Abstract
Regeneration of the endometrium after menstruation requires a rapid and highly organized vascular response. Potential regulators of this process include members of the vascular endothelial growth factor (VEGF) family of proteins and their receptors. Although VEGF expression has been detected in the endometrium, the relationship between VEGF production, receptor activation, and endothelial cell proliferation during the endometrial cycle is poorly understood. To better ascertain the relevance of VEGF family members during postmenstrual repair, we have evaluated ligands, receptors, and activity by receptor phosphorylation in human endometrium throughout the menstrual cycle. We found that VEGF is significantly increased at the onset of menstruation, a result of the additive effects of hypoxia, transforming growth factor-α, and interleukin-1β. Both VEGF receptors, FLT-1 and KDR, followed a similar pattern. However, functional activity of KDR, as determined by phosphorylation studies, revealed activation in the late menstrual and early proliferative phases. The degree of KDR phosphorylation was inversely correlated with the presence of sFLT-1. Endothelial cell proliferation analysis in endometrium showed a peak during the late menstrual and early proliferative phases in concert with the presence of VEGF, VEGF receptor phosphorylation, and decrease of sFLT-1. Together, these results suggest that VEGF receptor activation and the subsequent modulation of sFLT-1 in the late menstrual phase likely contributes to the onset of angiogenesis and endothelial repair in the human endometrium.
The cyclic regeneration of the endometrium during the female reproductive years requires a highly regulated angiogenic response. 1-5 Physiological changes associated with loss and reconstruction of the functional endometrium during the menstrual cycle are unique to the higher primate species. Humans undergo shedding of the upper spongy layer of the endometrium during menses. 4,6,7 Menstrual bleeding itself is brought on by tissue breakdown and damage surrounding superficial endometrial vessels. Within 5 days of menstrual onset, the damaged endometrial vessels have been repaired. 8 Hence, the initial phase of endometrial angiogenesis involves repair of the vascular bed in concert with the late stages of menstrual shedding and during the proliferative phase. Models of endometrial angiogenesis in the proliferative phase describe the growth of the vasculature under the influence of estrogen, whereas the secretory phase involves growth of the coiled arterioles mediated by progesterone. 9
It is evident that a large number of angiogenic growth factors might contribute to the initiation, progression, and morphogenesis of blood vessels associated with endometrial repair. Several angiogenic cytokines have been identified in the human endometrium including basic fibroblast growth factor-2, platelet-derived growth factor, epidermal growth factor, and transforming growth factor-β (TGF-β), although vascular permeability/vascular endothelial growth factor seems to be a major candidate for modulating the angiogenic response. 4,5,10,11
The role of VEGF as a mediator of angiogenesis during menstrual repair has been intensively investigated during the last decade. Most of the studies, however, have been descriptive in nature, providing either immunocytochemistry, in situ hybridization, or reverse transcriptase-polymerization chain reaction (RT-PCR) analysis for assessment of VEGF levels. This can be attributed, for the most part, to the tremendous limitations imposed by the uniqueness of the human endometrial cycle that cannot be reproduced in most animal models, except for a subset of primates. Functional studies addressing the relevance of the VEGF-signaling system to the female reproductive tract have been done in rodent models. Treatment of female rats with a truncated soluble form of the FLT-1 receptor resulted in virtually complete suppression of angiogenesis in the corpus luteum with associated maturation failure of the endometrium. 12 The truncated Flt-1 molecule acted in a dominant-negative manner, diminishing association of VEGF to its transmembrane receptors and impacting subsequent signaling pathways. 12 This elegant and important manuscript emphasized the relevance of the VEGF-signaling to the maintenance and normal physiology of the ovary and endometrium. However, these studies could not shed light on the participation of this signaling pathway in the human endometrial cycling.
In humans, VEGF mRNA is present throughout the endometrial cycle and seems to be increased in the secretory phase, 11,13 but this expression pattern does not parallel the predicted temporal stage associated with neovascularization and vascular repair that follows menstruation. 14 Serum levels of VEGF from woman at all stages of the endometrial cycle have been the focus of intense investigation. 15 Nonetheless, no significant changes and a complete lack of cyclicity was consistently found (M. L. Iruela-Arispe, unpublished results). 15 Interestingly, patients subjected to in vitro fertilization treatment, which involves significant doses of hormones, showed elevated levels of VEGF in serum during the luteal phase of the cycle. 15 This suggests that, at least in part, endometrial hormones contribute to VEGF levels. To support this concept, hormonal-based contraceptives have been shown to have an effect in the distribution of VEGF from the stroma to the glandular compartment 16 and also seem to predispose the endometrium to an increased vascular fragility. 17,18 Nonetheless, the relative contribution of VEGF to the normal physiology of the endometrial cycle remains elusive and additional investigations are required to: 1) concretely understand the contribution of steroid hormones in VEGF expression during normal physiology in concert with other endometrial confounding factors, in particular hypoxia; and 2) gain further insight as to the orchestrated regulation of VEGF, VEGF receptor expression, and signaling on endothelial cells during the endometrial cycle.
The present study was aimed at elucidating the role of the VEGF and VEGF receptors in modulating the initial wave of angiogenesis associated with postmenstrual repair. Using endometrial biopsy specimens from a cohort of normal ovulating women, our findings revealed a sophisticated regulatory control of VEGF-receptor activation that coincides with the onset of endothelial proliferation and vascular reconstitution of the tissue.
Materials and Methods
Study Participants
Entry criteria for this study included healthy, nonsmoking women aged 18 to 36 years, with regular menses, normal cervical cytology, no history of ovulatory dysfunction, endometriosis, infertility, or eating disorders. The study participants had no history of Norplant or Depo-Provera use in the last year, no oral contraceptive use in the past 3 months, and were more than 6 months postpartum and nonlactating. After receiving informed consent, patients meeting entry criteria underwent a physical examination, blood draw, urine pregnancy test, and endometrial aspiration biopsy using a Pipelle instrument (Cooper Surgical, Shelton, CT). The protocol was approved by the Committee on Clinical Investigations, Beth Israel Deaconess Medical Center. Endometrial tissue was placed in modified Eagle’s media and immediately processed for RNA extraction, cell isolation, and histology. Study participants’ serum was pooled and stored at −70°C until completion of recruitment.
Study Groups and Serum Hormone Assay
The study participants’ serum was measured for estradiol, progesterone, and luteinizing hormone in duplicate aliquots using an AutoDELPHIA immunoassay (Wallac, Turku, Finland) (courtesy of Dr. James Faix, Beth Israel Deaconess Core RIA Laboratory). The study participants were divided into study groups based on cycle day of biopsy, hormone levels, and endometrial dating. 19 The late proliferative phase group was assessed by serology as having pre-ovulatory progesterone levels (<7 nmol/L), and significantly higher estradiol and luteinizing hormone levels than the early proliferative group. Study participants in the secretory groups were considered when serum progesterone levels were indicative of ovulation (>7 nmol/L). Sixty-nine study participants entered the study, and eight could not have endometrial biopsy completed because of discomfort with procedure. Sixty-one endometrial biopsies were obtained for study: one late proliferative phase sample was excluded for a progesterone level suggestive of premature luteinization (>7 nmol/L), and one sample was excluded because of error in processing, leaving 59 biopsies available for study.
Materials
Paraformaldehyde, Ficoll-400, polyvinylpyrrolidone, salmon testicular DNA, dextran sulfate, RNase A, progesterone, 17-β-estradiol, TGF-α, and interleukin (IL)-1β were purchased from Sigma Chemical Co. (St. Louis, MO). Trypsin-ethylenediaminetetraacetic acid, collagenase, phenol-free medium, and antibiotics were purchased from Life Technologies, Inc. (Gaithersburg, MD). Charcoal-filtered fetal calf serum was supplied from Cocalico Biologicals (Reamstown, PA). The random-primed DNA-labeling kit was purchased from Amersham Corp. (Arlington Heights, IL). [32P]-dCTP was purchased from DuPont NEN (Boston, MA). Nylon membranes (Nytran) were purchased from Schleicher and Schuell (Keene, NH). All other reagents used in this study were of the highest purity available from Fisher Biochemicals (Pittsburgh, PA).
Northern Analysis
Total RNA was purified from either minced whole tissue or purified cells by guanidinum-isothiocyanate extraction. 20 Poly(A+) enriched RNA was isolated using an oligo(dT)-streptavidin magnetic bead separation kit from Boehringer Mannheim (Indianapolis, IN). Samples were subjected to electrophoresis on a denaturing 1% agarose gel and transferred to nylon membranes (Nytran). Northern blots were hybridized with [32P]-labeled cDNA probes. The following cDNA fragments were used in this study: 1) a 930-bp EcoRI fragment that recognizes all VEGF isoforms (a generous gift from N. Ferrara, Genentech); 2) a 1-kb EcoRI/XhoI fragments of VEGF-B and VEGF-C cDNAs (obtained by RT-PCR); 3) a 634-bp probe, generated from the HindIII-BamHI restriction fragment (bp1661 to 2294) of sFLT-1 (obtained by RT-PCR); 4) a KDR cDNA fragment that corresponds to a 300-bp EcoRI/BamHI fragment subcloned into pGEM3 (a gift from Kevin Claffey; University of Connecticut); 5) a 925-bp EcoRI TGF-α cDNA fragment (American Type Culture Collection, Rockville, MD); 6) a 1-kb SmaI/BamHI fragment for IL-1β; and 7) a 2.47-kb BamHI GLUT-1 cDNA fragment (a generous gift from Dr. Kevin Claffey, University of Connecticut). Membranes were exposed to Biomax MS film (Kodak, Rochester, NY), and densitometric analysis of autoradiograms were performed by scanning densitometry using a Vista S-12 scanner (UMAX, Taiwan, R.O.C.), and Molecular Analyst software (Bio-Rad, Hercules, CA) for Power Macintosh (Apple, Cupertino, CA). All densitometric data were normalized for loading and transfer efficiency to expression of 36B4 (a ribosome-associated protein). 21
Isolation of Human Endometrial Stromal Cells
We followed previously described procedures with few modifications. 21 Specifically, endometrial samples were minced into 1- to 2-mm 3 pieces under sterile conditions in a laminar flow hood and digested with collagenase at 37°C for 2 hours. After digestion, the cell suspension was filtered through a 70-μm nylon mesh to remove undigested fragments. Cells were spun and washed four times in Dulbecco’s modified Eagle’s medium containing a fivefold excess of antibiotics (penicillin, streptomycin, and gentamicin). Isolated cells were plated on tissue culture dishes previously coated with 50 μg/ml of vitrogen (Collagen Biomaterials, Palo Alto, CA). Cells were allowed to attach for 30 minutes; nonattached cells were removed by aspiration. Stromal cultures were evaluated for expression of estrogen receptor and progesterone receptor. Functional characterization of estrogen receptor and progesterone receptor was performed by transactivation of luciferase after steroid treatment, as previously performed. 22 Briefly, transfection of stromal cultures was performed with 1) a luciferase reporter construct containing six-tandem repeats of the estrogen receptor-binding motif (a generous gift from Dr. Miller, Dana Farber Cancer Institute, Boston, MA), or 2) a luciferase reporter construct driven by four-tandem repeats of the progesterone receptor CIS acting element (a generous gift from Dr. Sam Lee, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA), and 3) a plasmid containing CMV renilla to control for transfection efficiency. Lipofectamine (Life Technologies, Inc.) was used in all transfections following the manufacturer’s recommendations. Assays were performed in triplicate and were normalized for both transfection efficiency [with a renilla construct (Promega)] and total protein. Expression of luciferase and renilla was evaluated with a luminometer (Wallac, Gaithersburg, MD). Stromal cells were no longer used when functional levels of one or both receptors dropped 30% below the levels established for that culture at passage 2 (the decrease in receptor was seen in some cultures as early as passage 6 and as late as passage 10 in other cultures).
Treatment of stromal cultures with estradiol (Sigma), progesterone (Sigma), recombinant TGF-α, and IL-1β (Life Technologies, Inc.) was performed at the times and concentrations indicated in the figure legends. All cultures were incubated in serum-free and phenol-red-free media for 16 hours before treatment to remove potential confounding factors that could interact with steroid receptors. For neutralization experiments, antibodies against human TGF-α (polyclonal made in rabbit) and IL-1β (monoclonal made in mouse) were purchased from Leinco Technologies (St. Louis, MO). Antibodies were incubated with the related growth factor for 3 hours before cell treatment (10 ng of growth factor, 30 μg of antibody). Controls included treatment of cultures with antibodies alone and incubation of the growth factors with rabbit (for TGF-α) or mouse (for IL-1β) IgG. Treatment of cells followed for 4 hours and evaluation was performed by Northern analysis.
Hypoxia experiments were done using a Heraeus incubator using 3% O2 and 5% CO2 gas concentrations. We have selected this range of oxygen tension, as it has been shown that transcriptional response to hypoxia (particularly through induction of HIF-1α) is triggered at oxygen concentrations ranging from 3 to 5%. 23
Enzyme-Linked Immunosorbent Assays (ELISAs) for Detection of VEGF
VEGF protein was evaluated on sandwich ELISAs. Capture of VEGF was accomplished with a bound chicken IgY antibody to human VEGF (a gift from Dr. Don Senger, Beth Israel Deaconess Medical Center, Boston, MA). Followed by a mouse α-VEGF antibody (also from Don Senger). A peroxidase-conjugated goat anti-mouse IgG was used as secondary antibody (KPL, Gaithersburg, MD). Detection was aided by using KPL enhancer substrate, reactions were measured on a luminometer. Values were compared to a VEGF curve performed in parallel.
Immunoprecipitation and Western Blot Analysis
For immunoprecipitation of VEGF, tissue samples were solubilized in extraction buffer (50 mmol/L Tris, pH 7.5, 2% glycerol, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Triton X-100) containing proteinase inhibitors (2 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L NaF, 1 mmol/L NaVO4, and 10 μg/ml of each aprotinin, leupeptin, and pepstatin A). After centrifugation at 10,000 rpm for 30 minutes, protein extracts were mixed with a slurry of heparin-Sepharose CL-6B (Pharmacia) and incubated overnight rocking at 4°C. Beads were harvested by centrifugation, washed with 450 mmol/L NaCl, and eluted with 1.5 mol/L NaCl. Protein was dialyzed against phosphate-buffered saline (PBS) and quantified using the Bio-Rad protein assay system (Bio-Rad, Hercules, CA). Before immunoprecipitation bovine serum albumin was added to the precleared lysates (final concentration, 0.5%). Equal amounts of protein from lysates were always used for immunoprecipitation and Western blotting. Incubation of tissue lysate with chicken anti-human VEGF (a gift from Dr. Don Senger, Beth Israel Deaconess Medical Center, Boston, MA) followed by protein-G Sepharose beads was performed for 2 hours at 4°C. Immunoprecipitates were washed twice with Nonidet P-40 buffer (50 mmol/L Tris-HCl, pH 7.5, 10% glycerol, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40 and sodium vanadate). Immunoprecipitation of KDR was performed using a monoclonal antibody (Chemicon, Temecula, CA). For these experiments, endometrial samples were solubilized in a similar buffer to the one previously described for VEGF extraction, but containing 1% Triton X-114 for isolation of membranes. Further purification of membrane fractions was subsequently done by ultracentrifugation on sucrose gradients. Anti-phosphotyrosine PY-20 antibody (BD Transduction Labs., San Jose, CA) was used on Western blots and an antibody to the N-terminal sequence of sFLT-1 was used on immunoprecipitation and Western analysis. Immunodetection in all cases was performed by incubation with specific peroxidase-conjugated secondary antibodies followed by enhanced chemiluminescence (Pierce, Rockford, IL).
Immunocytochemistry
Tissue samples were fixed in 4% paraformaldehyde in PBS for 1 to 2 hours, embedded in paraffin, and sectioned at 5 μm. Sections were cleared, rehydrated, washed, and blocked with 1% goat serum. Tissue sections were subsequently incubated with a monoclonal antibody against Ki-67 (Immunotech, Westbrook, ME) that detects nuclear-associated antigen of proliferating cells and is absent in resting cells (G0) but is present through the rest of the cell cycle. 24 After several washes in PBS, sections were incubated with anti-Von Willebrand factor (DAKO, Glostrup, Denmark). The sections were washed and incubated simultaneously with two secondary antibodies: anti-rabbit alkaline phospatase-conjugated and anti-mouse peroxidase-conjugated. Development was performed according to the manufacturer’s protocol (Vectors Laboratories, Burlingame, CA). Quantification of endothelial proliferating cells was accomplished using ImagePro 3.1 software, scoring of positive nuclei in the stained (red) vessels was assessed within 1,000 endothelial nuclei per section.
Statistics
All categorical data are presented as a mean ± SD when repeated measures were done. Assuming normal distributions, data were analyzed by one-way analysis of variance, followed by either t-test with Dunnett test for comparisons between specific groups, or the Student-Newman-Keuls test for multiple comparisons between groups. 25 Statistical analysis was performed by In-Stat software (Graph Pad Software) for Macintosh.
Results
The endometrium from our cohort of normal, ovulatory women, was divided into six study groups based on cycle day of biopsy, and serum estradiol, progesterone, and luteinizing hormone levels (Table 1) ▶ . These included early menstrual, late menstrual, early proliferative, late proliferative, early secretory, and late secretory endometrium. As per study design, the late proliferative phase group had significantly higher estradiol (P < 0.001) and luteinizing hormone (P < 0.01) levels than menstrual or early proliferative phases. Additionally, the early secretory and late secretory phases had significantly higher progesterone levels (P < 0.001 and P < 0.05, respectively) than other phases of the cycle, indicative of ovulation.
Table 1.
Characterization of Endometrial Study Groups
| Study groups | n | Cycle day | Estradiol (nmol/L)* | Progesterone (nmol/L)† | Luteinizing hormone (mIU/ml) |
|---|---|---|---|---|---|
| Early menstrual | 8 | 1.7 ± 0.67 | 0.14 ± 0.15 | 1.77 ± 0.42 | 3.5 ± 0.23 |
| Late menstrual | 10 | 4.1 ± 0.37 | 0.19 ± 0.25 | 1.57 ± 0.32 | 4.3 ± 0.13 |
| Early proliferative | 9 | 9.8 ± 2.7 | 0.25 ± 0.09 | 1.34 ± 0.42 | 6.8 ± 1.9 |
| Late proliferative | 11 | 15.0 ± 3.7 | 0.71 ± 0.15‡ | 1.74 ± 0.35 | 29.6 ± 16.3¶ |
| Early secretory | 12 | 19.6 ± 1.2 | 0.39 ± 0.12 | 36.04 ± 18.41 | 5.49 ± 1.77 |
| Late secretory | 9 | 26.0 ± 0.81 | 0.34 ± 0.16 | 23.3 ± 11.4§ | 3.4 ± 0.57 |
Study participants were divided into six study groups based on cycle day of biopsy, and serum estradiol, progesterone, and luteinizing hormone levels. The late proliferative group was defined by significantly higher estradiol and luteinizing hormone levels. Additionally, significantly higher progesterone levels in the secretory groups are indicative of ovulation, a criteria necessary for study entry. Values are expressed as mean ± SD. Comparisons between groups evaluated by analysis of variance and Student-Newman test.
*1 nmol/L estradiol = 272.4 pg/ml.
†1 nmol/P = .314 ng/ml.
‡Late proliferative versus menstrual, early proliferative, late proliferative (P < 0.001); early secretory versus menstrual, early proliferative, late proliferative (P < 0.001).
§Late secretory versus menstrual, early proliferative, late proliferative, early secretory (P < 0.05).
¶Late proliferative versus all other groups (P < 0.01).
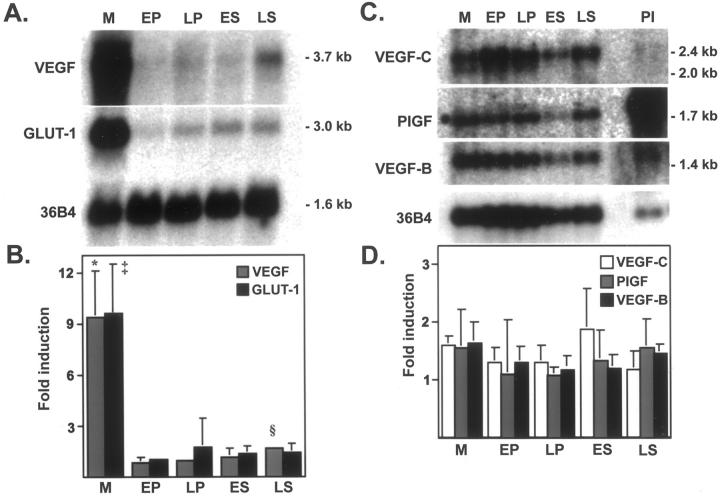
Steady-state mRNA levels for VEGF in endometrium throughout the menstrual cycle is shown in Figure 1A ▶ . Although VEGF mRNA is constitutively expressed at low levels throughout the menstrual cycle, a significant 9.3-fold induction was detected in the menstrual phase (P < 0.001) (Figure 1B) ▶ . Because of the sharp induction of VEGF mRNA, the menstrual phase was dropped out of the analysis of variance to analyze steady-state levels in the proliferative and secretory phases. After excluding the menstrual phase, a significant but small 1.6-fold induction was found in the late secretory phase (P < 0.05) as compared to all other phases in the cycle in agreement with previous studies. 13 To evaluate the potential role of hypoxia in the menstrual phase endometrium, we hybridized the same Northern blot with a cDNA probe for the glucose transporter GLUT-1, a marker of anaerobic metabolism and an ischemia-induced gene. 26 GLUT-1 is similarly co-expressed with a significant 9.6-fold induction of steady-state mRNA levels in the menstrual phase (P < 0.01) (Figure 1, A and B) ▶ . The induction of GLUT-1 signifies the hypoxic state of the endometrium and the potential contribution of hypoxia to the regulation of VEGF during the menstrual phase. We next evaluated the steady-state mRNA expression pattern of the VEGF-related proteins, VEGF-B, VEGF-C, and PlGF (Figure 1C) ▶ . Because of the low-level mRNA expression, we performed poly(A+) enrichment of 50 μg of total RNA in a subset of samples (n = 4/group). In Figure 1 ▶ (C and D), VEGF-B, VEGF-C, and PlGF were shown to be constitutively expressed at low levels throughout the endometrial cycle and did not seem to be regulated by either hypoxia or sex steroids, as per their lack of association with any particular phase. PlGF was highly expressed in the placental specimen used as control. There was no significant difference in steady-state RNA levels of any of these factors among endometrial groups.
Figure 1.
Expression of VEGF transcripts during human menstrual cycle. A: Northern blot of total RNA (20 μg/lane) extracted from menstrual (M), early proliferative (EP), late proliferative (LP), early secretory (ES), and late secretory (LS) endometrium, and hybridized to VEGF and GLUT-1 cDNA probes. B: Northern blots from independent biopsies (six per group) were scanned by densitometry, and normalized to signal from a 36B4 cDNA probe (ribosomal-associated protein). Fold induction values are expressed as means ± SD. *, M versus all other groups (P < 0.001), ‡, M versus all other groups (P < 0.01). The 1.6-fold induction of VEGF mRNA in LS phase is significantly greater than EP, LP, ES, after removing M phase from the analysis of variance (P < 0.05). C: Northern blot of poly(A+)-enriched RNA (isolated from 50 μg of total RNA) extracted from the same stages as in A and hybridized to VEGF-B, VEGF-C, and PlGF cDNA probes. D: Northern blots from independent biopsies (n = 4) were scanned by densitometry and normalized to signal from 36B4 mRNA (ribosomal-associated protein). Fold induction values between groups expressed as mean ± SD. Because of large variability in individual samples, no significant difference was detected between groups.
Given the menstrual phase induction of steady-state VEGF mRNA, we proceeded with in vitro experiments to elucidate potential regulators of VEGF expression in human endometrium. Human endometrial stromal cells were isolated, and tested for functional expression of estrogen and progesterone receptors by transfection with estrogen receptor-and progesterone receptor-responsive elements driving the luciferase reporter (data not shown). Hypoxia was the first condition tested as an obvious target for the regulation of VEGF.
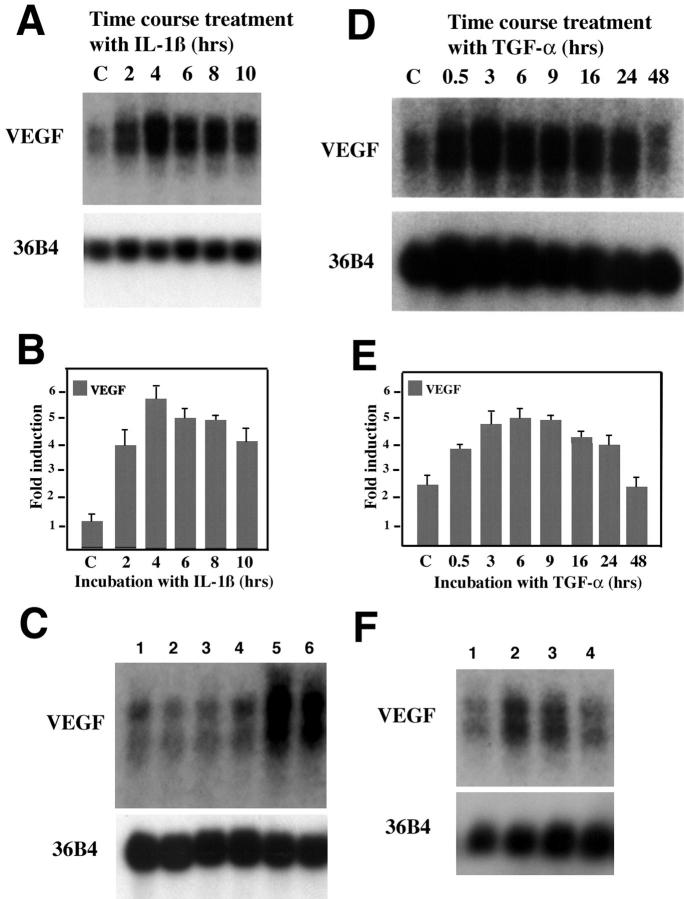
Figure 2A ▶ shows the induction of VEGF mRNA in human endometrial stromal cells after exposure to hypoxic conditions (3% O2). An increase in VEGF mRNA was detected as early as 6 hours (1.5-fold, P < 0.05). Maximal induction (2.4-fold more than control) was seen by 48 hours (P < 0.001). GLUT-1 mRNA was equally induced by hypoxia in vitro, demonstrating a 3.3-fold increase more than control at 48 hours (data not shown). An increase of VEGF mRNA levels on hypoxia has been shown to be variable among tumor cells. 27 While performing these studies, we evaluated the relative ability of endometrial fibroblasts to respond to hypoxia by their ability to induce VEGF compared to fibroblasts isolated from lung, skin, breast, bone marrow, and heart. Interestingly, endometrial fibroblasts were the most responsive followed by lung, heart, skin, bone marrow, and breast (data not shown).
Figure 2.
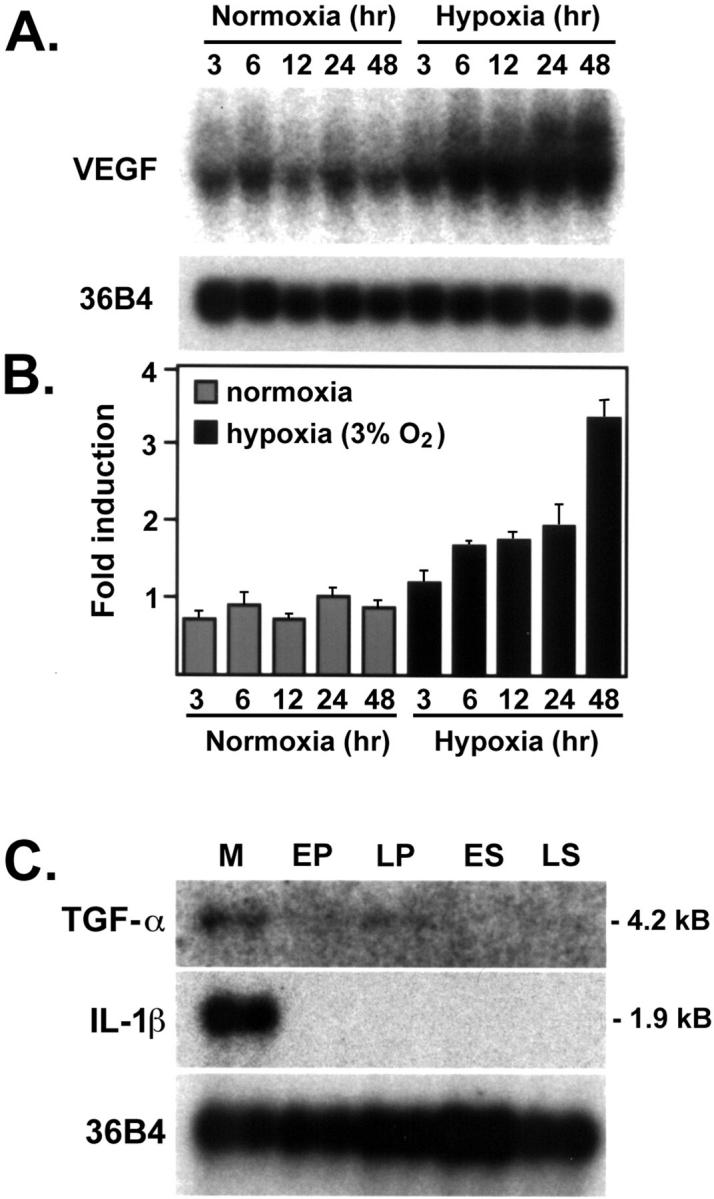
Effect of hypoxia on VEGF transcript levels in endometrial stromal cells. Possible contribution of local cytokines in the increase of VEGF mRNA during the menstrual phase. Stromal cells were isolated from endometrial biopsies by explant procedure and characterized by absence of endothelial and smooth muscle cell markers. A: Cultures of stromal endometrial cells were exposed to normoxia or hypoxia for the times indicated in the figure. Northern blots from 10 μg of total RNA were hybridized to VEGF cDNA. Loading and transfer efficiency was normalized by hybridization to 36B4. B: Scans of four independent experiments revealed a significant increase in VEGF levels throughout time. C: Expression of steady-state TGF-α and IL-β mRNA in human endometrium in menstrual (M), early proliferative (EP), late proliferative (LP), early secretory (ES), and late secretory (LS) phases. Poly(A+)-enriched human endometrial RNA (from 50 μg total RNA) was hybridized to cDNA probes for TGF-α, IL-1β, and 36B4 (ribosomal-associated protein) to demonstrate equivalent loading.
Given the nearly 10-fold induction of VEGF and GLUT-1 in menstrual endometrium in vivo (Figure 1) ▶ , our in vitro conditions either did not reproduce the degree of hypoxia/ischemia typical of the menstrual phase in vivo or additional confounding stimulators were missing in these experimental conditions. Hypoxic experiments were repeated under a variety of steroid treatments, including after steroid withdrawal in vitro, to best reproduce the physiological sex steroid withdrawal typical of the menstrual phase. Northern analysis was conducted for VEGF expression after exposure to 3 days of estradiol (10 nmol/L) and progesterone (10 μmol/L), followed by a steroid-free withdrawal period. No relative change was seen under normoxic conditions throughout a 48-hour period, revealing no direct effect of steroid withdrawal on VEGF mRNA levels (data not shown). Similarly, steroid withdrawal under hypoxic conditions did not seem to potentiate steady-state VEGF mRNA induction over the effect of hypoxia alone. Incubation with estradiol under normoxic conditions resulted in a modest, but significant 1.6-fold induction of VEGF mRNA (P < 0.05) at 6 hours, although mRNA levels quickly returned to baseline after estradiol withdrawal. Incubation with progesterone resulted in a 1.3-fold induction of VEGF mRNA at 6 hours, but this was not significant (data not shown). These data support previous reports that indicate a consistent but low contribution of steroids in VEGF regulation in the endometrium 28,29
We next hypothesized that additional cytokines could contribute to the enhancement in VEGF expression seen during the menstrual phase. Two cytokines in particular (TGF-α and IL-1) have been implicated in the regulation of VEGF mRNA in several cell types. 30,31 Interestingly, both cytokines were increased in menstrual endometrium and down-regulated during the remaining phases of the endometrial cycle (Figure 2C) ▶ following a profile similar to that of VEGF mRNA. These data temporally placed these cytokines as potential regulators of VEGF mRNA in this tissue. Neither TGF-α nor IL-1β was increased on hypoxic treatment of stromal cells (data not shown). It is likely that the major source of TGF-α and IL-1β in the endometrium is inflammatory cells, abundant in the menstrual endometrium, as compared to other phases of the cycle. 32
Induction of VEGF mRNA by IL-1β in vitro was dose- and time-dependent, with a maximal 4.2-fold induction greater than control after a 4-hour incubation of human endometrial stromal cells with IL-1β (10 ng/ml) (Figure 3, A and C) ▶ . Incubation of endometrial stromal cells with TGF-α (50 ng/ml) throughout a 48-hour time course resulted in a maximal 2.1-fold induction of VEGF mRNA by 3 hours. Transcript levels returned to baseline by 48 hours (Figure 3, B and D) ▶ . Induction of VEGF mRNA by TGF-α was dose-dependent with a maximal 4.7-fold induction at 200 ng/ml (data not shown). TGF-α induction was inhibited by pre-incubation with anti-TGF-α antibodies (Figure 3E) ▶ . Similarly the effect mediated by IL-1β was reversed if the cytokine was pre-incubated with neutralizing antibodies (Figure 3E) ▶ . As an important control, we found that fibroblast growth factor-2, a cytokine also present in the human endometrium was not able to enhance VEGF transcript levels (Figure 3F) ▶ .
Figure 3.
Effect of IL-1β and TGF-α on VEGF transcript levels. A: IL-1β induction of steady-state VEGF mRNA in human endometrial stromal cells. Northern blot of total RNA (10 μg) extracted from human endometrial stromal cells exposed to IL-1β (10 ng/ml) throughout a 10-hour time course. The blot was hybridized to VEGF and 36B4 cDNA probes as indicated. B: Effect of TGF-α (50 ng/ml) on VEGF transcripts. Stromal cultures were treated for the times indicated and total RNA was evaluated for hybridization with VEGF and 36B4 probes as indicated. C and D: VEGF signal from samples exposed to IL-1β (C) or TGF-α (D) were scanned by densitometry and normalized to expression of 36B4. Five independent experiments were scanned to provide the histogram shown. Lane labeled as C indicates control levels, ie, VEGF baseline expression. Bars indicate SD. E: Neutralization of cytokine-mediated VEGF induction. Neutralization of TGF-α and IL-1β was achieved by incubation of these cytokines with antibodies as indicated in Materials and Methods before treatment of cells. Controls included cytokine incubation with IgG alone and treatment of the cultures with anti-cytokine antibodies in the absence of either TGF-α or IL-1β. Treatment of the cultures was performed for 4 hours. RNA (10 μg) from cultures was extracted and VEGF transcript levels were evaluated by Northern analysis. Lanes indicate cultures incubated for 4 hours with: lane 1, anti-IL-1β antibody; lane 2, anti-TGF-α antibody; lane 3, complex—anti-IL-1β (30 μg/ml) and IL-β (10 ng/ml); lane 4, complex—anti-TGF-α (30 μg/ml) and TGF-α (50 ng/ml); lane 5, complex—mouse IgG (30 μg/ml) and IL-1β; lane 6, complex—rabbit IgG (30 μg/ml) and TGF-α. F: RNA (10 μg) from stromal cells isolated after 3 hours treatment with: lane 1, PBS; lane 2, IL-1β (10 ng/ml); lane 3, TGF-α (50 ng/ml); and lane 4, fibroblast growth factor-2 (20 ng/ml) were evaluated for VEGF transcript levels. 36B4 probe was used as loading control.
The combination of TGF-α, IL-1β, and hypoxia increased VEGF mRNA levels 15-fold (Figure 4A) ▶ . The effect mediated by these three factors together was clearly greater than the sum of each alone. Hypoxia also potentiated the effects mediated by TGF-α (Figure 4B) ▶ and IL-1β independently (data not shown). To best understand the relationship between hypoxia and growth factor regulation, we performed experiments in which stromal cultures were first subjected to hypoxia for 24 hours, followed by normoxia for 16 hours, and then treated with cytokines for an 16 additional hours. Levels of VEGF were compared to similar cultures that were first maintained in normoxia for 24 hours, followed by hypoxia for 16 hours, and then treated with cytokines in hypoxia (Figure 4C) ▶ . The increase of VEGF mRNA by hypoxia was shown to be transient and returned to baseline on normalization of O2 levels (Figure 4C ▶ , lane C). Previous exposure to hypoxia did not alter the response of VEGF transcript levels to TGF-α. Treatment with this cytokine alone induced VEGF levels by 2.5- to 3.5-fold, as shown in Figure 3 ▶ . Again, a small increase was seen when stromal cells were treated simultaneously with TGF-α and IL-1β even with previous exposure to hypoxia (Figure 4C, H ▶ + N). In contrast, constant hypoxic conditions showed an additive effect on treatment with TGF-α or IL-1β and clear potentiation when both of these cytokines were used in combination (Figure 4C, N ▶ + H). These results indicate that hypoxia can prolong the transient stimulation mediated by cytokines and provides further evidence for an additive effect of up to 15-fold when both cytokines are used in combination. Together, the temporal pattern of expression of TGF-α and IL-1β suggests that these cytokines in combination with hypoxia are likely responsible for the peak and sustained levels of VEGF transcripts in the menstrual phase of the endometrial cycle.
Figure 4.
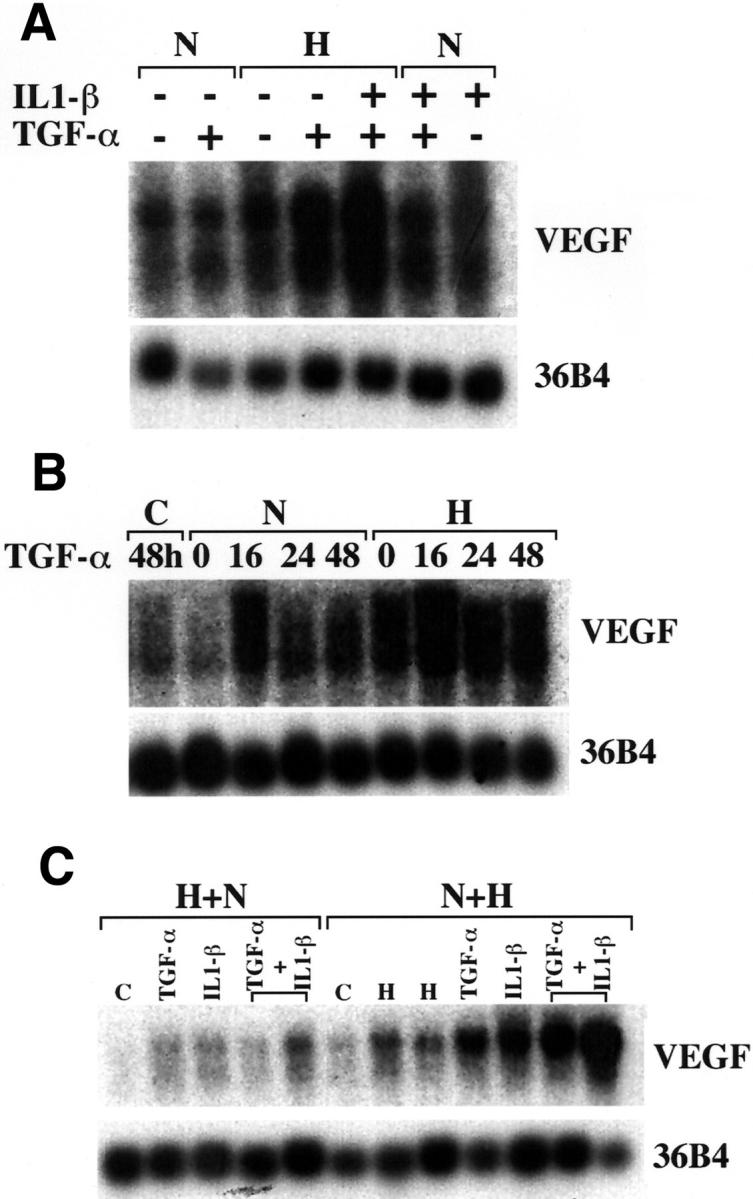
Combined effect of hypoxia, TGF-α, and IL-1β on VEGF expression. Northern blots of total RNA (10 μg/lane) extracted from stromal cells and probed with VEGF and 36B4 as indicated. Before extraction, cells were treated as follows. A: Stromal cultures were subjected to normoxia (N) or hypoxic conditions (H) in the presence or absence of TGF-α (50 ng/ml) and/or IL-1β (10 ng/ml) for 24 hours as indicated. B: Cells were maintained under normoxia (N) or hypoxia (H) and treated for variable amounts of time with TGF-α (50 ng/ml) as indicated. C-48 hours (lane 1) corresponds to baseline levels of VEGF in the absence of hypoxia and without exposure to TGF-α. C: Cultures were first incubated in hypoxia for 24 hours, then normoxia for 16 hours (H + N) or normoxia for 24 hours, then hypoxia for 16 hours (N + H). Cells were then incubated with TGF-α or IL-1β alone or in combination for the last 16 hours of treatment. In H + N, the lane labeled as C, corresponds to cells exposed to hypoxia for 24 hours followed by normoxia for 16 hours and no additional treatment. In N + H, the lane labeled as C, corresponds to cells exposed to 24 hours of normoxia. Lanes labeled H correspond to cultures maintained in normoxia for 24 hours followed by hypoxia for 16 hours.
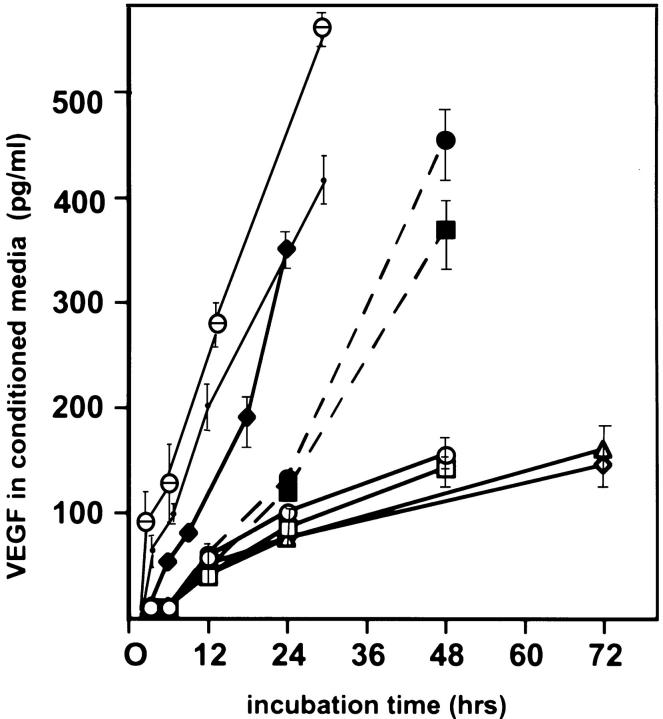
Conditioned media from in vitro experiments were also assayed for VEGF protein by ELISA to determine whether changes in transcription were followed by equivalent increases in protein levels (Figure 5) ▶ . VEGF detected by ELISA was normalized to total protein present in media. Hypoxia was associated with a 2.9-fold increase in VEGF protein more than normoxic controls at 48 hours. The addition of sex steroids did not significantly increase VEGF protein levels more than normoxic conditions, or augment the hypoxic induction in VEGF protein. Nonetheless, an impressive increase in protein was seen with the combination hypoxia, TGF-α, and IL-1β, confirming the data obtained by Northern analysis.
Figure 5.
VEGF secretion by cultured human endometrial stromal cells. VEGF secreted from stromal cells was detected by ELISA and normalized to total protein present in media. Aliquots were removed at 6, 12, 24, 36, and 72 hours. Culture conditions: open square, normoxia for 48 hours; open triangle, normoxia and incubation with estradiol (10 nmol/L) for 72 hours. Each time point was evaluated in six independent cultures isolated from six different patients. Bars indicate SE. Open diamond: normoxia and incubation with progesterone (10 μmol/L) for 72 hours. Open circle: normoxia and incubation with estradiol (10 nmol/L) plus progesterone (10 μmol/L) for 48 hours. Filled square: hypoxia (3%) exposure for 48 hours. Large filled circle: hypoxia (3%) and incubation with estradiol (10 nmol/L) plus progesterone (10 μmol/L) for 48 hours. Filled diamond: normoxia and incubation with TGF-α (50 ng/ml) for 24 hours. Small filled circle: normoxia in the presence of IL-1β (10 ng/ml) and TGF-α (50 ng/ml). θ, hypoxia (3%) in the presence of IL-1β (10 ng/ml) and TGF-α (50 ng/ml).
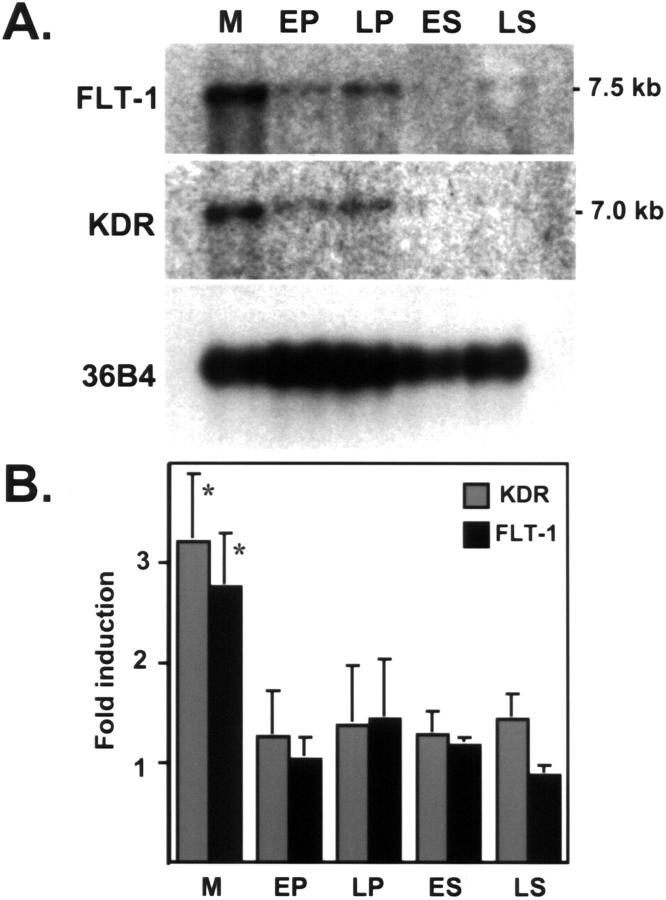
Because the VEGF-driven proliferative effects on endothelial cells are mediated by signaling via VEGF receptors, we evaluated transcript levels of FLT-1 and KDR throughout the menstrual cycle (Figure 6) ▶ . FLT-1 was increased by 2.8-fold (P < 0.01) and KDR by 3.2-fold (P < 0.01) in the menstrual as compared to other phases of the cycle. This pattern follows the one described earlier for VEGF (Figure 1) ▶ , yet these data did not provide further information as to the signaling status of these receptors.
Figure 6.
VEGF receptors are predominantly expressed during the menstrual phase. A: Northern blot of poly(A+)-enriched RNA, extracted from menstrual (M), early proliferative (EP), late proliferative (LP), early secretory (ES), and late secretory (LS) endometrium and hybridized to KDR and FLT-1 cDNA probes. B: Northern blots from independent biopsies (five per group) were scanned by densitometry and normalized to signal from 36B4 (ribosomal-associated protein). Values between groups are expressed as mean ± SD. *, M versus all other groups (P < 0.01).
To best assess the functional significance of VEGF and its receptors during the menstrual cycle, we examined levels of KDR protein by immunoprecipitation and performed phosphorylation studies to ascertain which portion of these receptors were actually functional/signaling (Figure 7) ▶ . KDR was increased in the menstrual phase and early in the proliferative phase (Figure 7A) ▶ . The peak during the menstrual phase was parallel to the high levels of VEGF. Consequently, we had anticipated a high phosphorylation state for this receptor during the menstrual phase. Interestingly, phosphorylation only peaked late in the menstrual phase with lower sustained phosphorylation levels during the proliferative phase (Figure 7A ▶ , PY antibody). The pattern was reproduced in all three independent samples evaluated. The reason for this lack of correlation between the peaks of KDR protein expression and phosphorylation levels implies an additional tier of regulation in receptor signaling. Because sFLT-1 has been implicated in the negative regulation of VEGF receptor pathway by binding to the growth factor and preventing interaction with its receptors, 12 we asked whether this mechanism of regulation might also be present in the human endometrium. Indeed, sFLT-1 was immunoprecipitated from endometrial samples (Figure 7B) ▶ . Major expression of sFLT-1 was detected in early menstrual specimens, but protein was also seen in the late secretory phase. The pattern of sFLT-1 expression provided a temporal correlation with the decrease of KDR phosphorylation in the early menstrual phase. Additional supporting data were obtained when anti-VEGF antibodies were used in early menstrual samples to immunoprecipitate the growth factor. Immunoprecipitated VEGF was bound to sFLT-1 (Figure 7C) ▶ . These results provided evidence that indeed during the early menstrual phase, at least some VEGF is bound to sFLT-1. This effect was transient, because sFLT-1 was not detected in late menstrual phase (Figure 7C ▶ , lane 2) and consistent with the low/undetectable levels of sFLT-1 in the late menstrual phase (Figure 7B) ▶ .
Figure 7.
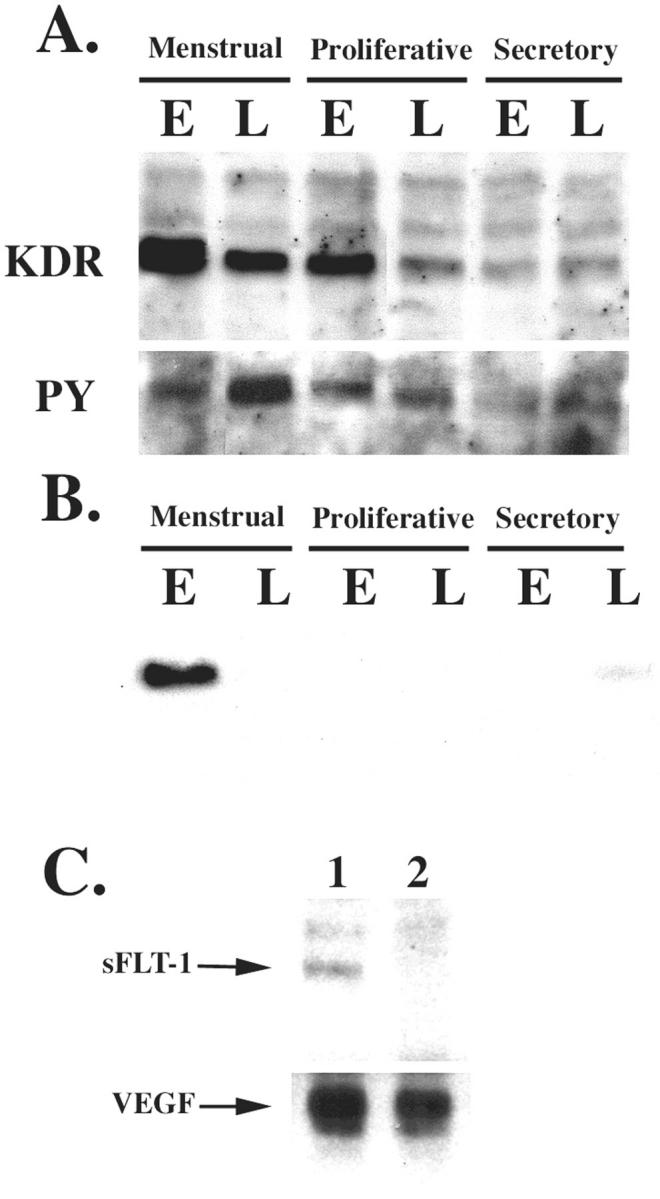
Phosphorylation of KDR is inversely correlated with levels of sFLT-1. A: Endometrial samples from all phases of the menstrual cycle (E, early; L, late) were lysed and purified to enrich the membrane fraction, as indicated in Materials and Methods. Samples were quantified and equal protein concentrations were immunoprecipitated with anti-KDR followed by immunoblotting with a monoclonal antibody against the same protein. After striping the membrane it was probed with anti-phosphotyrosine (PY) antibody. B: Endometrial samples were immunoprecipitated with anti-sFLT-1 receptor antibody followed by immunoblotting with an anti-Flt-1 monoclonal antibody. C: VEGF was immunoprecipitated with a chicken anti-mouse VEGF antibody from menstrual endometrial samples purified by heparin-affinity chromatography. The immunoprecipitated products were subsequently resolved on SDS-PAGE and the Western blot was incubated with anti-sFLT-1 or anti-VEGF (monoclonal antibody). Lane 1, Early menstrual sample; lane 2, late menstrual sample.
To further evaluate the biological relevance of KDR receptor phosphorylation we examined endometrial endothelial cell proliferation patterns during the endometrial cycle by dual immunocytochemistry. Vessels were detected with von Willebrand factor antibodies and proliferating cells were visualized with anti-Ki-67 (Figure 8) ▶ . The menstrual (day 3) biopsy seen in Figure 8, A and B ▶ , reveals a significant portion of viable, proliferating endothelial cells in the basal layer. Proliferation was also detected in the proliferative (Figure 8, C and D) ▶ and secretory phases (Figure 8E) ▶ . Quantification of proliferating endothelial cells revealed that these cells proliferate throughout the cycle, as previously shown, 9 nonetheless, detailed evaluation of the menstrual phase showed a statistically significant increase late in the menstrual phase and early in the proliferative phase (Figure 8E) ▶ .
Figure 8.
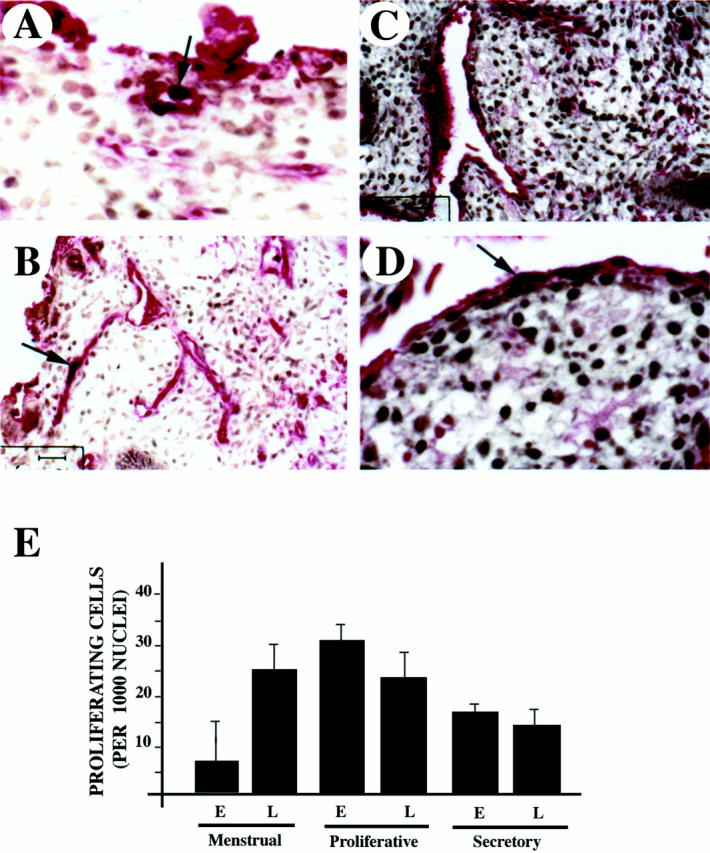
Proliferation of endothelial cells is initiated during the late menstrual phase. Capillaries were detected with anti-von Willebrand factor (in red) using an alkaline-phosphatase reaction and proliferating cells were detected with Ki67 antigen (in black) (light brown staining is background) in sections of menstrual (A and B) and early proliferative endometrium (C and D). Arrows indicate proliferating endothelial cells. E: Quantification of proliferating endothelial cells during early (E) and late (L) menstrual, proliferating and secretory phases. Six independent samples from each phase were used in the assessment. Numbers correspond to proliferating cells per 1,000 endothelial nuclei.
Discussion
The primate endometrium is a remarkable tissue for studying the regulation of angiogenesis. Cyclic patterns of rapid blood vessel growth are controlled in synchrony with stromal repair. However, little is known about the mediators of blood vessel growth and inhibition in this tissue. Among the causal suspects for induction of angiogenesis, VEGF has been a central focus of attention 13,14,16,17,28,33-35 , but previous studies have been unable to correlate the vascular reconstitution of the endometrium in the postmenstrual phase because the pattern of VEGF expression has been shown to peak in the secretory phase. 13 We hypothesized that the initial stimuli for angiogenesis most likely precedes the proliferative phase and therefore is initiated during menstruation, a phase in general not included in endometrial studies and not present in non-primate species. Here we demonstrate that, in fact, VEGF expression peaks during days 1 to 3 of the endometrial cycle. In addition, we provide evidence that physiological hypoxia in conjunction with TGF-α and IL-1β are most likely responsible for the induction and sustained levels of VEGF during menstruation. Finally we demonstrate that the KDR phosphorylation pattern does not follow the pattern of total receptor levels. Interestingly, phosphorylation of KDR is lower when sFLT-1 is present. We showed that this molecule can be found in association with VEGF in endometrial specimens providing a mechanism by which VEGF receptor phosphorylation might be regulated during the endometrial cycle.
Given the importance of oxygen tension as a regulator of blood vessel growth and development, 23,35 the contribution of physiological hypoxia associated with menstruation and its role in regulating endometrial angiogenesis has been poorly explored. Ischemic cellular stress leads to induction of hypoxia-inducible factor (HIF-1α) a transcription factor that regulates several genes, including VEGF. 23,36 Aside from an increase in transcription rates through HIF-1α, hypoxia also promotes increases in VEGF mRNA stability. 37 The overall effect is a sustained elevation in secreted VEGF protein with increased endothelial cell survival and proliferation, granted the appropriate receptors on the endothelial cell surface are expressed. Taking this background into consideration, it is not at all surprising that VEGF levels were raised in menstrual tissue. Our study shows that under hypoxic conditions endometrial stromal cells increase VEGF transcript levels. Our findings are in agreement with two recent studies that have examined the contribution of hypoxia to VEGF mRNA increase by endometrial cells. 38,39 However, alone, hypoxia could not account for the marked expression of VEGF seen in the menstrual phase. In fact, both our studies and the one reported previously 39 found no more than a 3.4-fold increase in VEGF levels in culture. Therefore contributing cytokines and/or hormones are likely to aid in the significant increase seen in the menstrual basal endometrium.
Two major cytokines have been implicated as potential regulators of VEGF expression in inflammatory states: TGF-α and IL-1β. In psoriasis, an inflammatory condition associated with overexpression of VEGF, the increased VEGF levels are most likely mediated by TGF-α, which has been shown to increase VEGF expression in epidermal keratinocytes in vitro. 40,41 IL-1β has been also shown to up-regulate VEGF mRNA in Kaposi’s sarcoma spindle cells 31 and to increase VEGF mRNA levels in aortic smooth muscle cells. 34 We sought out TGF-α and IL-β as candidate cytokines for VEGF regulation in human endometrium. TGF-α has been described in endometrium, with intense immunostaining noted around the spiral arterioles. 42 IL-1β protein and transcript have also been localized to the endothelium of the spiral arterioles. 43 Our studies indicated that both TGF-α and IL-1β regulate VEGF mRNA in human endometrial stromal cells. The combined effects of hypoxia, TGF-α, and IL-1β on VEGF mRNA in cultures of stromal cells were comparable with the increased levels of VEGF mRNA seen in the menstrual endometrium. Additional pro-inflammatory cytokines, in particular TNF-α and IFN-γ have also been examined for their ability to affect VEGF transcripts in endometrial stromal cells. These cytokines do not increase VEGF expression in stromal cultures (M. L. Iruela-Arispe, unpublished observations), 17 in fact, one study reported a decrease in VEGF transcripts after 6 hours of stromal cell exposure to IFN-γ. 44
In our endometrial stromal cultures, the positive contributions of estradiol and progesterone to VEGF up-regulation were modest, with maximal 1.6- to 1.8-fold increases, as has been reported by others. 13,28,29,45 Although VEGF up-regulation in the secretory phase may be explained by progesterone effects, it is unlikely sex steroids play a direct role on VEGF regulation during postmenstrual repair as circulating estrogen and progesterone levels are physiologically low at this point in the cycle.
Additional members of the VEGF cytokine family were constitutively expressed at low levels during the menstrual cycle and do not seem to be either hypoxia- or steroid-regulated. These data concur with previous findings that VEGF-B and VEGF-C were not increased by hypoxia in vitro in cell types other than fibroblasts. 46 Constitutive expression of additional VEGF family members may provide a positive angiogenic stimulus for the endometrium during all phases of the cycle. Hence, the potential for endometrial blood vessel growth is always present. 9,47 Regulation of endometrial angiogenesis may then occur through a rise in angiogenic inhibitors. We have previously shown that thrombospondin-1, an inhibitor of angiogenesis, is increased during the early secretory phase of the human endometrial cycle, and is regulated by progesterone in vitro. 21 It is possible that steroid contribution to the vascular repair in endometrium occurs predominantly through the regulation of inhibitory pathways, eg, via thrombospondin-1.
Relevant to our understanding of VEGF function during the endometrial cycle are also the patterns displayed by its receptors: FLT-1 and KDR. Our findings indicate that both receptors are present mostly during the menstrual and proliferative phases, decreasing thereafter. Others have reported presence of full length FLT-1 and KDR at “almost constant levels” throughout the endometrial cycle by RT-PCR, 48 however the menstrual phase was not part of these analyses. As for the distribution of VEGF receptors by immunocytochemistry, expression of FLT-1, in contrast to KDR, was observed primarily in dilated capillaries during the premenstrual period. 49 Interestingly, recent studies have co-localized KDR in stromal cells of the superficial endometrial zones during the premenstrual phase 50 and in epithelial cells. 49 The functional significance of this expression remains unclear. Considering that our Northern analysis included the entire endometrium, it is likely that we were also detecting receptor-expressing nonendothelial cells.
In addition, and perhaps more pertinent, to VEGF regulation are the levels of sFLT-1. The soluble form of FLT-1 was identified initially in the conditioned media of human umbilical vein endothelial cells. 51,52 This protein was shown to act as a dominant-negative effector by forming inactive heterodimers with transmembrane receptors and by sequestering VEGF. 53 sFLT-1 has been found in the conditioned media of tumor cells, endothelial cells, and in amniotic fluid. 54 More directly relevant to our findings, sFLT-1 has been found in placenta and in the serum of pregnant women indicating that regulation of VEGF by sFLT-1 is an important event in implantation and the maintenance of pregnancy. 55 A recent study has described the presence of sFLT-1 by RT-PCR in endometrium mostly during the secretory phase. 48 Our data agrees with these findings, at the protein level, but in addition we showed a significant increase during the early menstrual phase. More interestingly, we found a correlation between decreased KDR phosphorylation and presence of sFlt-1. Given the strong body of literature 12,53,55 implicating sFLT-1 in the sequestration of VEGF from its transmembrane receptors, it is tempting to speculate that expression of sFLT-1 during the menstrual phase might modulate KDR receptor signaling by retarding the wave of vascular repair. In addition, we found that VEGF is bound to sFLT-1 early in the menstrual phase. Consistent with these correlations, we found a small, yet statistically significant, peak of endothelial cell proliferation that coincides with time of KDR phosphorylation. Clearly further experimentation and additional mechanistic studies will be required to support this model.
Acknowledgments
We thank Xin-hua Lee for her technical assistance; and Kevin Claffey and Michael Detmar for their helpful input and advice.
Footnotes
Address reprint requests to Dr. Luisa Iruela-Arispe, Molecular Biology Institute, 611 Charles Young Drive East, Los Angeles, CA 90095. E-mail: arispe@mbi.ucla.edu.
Supported by the National Institutes of Health (grant R29C65624 and RO3CA70559-02) and by the Division of Reproductive Endocrinology, Department of Obstetrics and Gynecology, Beth Israel Deaconess Medical Center, Boston.
In memory of Joseph F. Mortola.
This work was presented in part at the 44th Annual Meeting of the Society for Gynecologic Investigation, San Diego, California, 1997.
Current address of M. D. Graubert: Palmetto Fertility Center of South Florida, Miami, FL 33016.
References
- 1.Reynolds LP, Killilea SD, Redmer DA: Angiogenesis in the female reproductive system. FASEB J 1992, 6:886-892 [PubMed] [Google Scholar]
- 2.Gordon JD, Shifren JL, Foulk RA, Taylor RN, Jaffe RB: Angiogenesis in the human female reproductive tract. Obstet Gynecol Surv 1995, 50:688-697 [DOI] [PubMed] [Google Scholar]
- 3.Kamat B, Brown LF, Manseau EJ, Senger DR, Dvorak HF: Expression of vascular permeability factor/vascular endothelial growth factor by human granulosa and theca lutein cells. Role in corpus luteum development. Am J Pathol 1995, 146:157-165 [PMC free article] [PubMed] [Google Scholar]
- 4.Goodger-Macpherson AM, Rogers PAW: Blood vessel growth in the endometrium. Microcirculation 1995, 4:329-343 [DOI] [PubMed] [Google Scholar]
- 5.Smith SK: Growth factors in human endometrium. Hum Reprod 1994, 9:936-946 [DOI] [PubMed] [Google Scholar]
- 6.Markee JE: Menstruation in intraocular endometrial transplants in the rhesus monkey. Contrib Embryol Carnegie Inst 1940, 28:219-308 [Google Scholar]
- 7.Ferenczy A, Gualnick M: Endometrial microstructure: structure-function relationships throughout the menstrual cycle. Semin Reprod Endocrinol 1983, 1:205-219 [Google Scholar]
- 8.Ludwig H, Metzger H: The repithelialization of endometrium after menstrual desquamation. Arch Gynecol 1973, 221:51-60 [DOI] [PubMed] [Google Scholar]
- 9.Rogers PAW, Abberton KM, Susil B: Endothelial cell migratory signal produced by human endometrium during the menstrual cycle. Hum Reprod 1992, 7:1061-1066 [DOI] [PubMed] [Google Scholar]
- 10.Tazuke SI, Giudice LC: Growth factors and cytokines in endometrium, embryonic development, and maternal: embryonic interactions. Semin Reprod Endocrinol 1996, 14:231-245 [DOI] [PubMed] [Google Scholar]
- 11.Charnock-Jones SD, Sharkey AM, Raiput-Williams J, Burch D, Schofield PJ, Fountain SA, Boocock CA, Smith SK: Identification and localization of alternatively spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod 1993, 48:1120-1128 [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Crisholm V, Hillan KJ, Schwall RH: Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med 1998, 4:336-340 [DOI] [PubMed] [Google Scholar]
- 13.Torry DS, Holt VJ, Keenan JA, Harris G, Caudle MR, Torry RJ: Vascular endothelial growth factor expression in cycling human endometrium. Fertil Steril 1996, 66:72-80 [PubMed] [Google Scholar]
- 14.Gargett CE, Lederman FL, Lau TM, Taylor NH, Rogers PA: Lack of correlation between vascular endothelial growth factor production and endothelial cell proliferation in the human endometrium. Hum Reprod 1999, 14:2080-2088 [DOI] [PubMed] [Google Scholar]
- 15.Unkila-Kallio L, Vuorela-Vepsalainen P, Tiitinen A, Halmesmaki E, Ylikorkala O: No cyclicity in serum vascular endothelial growth factor during normal menstrual cycle but significant luteal phase elevation during an in vitro fertilization program. Am J Reprod Immunol 2000, 43:25-30 [DOI] [PubMed] [Google Scholar]
- 16.Charnock-Jones DS, Macpherson AM, Archer DF, Leslie S, Makkink WK, Sharkey AM, Smith SK: The effect of progestins on vascular endothelial growth factor, oestrogen receptor and progesterone receptor immunoreactivity and endothelial cell density in human endometrium. Hum Reprod 2000, 15(Suppl. 3):85-95 [DOI] [PubMed] [Google Scholar]
- 17.Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, Taylor RN: Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod 2000, 15(Suppl. 3):67-77 [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Manzaneque JC, Graubert M, Iruela-Arispe ML: Vascular dysfunction by prolonged activation of progesterone receptor. Hum Reprod 2000, 15:39-47 [DOI] [PubMed] [Google Scholar]
- 19.Noyes RW, Hertig AT, Rock J: Dating the endometrial biopsy. Fertil Steril 1950, 1:23-27 [DOI] [PubMed] [Google Scholar]
- 20.Chomczynski P, Sacchi N: Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 21.Iruela-Arispe ML, Porter P, Bornstein P, Sage H: Thrombospondin-1, an inhibitor of angiogenesis, is regulated by progesterone in the human endometrium. J Clin Invest 1996, 97:403-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G: Endometrial endothelia cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation 1999, 6:127-140 [PubMed] [Google Scholar]
- 23.Maltepe E, Simon MC: Oxygen, genes, and development: an analysis of the role of hypoxic gene regulation during murine vascular development. J Mol Med 1998, 76:391-401 [DOI] [PubMed] [Google Scholar]
- 24.Brown DC, Gatter KC: Monoclonal antibody Ki-67: its use in histopathology. Histopathology 1990, 17:489-503 [DOI] [PubMed] [Google Scholar]
- 25.Pagano M, Gauvreau K: Analysis of variance. Principles of Biostatistics. 1993, :pp 257-263 Duxbury Press, Belmont [Google Scholar]
- 26.Brogi E, Wu T, Namiki A, Isner JM: Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 1994, 90:649-652 [DOI] [PubMed] [Google Scholar]
- 27.Claffey KP, Robinson GS: Regulation of VEGF/VPF expression in tumor cells: consequences for tumor growth and metastasis. Cancer Met Rev 1996, 15:165-176 [DOI] [PubMed] [Google Scholar]
- 28.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng GY, Ferrara N, Jaffe RB, Taylor RN: Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis for endometriosis. J Clin Endocrinol Metab 1996, 81:3112-3118 [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Rees MC, Bicknell R: The isolation and long-term culture of normal human endometrial epithelium and stroma. Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci 1995, 108:323-331 [DOI] [PubMed] [Google Scholar]
- 30.Detmar M, Yeo KT, Nagy J, Van De Water L, Brown LF, Berse B, Elicker BM, Ledbeter S, Dvorak HF: Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol 1995, 105:44-50 [DOI] [PubMed] [Google Scholar]
- 31.Cornali E, Zietz C, Benelli R, Weninger W, Masiello L, Bier G, Tschachler E, Albini A, Sturzl M: Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi’s sarcoma. Am J Pathol 1996, 149:151-169 [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller MD, Lebovic DI, Garrett E, Taylor RN: Neutrophils infiltrating the endometrium express vascular endothelial growth factor: potential role in endometrial angiogenesis. Fertil Steril 2000, 74:107-112 [DOI] [PubMed] [Google Scholar]
- 33.Cullinan-Bove K, Koos RD: Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinol 1993, 133:829-837 [DOI] [PubMed] [Google Scholar]
- 34.Li J, Perrella MA, Tsai JC, Yet SF, Hsieh CM, Yoshizumi M, Patterson C, Endege WO, Zhou F, Lee ME: Induction of vascular endothelial growth factor gene expression by interleukin-I beta in rat aortic smooth muscle cells. J Biol Chem 1995, 270:308-312 [DOI] [PubMed] [Google Scholar]
- 35.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843-845 [DOI] [PubMed] [Google Scholar]
- 36.Levy AP, Levy NS, Wegner S, Goldberg MA: Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 1995, 270:13333-13340 [DOI] [PubMed] [Google Scholar]
- 37.Claffey KP, Shih SC, Mullen A, Dziennis S, Cusick JL, Abrams KR, Lee SW, Detmar M: Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell 1998, 9:469-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popovici RM, Irwin JC, Giaccia AJ, Giudice LC: Hypoxia and cAMP stimulate vascular endothelial growth factor (VEGF) in human endometrial stromal cells: potential relevance to menstruation and endometrial regeneration. J Clin Endocrinol Metab 1999, 84:2245-2248 [DOI] [PubMed] [Google Scholar]
- 39.Sharkey AM, Day K, McPherson A, Malik S, Licence D, Smith SK, Charnock-Jones DS: Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab 2000, 85:402-409 [DOI] [PubMed] [Google Scholar]
- 40.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF: Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med 1994, 180:1141-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Detmar M, Yeo KT, Nagy JA, Van de Water L, Brown LF, Berse B, Elicker BM, Ledbetter S, Dvorak HF: Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol 1995, 105:44-50 [DOI] [PubMed] [Google Scholar]
- 42.Horowitz GM, Scott RT, Drews MR, Navot D, Hofmann GE: Immunohistochemical localization of transforming growth factor-alpha in human endometrium, decidua, and trophoblast. J Clin Endocrinol Metab 1993, 76:786-792 [DOI] [PubMed] [Google Scholar]
- 43.Simon C, Piquette GN, Frances A, el-Danasouri I, Irwin JC, Polan ML: Localization of interleukin type I receptor and interleukin-I b in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab 1993, 77:549-555 [DOI] [PubMed] [Google Scholar]
- 44.Kawano Y, Matsui N, Kamihigashi S, Narahara H, Miyakawa I: Effects of interferon-gamma on secretion of vascular endothelial growth factor by endometrial stromal cells. Am J Reprod Immunol 2000, 43:47-52 [DOI] [PubMed] [Google Scholar]
- 45.Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E: Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest 1993, 91:2235-2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enholm B, Paavonen K, Ristimaki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K: Comparison of VEGF, VEGF-B, VEGF-C, and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14:2475-2483 [DOI] [PubMed] [Google Scholar]
- 47.Rogers PA, Lederman F, Taylor N: Endometrial microvascular growth in normal and dysfunctional states. Hum Reprod Update 1998, 4:503-508 [DOI] [PubMed] [Google Scholar]
- 48.Krussel JS, Casan EM, Raga F, Hirchenhain J, Wen Y, Huang HY, Bielfeld P, Polan ML: Expression of mRNA for vascular endothelial growth factor transmembraneous receptors Flt1 and KDR, and the soluble receptor sflt in cycling human endometrium. Mol Hum Reprod 1999, 5:452-458 [DOI] [PubMed] [Google Scholar]
- 49.Meduri G, Bausero P, Perrot-Applanat M: Expression of vascular endothelial growth factor receptors in the human endometrium: modulation during the menstrual cycle. Biol Reprod 2000, 62:439-447 [DOI] [PubMed] [Google Scholar]
- 50.Nayak NR, Critchley HO, Slayden OD, Menrad A, Chwalisz K, Baird DT, Brenner RM: Progesterone withdrawal up-regulates vascular endothelial growth factor receptor type 2 in the superficial zone stroma of the human and macaque endometrium: potential relevance to menstruation. J Clin Endocrinol Metab 2000, 85:3442-3452 [DOI] [PubMed] [Google Scholar]
- 51.Kendall RL, Thomas KA: Protein, nucleotide inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993, 90:10705-10709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendall RL, Wang G, Thomas KA: Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 1996, 226:324-328 [DOI] [PubMed] [Google Scholar]
- 53.Goldman CK, Kendall RL, Cabrera G, Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ, Huckle WR, Thomas KA, Curiel DT: Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastatis, and mortality rate. Proc Natl Acad Sci USA 1998, 95:8795-8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horning C, Barleo B, Ahmad S, Vuorela P, Ahmed A, Weich HA: Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest 2000, 80:443-454 [DOI] [PubMed] [Google Scholar]
- 55.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones S: A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998, 59:1540-1548 [DOI] [PubMed] [Google Scholar]