 days after treatment. The thickness of the outer nuclear layer decreases throughout time. A: 6 hours after treatment; B, 12 hours; C, 1 day, D, 3 days; E, 5 days; F, 7 days; G, 14 days; H, 28 days. Original magnifications, ×200.
Figure 3.
Time course of photoreceptor apoptosis in experimental RD detected by TdT-dUTP TUNEL. The ratio of the number of apoptotic photoreceptors/total photoreceptors increases for 3 days after detachment and then gradually decreases. Apoptotic photoreceptor ratio (%) was evaluated as the ratio of total number of TUNEL-positive photoreceptors/total number of photoreceptors in each section. The sections for each eye specimen were randomly selected and observed by masked observers (six eyes for each time point). The representative results of three independent experiments are presented.
AIF, GFAP, and Cytochrome c in Experimental RD
Immunohistochemistry showed specific AIF staining in ganglion cells, inner nuclear layer, outer plexiform layer and photoreceptors (Figure 4A ▶ , arrows). Immunostaining of control retinas revealed a multilinear pattern of AIF (Figure 4A ▶ , arrowheads) within the inner segment of photoreceptors, that is the mitochondrion-rich portion of the cells. This AIF-staining pattern did not change during the first 6 hours after RD. However, 12 hours after RD, we observed in some photoreceptors that AIF distributed in a diffuse manner throughout the cytosol and the nucleus (Figure 4B ▶ , arrows). No positive staining was found either by nonimmune serum or a pre-absorbed antiserum (with 1 μg/μl recombinant AIF; Figure 4, C and D ▶ ). The double immunostaining showed that AIF was not localized in the GFAP-positive cells (Figure 4E) ▶ . On the other hand, a double staining for AIF and TUNEL experiments revealed that AIF-positive nuclei from photoreceptor cells mostly are TUNEL-positive, at least during the first days after RD (Figure 5 ▶ and Table 1 ▶ ). Electron microscopy confirmed RD-induced morphological alterations in the photoreceptor layer, including chromatin condensation and cell shrinkage (Figure 6, A–E) ▶ . Photoreceptors from control retinas contain AIF in their mitochondria but not in the nucleus, as revealed by immunogold electron microscopy (Figures 7 and 8) ▶ ▶ . In contrast, dense AIF staining of the nucleus was observed in apoptotic cells, 3 days after RD (Figure 7) ▶ . Thinning of the outer nuclear layer because of loss of photoreceptors (Figure 2, F–H) ▶ and deconstruction of the inner and outer segments of the photoreceptors were also observed (Figure 8, A and B) ▶ . The regular arrangement of mitochondria observed in the control retinas was lost, and AIF staining dispersed from mitochondria to cytosol (Figure 8, A and B) ▶ . The immunostaining for cytochrome c showed that cytochrome c was localized in the inner segment of photoreceptors of nondetached retina, whereas it was diffusely dispersed in the cytosol of photoreceptors in the detached (Figure 9) ▶ . In conclusion, in dying photoreceptor cells both AIF and cytochrome c localization altered from mitochondria to an extramitochondrial localization.
Figure 4.
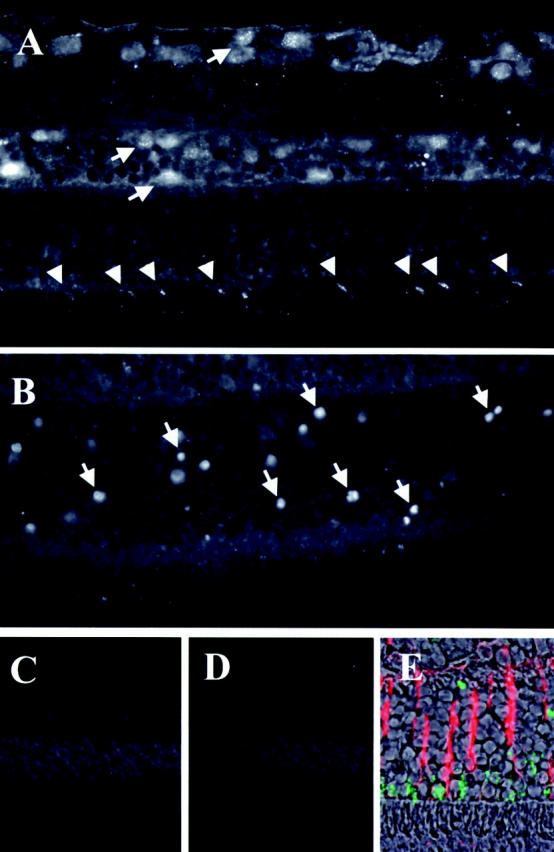
Fluorescent micrographs of AIF immunohistochemistry. The eyes were stained by AIF antiserum and visualized by fluorescent microscopy (see Materials and Methods). A: Control retina stained by AIF-antiserum. B: Detached retina on day 3 stained by AIF-antiserum. C: Control for staining by pre-immune serum. D: Control by pre-absorbed antiserum (with 1 μg/μl recombinant AIF). E: Double staining for AIF (green) and GFAP (red) on day 1 after RD. Specific AIF-staining is shown in ganglion cells, inner nuclear layer, outer plexiform layer, and photoreceptors (A, arrows). In normal photoreceptors, AIF staining is localized in inner segment, showing a multilinear pattern (A, arrowheads). Note the AIF-positive photoreceptor nuclei in the outer nuclear layer of detached retina (B, arrows). AIF staining decreased from the inner segment and appeared in the nucleus. Original magnifications: ×400 (A, B, and E), ×200 (C and D).
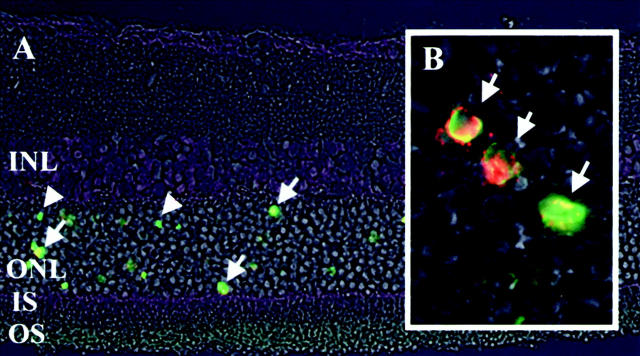
Figure 5.
Fluorescent microphotographs of the detached retina, double stained by AIF and TUNEL. Each section was double stained by TUNEL (green) and with AIF antiserum (red), and a micrograph was made using a phase-contrast microscope. A: Detached retina (INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment). Most of AIF-positive photoreceptors (arrow) are also TUNEL-positive (arrowhead), indicating apoptotic photoreceptors. B: High magnification of the outer nuclear layer. Co-localization of TUNEL and AIF staining is shown in detail. Original magnifications: ×400 (A), ×1000 (B).
Table 1.
Ratio of TUNEL- and AIF-Positive Photoreceptors in Total Photoreceptors in BN Rats
| 12 hours | 1 day | 3 days | |
|---|---|---|---|
| TUNEL-positive (%) | 0.820% | 1.93% | 5.38% |
| AIF-positive (%) | 0.724% | 1.60% | 2.87% |
Ten sections for each eye specimen were randomly selected for the evaluation (six eyes for each time point).
Figure 6.
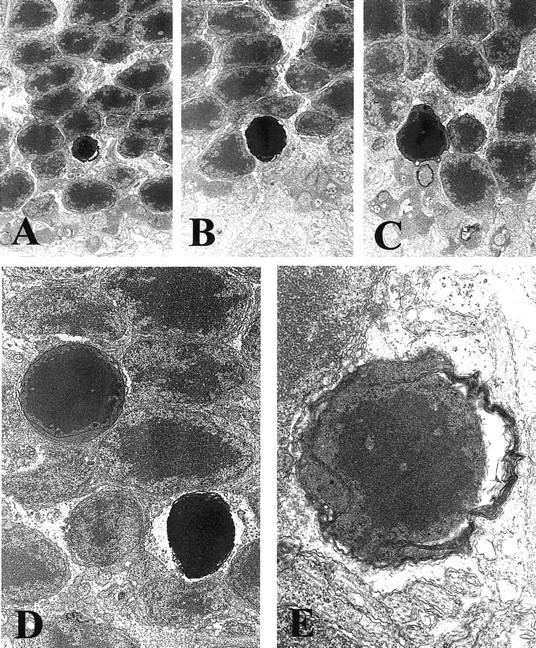
Electron microscopic photographs of the apoptotic photoreceptors. Eyes were fixed in 1% glutaraldehyde and 1% paraformaldehyde in PBS, and embedded in Epon. Major characteristic changes occurring in apoptosis, such as chromatin condensation, alteration of nuclear shape, and cell shrinkage, are seen in the photoreceptors. Original magnification, ×10,000.
Figure 7.
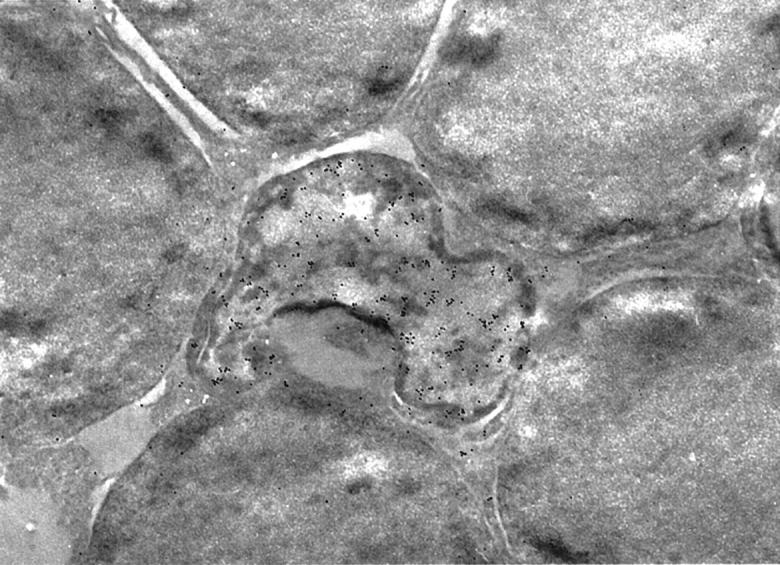
Immunoelectron microscopic photograph of the apoptotic photoreceptors. Apoptotic photoreceptor nuclei shown in Figure 6 ▶ are seen as irregularly shaped nuclei in the sections fixed in 1% paraformaldehyde and embedded in LR white. AIF is positive in the cytoplasm and nucleus of apoptotic photoreceptors. The dense staining of AIF on the nucleus is remarkable. Normal photoreceptors are negative in AIF staining. Original magnification, ×10,000.
Figure 8.
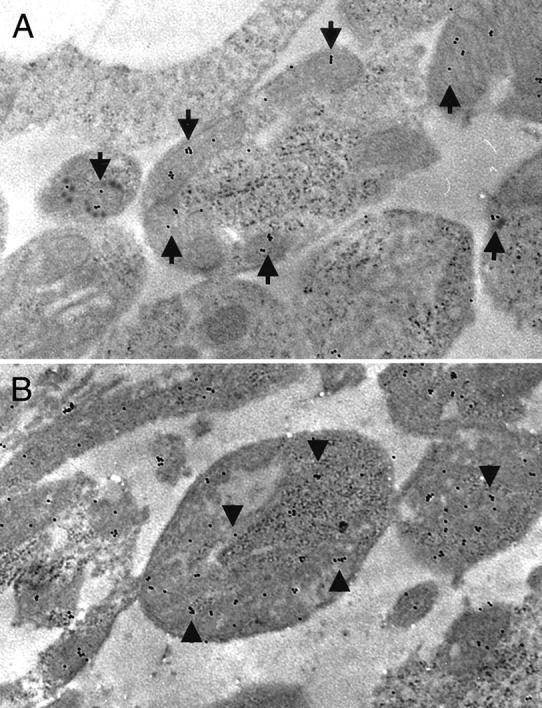
Immunoelectron microscopic photographs of the inner segment of the photoreceptors. A: Inner segment of the photoreceptor from the control retina (nondetached). B: Inner segment of the photoreceptor from the detached retina (day 3). Mitochondria is placed regularly and the cell structure is well preserved, and AIF staining is confined to mitochondria (A, arrows). In detached retina, mitochondria degenerates and AIF staining is diffusely distributed in the cytosol of destructive cells (B, arrowheads). Original magnifications, ×13,000).
Figure 9.
Fluorescent micrographs of cytochrome c immunohistochemistry. The eyes were stained by cytochrome c antibody and visualized by fluorescent microscopy. A: Control for staining without primary antibody. B: Control retina stained by cytochrome c antibody. Cytochrome c is positive in multilinear pattern in the inner segment of the photoreceptor (arrows). C: Detached retina stained by cytochrome c antibody. Cytochrome c is positive in the cytosol and nucleus in the outer nuclear layer (arrowheads).
Modulation of RD-Induced Photoreceptor Apoptosis by the CD95/CD95 Ligand System and Neurotrophic Factors
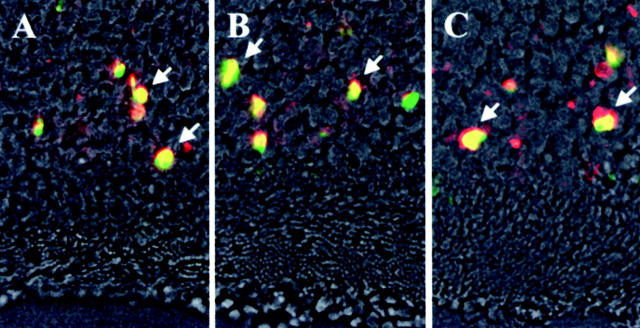
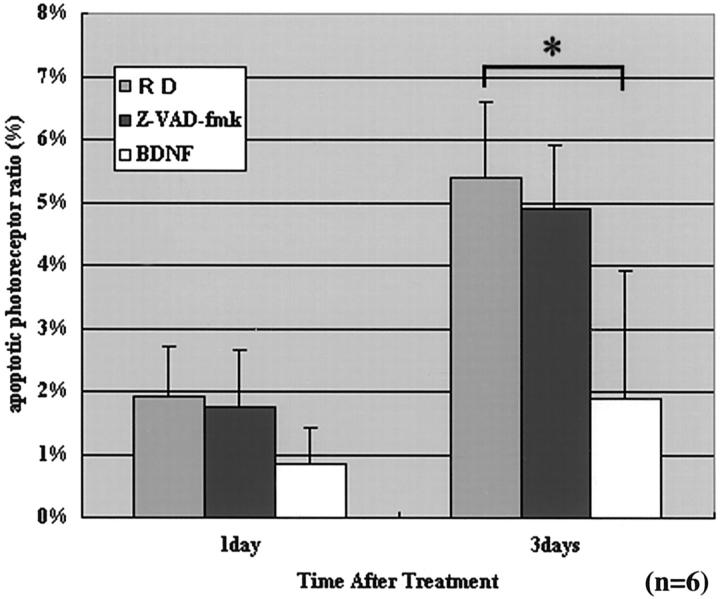
In C3H mice, RD induced photoreceptor apoptosis, following a similar time course as that observed in Brown Norway rats, with a maximum of TUNEL-positive cells 3 days after RD. The double staining of AIF and TUNEL confirmed that most of the nuclei from TUNEL-positive photoreceptors also reacted with the AIF-specific antiserum (Figure 10A ▶ , arrows). No significant difference was found between these mouse strains as far as the RD-induced AIF- and TUNEL-positive nuclei of photoreceptors (Table 2) ▶ . The CD95 death receptor is concerned to rapid activation of the caspases. To further exclude the implication of CD95-like receptors in RD-induced apoptosis, RD was induced in rats by subretinal injection of sodium hyaluronate, in the presence or absence of the wide-ranging caspase inhibitor Z-VAD.fmk. No inhibitory effect of Z-VAD.fmk was detected, both at the level of AIF relocalization and at the level of TUNEL positivity. In strict contrast, we found that subretinal application of BDNF significantly reduced the frequency of mitochondrio-nuclear AIF relocalization and TUNEL positivity (Figure 11 ▶ ; day 3, P < 0.05). Altogether, these data indicate that the CD95/CD95 ligand system does not control photoreceptor apoptosis, at least in this model. However, local shortage of trophic factors such as BDNF is likely to be involved in RD-induced photoreceptor apoptosis.
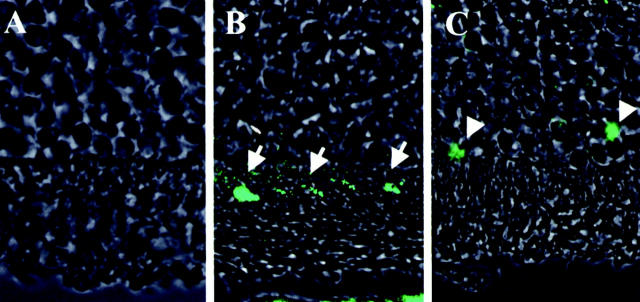
Figure 10.
Fluorescent microphotographs of double staining by AIF and TUNEL in CD95/CD-95 ligand gene mutation mice. A, C3H, control mice; B, C3H-lpr, CD95 gene mutation; C, C3H-gld, CD95 ligand gene mutation. AIF immunohistochemistry (red), TUNEL method (green), and phase-contrast microscopy on day 3 after RD. Double-positive photoreceptors (yellow, arrows) by TUNEL and AIF are seen in all groups.
Table 2.
Ratio of TUNEL- and AIF-Positive Photoreceptors in Total Photoreceptors in C3H Mice on Day 3
| Wild-type | gld | lpr | |
|---|---|---|---|
| TUNEL-positive (%) | 5.33% | 5.62% | 5.11% |
| AIF-positive (%) | 2.92% | 3.11% | 2.98% |
Ten sections for each eye specimen were randomly selected for the evaluation (n = 6). No significant difference was found between these mouse strains as far as the RD-induced AIF- and TUNEL-positive nuclei of photoreceptors.
Figure 11.
The effect of Z-VAD.fmk and BDNF on photoreceptor apoptosis on days 1 and 3. Ten sections of each eye specimen were randomly selected and observed in a masked manner (n = 6). The wide-ranging caspase inhibitor Z-VAD.fmk does not inhibit photoreceptor apoptosis significantly, but the neurotrophic factor BDNF does (on day 3) (P < 0.05).
Discussion
To our knowledge, this is the first report showing clear evidence of the transition of AIF localization from mitochondria to nucleus in the apoptotic process in vivo.
Immunohistochemistry and immunoelectron microscopy revealed AIF to be normally confined to mitochondria, yet to be relocated to the extramitochondrial compartment (cytosol plus nucleus) when nuclear chromatin condensation is occurring. After RD, AIF was found diffusely in the cytosol and in a more patchy manner within areas of condensed chromatin of the nucleus. The double staining for AIF and GFAP showed that most of AIF-positive cells are not Müller cells. Thus, AIF might be involved in the apoptotic process affecting photoreceptor cells.
Susin and colleagues 9 proved the subcellular translocation of AIF during apoptotic processes by comparing subcellular fractions of mouse liver cells in vitro. We also performed both Western blotting of total retinal extracts (with RD) and that of subcellular fraction of the retinal extract for AIF. The results showed that the amounts of total AIF protein remained unchanged for 7 days. We also examined reverse transcriptase-polymerase chain reaction for AIF, and the amount of mRNA for AIF was not significantly changed either. These data might support the translocation of AIF during apoptosis in RD. However the retinal extracts contain not only apoptotic photoreceptors but also photoreceptors with no apoptosis (<5.3% of photoreceptor shows apoptosis at greatest) and many other cellular components, and such other components might mask the intracellular changes of AIF of photoreceptors. Because it is practically impossible to collect the apoptotic photoreceptors exclusively from the detached retina, we could not show the perfect data to prove the translocation of AIF of photoreceptor apoptosis in RD. Therefore, we use the term relocalization of AIF, not the translocation of AIF in this article. From the previous in vitro study 9 and the present morphological results, it is conceivable that AIF translocated from the mitochondria to nucleus in apoptosis, however, more evidence is necessary to prove this hypothesis.
Identifying the upstream signal that leads to the mitochondrial and nuclear signs of apoptosis in photoreceptor cells is obviously an important goal. Nonetheless, the mechanisms of photoreceptor apoptosis in experimental RD remains unknown, 18,33-37 and the regulatory mechanism controlling neuronal survival and apoptosis is just the beginning to be defined. In the present study, we found that apoptosis was induced by experimental RD even in CD95/CD95 ligand-deficient animals and that AIF relocalization occurred in these animals in a way undistinguishable from control animals. Photoreceptor apoptosis also developed while downstream caspases were inhibited by the wide-ranging caspase inhibitor, Z-VAD.fmk. Thus, apparently the CD95/CD95 ligand system and downstream caspases are not involved in the photoreceptor apoptosis. In addition, cytochrome c immunohistochemistry indicated that photoreceptor apoptosis was mediated by mitochondria via a caspase-independent pathway (Figure 9) ▶ .
Because the inner layers of the retina are supplied by retinal circulation, their nutrient supply is not affected by RD. However, the outer layers, including the photoreceptor layer, are supplied by diffusion from the choriocapillaris, implying that RD greatly compromises their trophic supply and causes nutrients to be diluted in the subretinal space. Mervin and colleagues 33 and Lewis and colleagues 36 already reported that oxygen supplementation (hyperoxia) during detachment reduced photoreceptor death, maintaining the specialized structures of surviving photoreceptors, which is good evidence that photoreceptor death in detached retina is nutrition related. Interestingly, the supplement of a neurotrophic factor such as BDNF into the subretinal space inhibited both the AIF relocalization and the signs of nuclear apoptosis. Neuroprotection by growth factors and neurotrophins such as insulin, insulin-like growth factor-1, nerve growth factor, and BDNF has been studied as a means of regulating neuronal apoptosis. 37 BDNF has been reported to activate extracellular signal-regulated kinase and the phosphatidylinositol-3 kinase (PI3-kinase) pathways. IGF-1-activated phosphoinositide 3-kinase (PI3-K) triggered the activation of the serine-threonine kinase Akt. Akt phosphorylation of the Bcl-2 family member BAD has been demonstrated to promote cell survival. 38,39 The ras mitogen-activated protein kinase signaling pathway also apparently mediates growth-factor-dependent cell survival. 40 However, at the present stage, it remains elusive which among these survival pathways is activated by BDNF in photoreceptor cells.
It remains also an open question whether inhibition of photoreceptor apoptosis will actually restore normal vision. Indeed, the decreased nutrition demands resulting from limited apoptosis might actually reduce total tissue damage. As a result, it will be important to study the long-term effects of apoptosis inhibition by BDNF, overexpression of Bcl-2-like mitochondrioprotective proteins, neutralization of AIF, and similar interventions, not only at the level of cell survival but also in functional terms.
Acknowledgments
We thank Dr. Kenneth W. Parker for editorial assistance, and Drs. Shozo Shimokawa, Masao Uehara, and Hiroki Sanui for financial support.
Footnotes
Address reprint requests to Dr. Taiji Sakamoto M.D., Department of Ophthalmology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka, 812-8582, Japan. E-mail: tsakamot@med.kyushu-u.ac.jp.
Supported in part by grant-in-aid no. 09671804 and no. 09470382 for Scientific Research from the Ministry of Education, Science, Sports, and Culture of the Japanese Government; the Japan National Society for the Prevention of Blindness (Tokyo); Fondation pour la Recherche Médicale; and a Special Grant from the League Nationale contre le Cancer (to G. K.).
References
- 1.Kerr JF, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972, 26:239-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huppertz B, Frank HG, Kaufmann P: The apoptosis cascade—morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999, 200:1-18 [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Dallaporta B, Resche-Rigon M: The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 1998, 60:619-642 [DOI] [PubMed] [Google Scholar]
- 4.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F: Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem 1999, 264:687-701 [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, Reed JC: Mitochondrial control of cell death. Nat Med 2000, 6:513-519 [DOI] [PubMed] [Google Scholar]
- 6.Green DR, Amarante-Mendes GP: The point of no return: mitochondria, caspases, and the commitment to cell death. Results Probl Cell Differ 1998, 24:45-61 [DOI] [PubMed] [Google Scholar]
- 7.Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, Debatin KM: Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem 1998, 273:33942-33948 [DOI] [PubMed] [Google Scholar]
- 8.Crompton M: The mitochondrial permeability transition pore and its role in cell death. Biochem J 1999, 341:233-249 [PMC free article] [PubMed] [Google Scholar]
- 9.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G: Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397:441-446 [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo HK, Susin SA, Penninger J, Kroemer G: Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ 1999, 6:516-524 [DOI] [PubMed] [Google Scholar]
- 11.Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prevost MC, Leber B, Andrews D, Penninger J, Kroemer G: Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J 2000, 14:729-739 [PubMed] [Google Scholar]
- 12.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost MC, Alzari PM, Kroemer G: Mitochondrial release of caspase-2 and -9 during the apoptotic process. J Exp Med 1999, 189:381-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MR: Apoptotic signal transduction: emerging pathways. Biochem Cell Biol 1998, 76:573-582 [DOI] [PubMed] [Google Scholar]
- 14.Luthert PJ, Chong NH: Photoreceptor rescue. Eye 1998, 12:591-596 [DOI] [PubMed] [Google Scholar]
- 15.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA: Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci 1983, 24:906-926 [PubMed] [Google Scholar]
- 16.Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA: Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci 1983, 24:927-942 [PubMed] [Google Scholar]
- 17.Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK: Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci 1986, 27:168-183 [PubMed] [Google Scholar]
- 18.Cook B, Lewis GP, Fisher SK, Adler R: Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 1995, 36:990-996 [PubMed] [Google Scholar]
- 19.Berglin L, Algvere PV, Seregard S: Photoreceptor decay over time and apoptosis in experimental retinal detachment. Graefes Arch Clin Exp Ophthalmol 1997, 235:306-312 [DOI] [PubMed] [Google Scholar]
- 20.Hatae T, Ishibashi T, Yoshitomi F, Shibata Y: Immunocytochemistry of types I-IV collagen in human anterior subcapsular cataracts. Graefes Arch Clin Exp Ophthalmol 1993, 231:586-590 [DOI] [PubMed] [Google Scholar]
- 21.Nagata S: Apoptosis by death factor. Cell 1997, 88:355-365 [DOI] [PubMed] [Google Scholar]
- 22.Roths JB, Murphy ED, Eicher EM: A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med 1984, 159:1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 1992, 356:314-317 [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ: Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 1995, 377:348-351 [DOI] [PubMed] [Google Scholar]
- 25.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 26.Ferguson TA, Griffith TS: A vision of cell death: insights into immune privilege. Immunol Rev 1997, 156:167-184 [DOI] [PubMed] [Google Scholar]
- 27.Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N: Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 1999, 104:13-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unoki K, LaVail MM: Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci 1994, 35:907-915 [PubMed] [Google Scholar]
- 29.Liu ZZ, Zhu LQ, Eide FF: Critical role of TrkB and brain-derived neurotrophic factor in the differentiation and survival of retinal pigment epithelium. J Neurosci 1997, 17:8749-8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazawa H, Kamei M, Imafuku I, Kanazawa I: Gene regulation of trkB and trkC in the chick retina by light/darkness exposure. Oncogene 1994, 9:1813-1818 [PubMed] [Google Scholar]
- 31.LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH: Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA 1992, 89:11249-11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaVail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CH, Steinberg RH: Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci 1998, 39:592-602 [PubMed] [Google Scholar]
- 33.Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J: Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol 1999, 128:155-164 [DOI] [PubMed] [Google Scholar]
- 34.Egensperger R, Maslim J, Bisti S, Hollander H, Stone J: Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Müller cells). Brain Res Dev Brain Res 1996, 97:1-8 [DOI] [PubMed] [Google Scholar]
- 35.Lewis GP, Linberg KA, Geller SF, Guerin CJ, Fisher SK: Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 1999, 40:1530-1544 [PubMed] [Google Scholar]
- 36.Lewis G, Mervin K, Valter K, Maslim J, Kappel PJ, Stone J, Fisher S: Limiting the proliferation and reactivity of retinal Müller cells during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol 1999, 128:165-172 [DOI] [PubMed] [Google Scholar]
- 37.Hetman M, Kanning K, Cavanaugh JE, Xia Z: Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem 1999, 274:22569-22580 [DOI] [PubMed] [Google Scholar]
- 38.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME: Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997, 275:661-665 [DOI] [PubMed] [Google Scholar]
- 39.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91:231-241 [DOI] [PubMed] [Google Scholar]
- 40.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME: Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999, 286:1358-1362 [DOI] [PubMed] [Google Scholar]