Abstract
Hyperparathyroidism may result from parathyroid hyperplasia or adenoma, or rarely from parathyroid carcinoma. Pericentromeric inversion of chromosome 11 that results in activation of the PRAD1/cyclin D1 gene and tumor suppressor gene loss have been described as genetic abnormalities in the evolution of parathyroid neoplasms. We studied tissue samples taken from primary parathyroid hyperplasia, parathyroid adenoma, and histologically normal parathyroid tissue by comparative genomic hybridization, fluorescent in situ hybridization, and immunohistochemistry for cyclin D1. DNA copy number changes were infrequent in primary hyperplasia (4 of 24, 17%), but common in adenomas (10 of 16, 63%; P = 0.0059). The most common change was deletion of the entire chromosome 11 or a part of it, with a minimal common region at 11q23. This change was present in five (31%) adenomas and two (8%) primary hyperplasias. Fluorescent in situ hybridization confirmed the presence of both MEN1 alleles located at 11q13 despite deletion of 11q23 in all three cases studied. Cyclin D1 was overexpressed in six (40%) of the 15 adenomas studied, whereas none of the 27 hyperplasias (P = 0.0010) nor the five histologically normal tissue samples overexpressed cyclin D1. Either DNA copy number loss or cyclin D1 overexpression was present in 13 (81%) of the 16 adenomas. We conclude that DNA copy number loss and cyclin D1 overexpression are common in parathyroid adenomas. The region 11q23 is frequently lost in parathyroid adenomas and occasionally in parathyroid hyperplasias, and this suggests the possibility that a tumor suppressor gene that is important in their pathogenesis is present on 11q23.
Hyperparathyroidism may be caused by parathyroid gland hyperplasia or adenoma, or very rarely by parathyroid carcinoma. The clinical manifestations of hyperparathyroidism vary, but the patient may present with recurrent nephrolithiasis, mental changes, peptic ulcers, and sometimes with extensive bone resorption. The annual incidence is estimated to be 0.03% in patients older than 60, but the estimated prevalence including undiscovered asymptomatic patients is 1 to 2% or higher. The diagnosis is made primarily on clinical grounds and presence of elevated serum parathyroid hormone, which is usually associated with elevated serum calcium levels and urine calcium excretion. Primary hyperparathyroidism is usually caused by a single parathyroid adenoma, but multiglandular parathyroid hyperplasia accounts for ∼10 to 15% of primary hyperparathyroidism. 1 Secondary hyperparathyroidism occurs in patients with chronic renal failure, and is usually associated with multiglandular parathyroid hyperplasia. Primary hyperparathyroidism can be cured by surgery in >90% of cases, but it is important to know for surgical decision-making whether an abnormal parathyroid represents a single adenoma, or whether hyperplasia affecting the parathyroids is present.
Histological distinction between hyperplastic and adenomatous parathyroid glands is difficult, and there are no specific abnormalities that distinguish between hyperplastic and adenomatous glands. 2 Hyperplasia is generally considered as a nonneoplastic condition, whereas adenomas are neoplasms. However, these two entities may not be distinctly different, and at least some adenomas might have their origin in antecedent parathyroid hyperplasia, and develop from the latter via a series of somatic mutations. Clonal analyses have suggested that in renal hyperparathyroidism parathyroid glands initially grow diffusely and polyclonally, after which the foci of nodular hyperplasia are transformed to monoclonal neoplasia. 3 Monoclonality has been found also in a minority of primary parathyroid hyperplasias by X-chromosome inactivation analysis. 4
Several genes and chromosomal changes seem to be involved in the molecular pathogenesis of parathyroid adenomas. The cyclin D1/PRAD1 oncogene, 5-7 and the MEN1 tumor suppressor gene on chromosome 11q13 8,9 have been reported to be involved in the pathogenesis of some parathyroid adenomas. Cyclin D1 is also located at 11q13, and in a subset of parathyroid adenomas pericentromeric inversion of chromosome 11 places the parathyroid hormone (PTH) gene transcriptional regulatory sequences on 11p15 immediately upstream of the cyclin D1 proto-oncogene promoter and its five exons. This rearrangement results in unregulated or inappropriate expression of cyclin D1 that has an important role in the control of cell cycle progression through the G1/S checkpoint. Loss or inactivation of both copies of the tumor suppressor gene MEN1 contributes to the genesis of parathyroid neoplasms in the familial MEN1 syndrome, but allelic losses at 11q13 have been observed in up to 40% of parathyroid adenomas suggesting an important role for MEN1 in molecular evolution of sporadic parathyroid adenomas. 9-11 In addition, loss of heterozygosity on chromosome arms 1p, 6q, 11p, and 15q have been found in ∼30%, and loss of chromosome 3q markers in ∼10% of parathyroid adenomas. 12-15 Allelic loss of chromosome 13, including the tumor suppressor gene RB1, has been detected in 16 to 30% of parathyroid adenomas, and even more frequently in carcinomas. 15-19 DNA alterations involving the parathyroid hormone gene PTH locus on 11p15 may also be important in the tumorigenesis or clonal evolution of some parathyroid adenomas. 20
Comparative genomic hybridization (CGH) has recently been used to identify chromosomal changes in parathyroid adenomas. These studies, as well as conventional cytogenetics analysis, indicate losses on many chromosomes including 1, 3, 6, 9, 11, 13, 15, 17, 18, 19, and 22. Loss of 11q has been particularly common with a frequency of 34 to 50% in parathyroid adenomas studied by CGH. 19,21,22 To the best of our knowledge, no similar data on parathyroid hyperplasias is available, and none of the studies have compared the DNA profiles of parathyroid hyperplasias and adenomas by CGH. In the present study we compared DNA copy number changes in parathyroid hyperplasias and adenomas with CGH. Because pericentromeric inversion of chromosome 11 contributing to overexpression of cyclin D1 is unlikely to result in a large DNA copy number change that could be detected by CGH, we assessed cyclin D1 overexpression by immunohistochemistry.
Materials and Methods
Patients
The series is based on 47 patients who underwent parathyroid surgery at the Department of Surgery, Helsinki University Central Hospital, Helsinki, Finland. Eleven were male, and the median age was 61 years (range, 29 to 90 years). Sixteen patients had primary parathyroid hyperplasia, four patients had MEN1 syndrome, three had secondary hyperplasia related to chronic renal failure, and three had uremic parathyroid hyperplasia that had become refractory to medical treatment (tertiary hyperplasia). The patients with secondary hyperplasia because of renal disease had been subjected to parathyroid surgery to make the disease less difficult to control by medical therapy (n = 2) or the parathyroids were removed in conjunction with thyroid surgery (n = 1). Sixteen further patients had been diagnosed with parathyroid adenoma (Table 1) ▶ . In addition, we studied five cases in which the excised sample contained histopathologically normal parathyroid tissue only. One parathyroid gland was examined by CGH in each of the 47 patients except for five patients with hyperplasia, in which two to four glands were examined (cases 2, 3, 5, 7, and 8; Table 1 ▶ ). A total of 34 hyperplastic parathyroids from 26 patients were investigated, and the total number of parathyroid glands studied by CGH in the entire series was 55.
Table 1.
Clinical Characteristics, CGH Findings, and Cyclin D1 Overexpression
| No. | Age/sex | Histology | Parathyroid gland weight (mg) | Serum Ca (mmol/L)* | Serum PTH (ng/L)† | CGH results | Cyclin D1 overexpression | |
|---|---|---|---|---|---|---|---|---|
| 1 | 61 /F | Hyperplasia | 250 | 2.79 | 121 | Normal | No | |
| 55 | - | - | ||||||
| 2a | 56 /M | Hyperplasia | 400 | 3.25 | 181 | −6q14−qter, −11q14−q23.1,+19 | No | |
| 2b | Hyperplasia | 200 | Normal | No | ||||
| 2c | Hyperplasia | - | Normal | - | ||||
| 2d | Hyperplasia | 100 | Normal | - | ||||
| 3a | 90 /F | Hyperplasia | 950 | 2.73 | 180 | Normal | No | |
| 3b | Hyperplasia | 200 | Normal | - | ||||
| 4 | 79 /F | Hyperplasia | 800 | 2.85 | 88 | Normal | No | |
| 250 | - | - | ||||||
| 5a | 64 /F | Hyperplasia | 180 | - | 100 | Normal | No | |
| 5b | Hyperplasia | - | Normal | - | ||||
| 6 | 42 /M | Hyperplasia, water-clear cell | 1275 | 3.50 | 478 | Normal | No | |
| 7a | 76 /M | Hyperplasia | 250 | 3.29 | 650 | −4q25−q30,−13 | No | |
| 7b | Hyperplasia | 8600 | −13 | - | ||||
| 7c | Hyperplasia | 250 | Normal | - | ||||
| 8a | 64 /F | Hyperplasia | 1400 | 2.95 | 214 | Normal | No | |
| 8b | 400 | Normal | No | |||||
| 9 | 51 /F | Hyperplasia | - | 2.51 | 29 | Normal | No | |
| 10 | 61 /F | Hyperplasia | 600,200 | 2.64 | 77 | Normal | No | |
| - | - | |||||||
| 11 | 70 /F | Hyperplasia | 300 | 2.74 | 80 | Normal | No | |
| 550 | - | - | ||||||
| 12 | 77 /F | Hyperplasia | 1200 | 3.18 | 237 | Normal | No | |
| 100 | - | - | ||||||
| 50 | - | - | ||||||
| 13 | 78 /F | Hyperplasia | 100 | 2.71 | 104 | Normal | No | |
| 70 | - | - | ||||||
| 300 | - | - | ||||||
| 14 | 70 /F | Hyperplasia | 500 | 2.71 | 122 | Normal | No | |
| 100 | - | - | ||||||
| 15 | 44 /F | Hyperplasia | 200 | 2.98 | 200 | Normal | No | |
| 16 | 41/F | Hyperplasia | 1000,50 | 2.63 | 56 | −11 | No | |
| 17 | 68 /F | Adenoma | 550 | 3.00 | 116 | −4q22−qter, −6q16−qter,+12,−15p−q26 | Yes | |
| 18 | 64 /F | Adenoma, atypical | - | - | - | +7/q21−q35,+8/p12−p22,q21.2−qter, −9,−15,−18,+19,+20,−21 | No | |
| 19 | 52 /M | Adenoma | 5100 | 2.63 | - | Normal | Yes | |
| 20 | 50 /F | Adenoma | 150 | 2.59 | 107 | Normal | No | |
| 21 | 37 /F | Adenoma | 600 | 2.60 | 84 | Normal | Yes | |
| 22 | 63 /F | Adenoma | 550 | 3.12 | 130 | Normal | Yes | |
| 23 | 70 /F | Adenoma | 400 | 2.68 | 89 | Normal | No | |
| 24 | 63 /F | Adenoma | 1000 | 2.75 | 62 | −6 | No | |
| 25 | 61 /F | Adenoma | 900 | - | 131 | +5,−6,+7 | No | |
| 26 | 65 /F | Adenoma | 350 | 2.71 | 91 | +16 | Yes | |
| 27 | 65 /F | Adenoma | 1200 | 2.61 | 111 | −11 | No | |
| 28 | 72 /F | Adenoma | 1050 | 2.90 | 107 | −X,−11p,−11q14−qter, −13 | No | |
| 29 | 58 /M | Adenoma | 400 | 2.77 | 109 | −11q22−q23, −13q21−qter | Yes | |
| 30 | 61 /M | Adenoma | 250 | - | 81 | Normal | No | |
| 31 | 68 /F | Adenoma | 1800 | 3.14 | 230 | +7,−11,−13 | - | |
| 32 | 49 /F | Adenoma | 900 | 2.69 | 128 | −11,−18 | No | |
| 33- | 61 /F | Secondary hyperplasia | Normal | No | ||||
| 35 | 29 /F | Secondary hyperplasia | Normal | - | ||||
| 33 /F | Secondary hyperplasia | Normal | No | |||||
| 36- | 38 /F | Tertiary hyperplasia | Normal | No | ||||
| 38 | 63 /F | Tertiary hyperplasia | −11 | No | ||||
| 59 /M | Tertiary hyperplasia | −4q13.1−qter, −6q22.3−q24,−13q21.3−q31.3 | No | |||||
| 39- | 50 /F | MENI-related hyperplasia | −11,+12q | No | ||||
| 42 | 38 /M | MENI-related hyperplasia | −11 | No | ||||
| 39 /M | MENI-related hyperplasia | −11 | No | |||||
| 52 /F | MENI-related hyperplasia | −11, −13q22.3−q31.1,−15q12−qter,−18,−X | No | |||||
| 43- | 59 /F | Normal | Normal | No | ||||
| 47 | 72 /F | Normal | Normal | No | ||||
| 35 /M | Normal | Normal | No | |||||
| 49 /F | Normal | Normal | No | |||||
| 35 /F | Normal | Normal | No | |||||
*Normal reference range, 2.25 to 2.65 mmol/L.
†Normal reference range, 15 to 60 ng/L.
Histopathology
All original histological sections were reexamined (KF). The differential diagnosis between adenoma and hyperplasia was based on the following criteria: 1) parathyroid adenoma was diagnosed when there was an encapsulated tumorous parathyroid lesion with no fat cells. Outside the lesion capsule areas of normal appearing parathyroid tissue with fat cells was seen in all cases. 2) Parathyroid hyperplasia, in turn, was diagnosed when at least two enlarged parathyroid glands were present with no normal parathyroid tissue identified outside the capsule of the lesion. In the lesion, either diffuse proliferation of enlarged parathyroid chief cells with no fat cells or nodular proliferation of chief cells sometimes with some fat cells between the nodules was seen. Two MEN1-related hyperplasias were classified as nodular and the rest as diffuse. All lesions with hyperplasia represented chief cell hyperplasia except for one case (case 6, Table 1 ▶ ), which was a water-clear cell hyperplasia.
DNA Isolation and CGH
Standard methods were used to extract genomic DNA from frozen tumor tissue or paraffin-embedded tissue (test DNA), and from the peripheral blood of a healthy male or female (normal reference DNA). 23,24 CGH using directly fluorochrome-conjugated nucleotides was performed according to the protocol by Kallioniemi and colleagues, 25 modified by us as described elsewhere. 26 Briefly, 1 μg of tumor DNA was labeled with fluorescein isothiocyanate-dUTP and fluorescein isothiocyanate-dCTP (1:1; Dupont, Boston, MA), and 1 μg of normal DNA was labeled with Texas-red-dUTP and Texas-red-dCTP (1:1, Dupont) in a standard nick-translation reaction. Equal amounts of labeled test and reference DNA were hybridized to normal metaphase spreads. The slides were counterstained with 4′, 6-diamidino-2-phenylindole (Sigma, St. Louis, MO) for the identification of the chromosomes.
In three cases in which histologically normal parathyroid tissue was studied the starting material did not contain enough DNA for CGH (cases 44, 46, and 47). In these cases we amplified the genomic DNA by using degenerate oligonucleotide-primed PCR as described. 27
The results were analyzed using an Olympus fluorescence microscope and an ISIS digital image analysis system (MetaSystems GmbH, Altlussheim, Germany). Three-color images (green for tumor DNA, red for normal reference DNA, and blue for DNA counterstain) were acquired from 8 to 10 metaphases per sample. Green-to-red ratio profiles along the chromosome axis were displayed. Chromosomal regions with a green-to-red ratio exceeding 1.17 were considered to be overrepresented (gains), whereas regions with a ratio below 0.85 were considered underepresented (losses). These values were set on the basis of the results of negative control experiments, in which two differently labeled normal DNAs were hybridized together. In the negative controls, the ratios varied within these limits. Reverse-labeling CGH was performed on selected cases (cases 18, 26, and 27; Table 1 ▶ ), which confirmed the alterations detected by the standard technique. 28 The findings were confirmed using a confidence interval of 99%. The cut-off level for high-level amplification was 1.5. Heterochromatic areas, the short arm of the acrocentric chromosomes and chromosome Y were discarded from the analysis.
Nuclei Extraction and Fluorescence in Situ Hybridization (FISH)
FISH was performed to confirm the CGH results. The nuclei from paraffin-embedded tissue were extracted as described elsewhere 29,30 with slight modifications. Briefly, four 30-μm sections were deparaffinized and incubated in 1 ml of Carlsberg’s solution (0.1% Sigma protease XXIV, 0.1 mol/L Tris, 0.07 mol/L NaCl, pH 7.2) for 1 hour at 37°C and vortexed vigorously for 20 minutes. The nuclear suspension was filtered through a nylon mesh (pore size, 55 μm), centrifuged, and diluted in 0.1 mol/L Tris. Following this the nuclei suspension was spread on slides and checked microscopically.
Yeast artificial chromosome (YAC) clone 755b11 obtained from the Center d’Etude Polymorphisme d’Humain (CEPH, Paris, France) was used to detect deletion of chromosome 11q23.1 by FISH. Four cases that had 11q deletions by CGH (cases 2a, and 27–29; Table 1 ▶ ) and 14 cases without 11q deletion (cases 1, 2b, 3a, 4, 17, 19–26, and 30) were investigated. The YAC probe 953e4, which hybridizes to 11p13, was used as a control for hybridization efficiency and for evaluation of the chromosome copy number in these experiments. A MEN1 gene-specific cosmid clone c10B11 (a kind gift from Dr. D. Gisselsson, Department of Clinical Genetics, University Hospital, Lund, Sweden) was used in 9 cases (cases 2a, 3a, 4, 17, 19, 21, 22, 28, and 29) to determine the MEN1 gene copy number. 31
All probes were labeled with biotin-14-dATP (Life Technologies, Inc., Gaithersburg, MD) by nick-translation, precipitated with herring sperm DNA (0.62 μg/μl, Sigma) and human Cot-1 DNA (0.62 μg/μl, Life Technologies, Inc.), and dissolved in a hybridization buffer containing 50% formamide, 20% dextran sulfate, and 2× standard saline citrate (SSC). To allow for penetration of the probe, the slides were treated in 1 mol/L sodium thiocyanate at 70°C for 15 minutes, followed by treatment in 0.05 N HCl at 37°C for 10 minutes, and by 5 mg/ml pepsin in 0.05 N HCl at 37°C for 20 minutes. The slides were then dehydrated in a rising alcohol series (70, 85, and 100%) and denatured in 70% formamide/2× SSC, pH 7, at 75°C for 5 minutes, followed by dehydration in a cold alcohol series. The probes were denatured at 75°C for 5 minutes and applied onto the slides. Hybridizations were performed at 37°C for 2 days. Posthybridization washes were performed at 45°C in 50% formamide, three times in 2× SSC, pH 7.0, for 5 minutes each; once in 2× SSC, pH 7.0, for 5 minutes; twice in 0.1× SSC, pH 7.0, for 5 minutes each; and finally in 4× SSC, 0.2% Tween, pH 7.0 (Sigma), at room temperature for 5 minutes. For detection of signals, fluorescein isothiocyanate-conjugated avidin (Vector Laboratories Inc., Burlingame, CA) was used. The signals were then further amplified with anti-avidin D/avidin-fluorescein isothiocyanate (Vector Laboratories Inc.). Finally, slides were counterstained with diamidino-2-phenylindole (Sigma), and mounted with an anti-fade solution (Vector Laboratories Inc.). From each preparation a minimum of 100 morphologically intact and nonoverlapping nuclei were scored using a Leitz fluorescence microscope (Laborlux D, Germany).
Immunohistochemistry
Immunohistochemistry for cyclin D1 was performed on deparaffinized 3-μm sections. Deparaffination was done at room temperature with Autostainer XL version 1.10 (Leica, Nussloch, Germany). The procedure involves treatment with xylene 2 × 7 minutes, a descending alcohol series (100% for 2 minutes, 100% for 1 minute, 94% for 30 seconds, 50% for 30 seconds, and aqua for 30 seconds). Antigen demasking was performed by heating the samples in a microwave oven in 1 mmol/L of ethylenediaminetetraacetic acid buffer, pH 8.0, 2 × 5 minutes with 1000 W and 5 minutes with 700 W. Methanol-peroxidase (1.6%) was used to inhibit endogenous peroxidase activity. For immunohistochemistry, the specimens were incubated overnight at room temperature with a 1:100 diluted mouse monoclonal antibody for human cyclin D1 (Novocastra Laboratories Ltd., Newcastle, UK). A peroxidase-conjugated secondary antibody was used to detect binding of the primary antibody using the Vectastain Elite ABC kit (Vector Laboratories, Inc.). The sections were counterstained with hematoxylin. A positive control with cyclin D1 expression was included in each experiment. Overexpression of cyclin D1 was considered to be present if ∼40% or more of the sample cells showed nuclear immunopositivity. In cases classified as negative, the proportion of the stained cells never exceeded 10%.
Statistical Analysis
Frequency tables were analyzed with Fisher’s exact test. Differences between two groups in parathyroid gland weight, serum parathyroid hormone levels, and serum calcium levels were compared using Mann-Whitney’s U test because of nonnormal distributions. All P values are two-tailed.
Results
DNA Copy Number Changes Detected by CGH
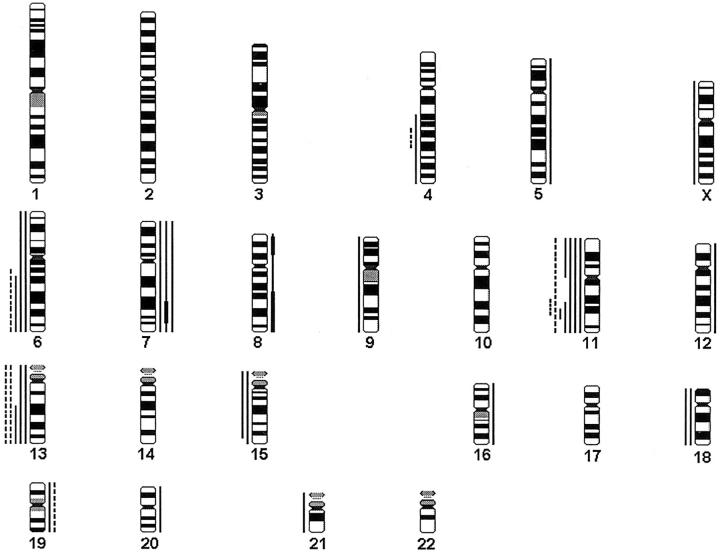
A summary of gains and losses detected by CGH is shown in Table 1 ▶ . DNA copy number changes were more frequent in adenomas (10 of 16, 63%) than in primary hyperplasias (4 of 24, 17%; P = 0.0059). In hyperplasias and adenomas the changes were clearly associated with particular chromosomes (Figure 1) ▶ .
Figure 1.
A summary of gains and losses found in parathyroid hyperplasias and adenomas. Gains are shown on the right side of the chromosomes and losses on the left. Each line represents a genetic aberration seen in one tumor. Primary hyperplasias are marked with dashed lines and adenomas with solid lines. High-level amplifications are marked with a thick bar.
In primary parathyroid hyperplasias six of the seven changes detected were losses, and they were found in four different chromosomes only, 4, 6, 11, and 13. Losses dominated also in tertiary and MEN1-associated hyperplasias. Loss of chromosome 11 or a part of it (11q14-q23.1) was found in two (8%) of the 24 glands with primary hyperplasia and in one of the three glands with tertiary hyperplasia. Loss of chromosome 11 was present in all four MEN1-related cases. Loss of chromosome 13 was found in two glands of one patient with primary hyperplasia, and in one tertiary and one with MEN1-related hyperplasia. Other losses found were losses of 4q (one primary and one tertiary hyperplasia), 6q (one primary and one tertiary hyperplasia), and 15q, 18, and X (in one MEN1-related hyperplasia). Gain of chromosome 12q (1 MEN1-associated hyperplasia) and 19 (one primary hyperplasia) were also detected.
Two to four glands of the same patient were studied by CGH in five cases with primary hyperplasia (cases 2, 3, 5, 7, and 8, Table 1 ▶ ), and in three of these five patients no DNA copy number changes were present in any of the glands. However, in one case (case 2) deletions of 6q14-qter and 11q14-q23.1 and a gain of chromosome 19 were found in one of the four glands investigated, whereas no DNA copy number changes were present in the three other glands. In another case (case 7) deletion of 4q25-q30 and loss of chromosome 13 were found in one gland, a loss of chromosome 13 in another gland, but no changes were found in the third gland investigated.
We found as many as 29 DNA copy number changes in the 16 adenomas investigated. As in hyperplasias, losses (n = 20, 69%) were more common than gains (n = 9, 31%). Chromosome 11q or a part of it was the most common loss (5 of 16, 31%) with the minimum common deleted region at 11q22-q23. Other frequent losses were detected at chromosomes 13q and 6q (n = 3 for each, 19%), 15 and 18 (n = 2 for each, 13%). The most frequent gain was gain of chromosome 7 (n = 3). In one case (case 18) high-level amplifications were found at 7q21-q35, 8p12-p22, and 8q21.2-qter.
None of the five histologically normal parathyroid glands had any DNA copy number changes by CGH.
FISH Results
We investigated 18 parathyroid lesions by FISH for the presence of 11q23 deletion or amplification to confirm the results obtained with CGH. In all four cases with a deletion of 11q in a CGH analysis (cases 2a, 27, 28. and 29; Table 1 ▶ ), only one hybridization signal was obtained one in >75% of the cells with YAC 755b11 (1.6 Mb) that hybridizes to 11q23. Two of these cases (cases 27 and 28) with a deletion of also 11p in CGH showed only one hybridization signal for YAC 953e4 that hybridizes to 11p13. In the rest of the cases that consisted of four hyperplasias (cases 1, 2b, 3a, and 4) and 10 adenomas (cases 17, 19–26, and 30) and with no detectable changes in chromosome 11 in CGH analyses, two signals for both YAC 755b11 and 953e4 were seen in >80% of the cells.
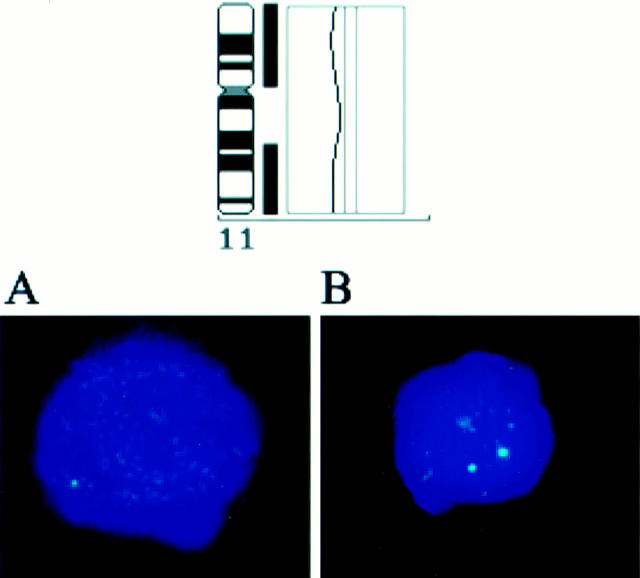
We also analyzed nine lesions that did not have deletion of 11q13 in CGH with FISH to investigate the copy numbers of the MEN1 gene. In all nine cases two hybridization signals were present in >85% of the cells. Although 11q23 was deleted in three of these cases (cases 2a, 28, and 29) by both CGH and FISH, both alleles of MEN1 were present (Figure 2) ▶ . Hence, the results obtained by FISH were fully concordant with those of CGH.
Figure 2.
A CGH profile from a patient with parathyroid adenoma (case 28, Table 1 ▶ ) showing a deletions of −11p and −11q14-qter. A: FISH of the same case demonstrating deletion of 11q23.1 (only one green signal obtained using Yac 755b11). B: No deletion of 11q13 is present (location of the MEN1 gene, two green signals obtained with cosmid c10B11). Diamidino-2-phenylindole blue counterstaining.
Associations between Serum Calcium and Parathyroid Hormone Levels, Gland Weight, and DNA Copy Number Changes
There was no difference in the parathyroid gland weight, serum calcium levels, or serum parathormone levels measured at diagnosis between primary parathyroid hyperplasia patients with a DNA copy number change and those without such a change (P = 0.23, 0.47, and 0.64, respectively). Similarly, no difference in gland weight or serum calcium or parathormone levels was found among parathyroid adenoma patients between those with a DNA copy number change and those without (P = 0.19, 0.11, and 0.18, respectively).
Cyclin D1 Expression
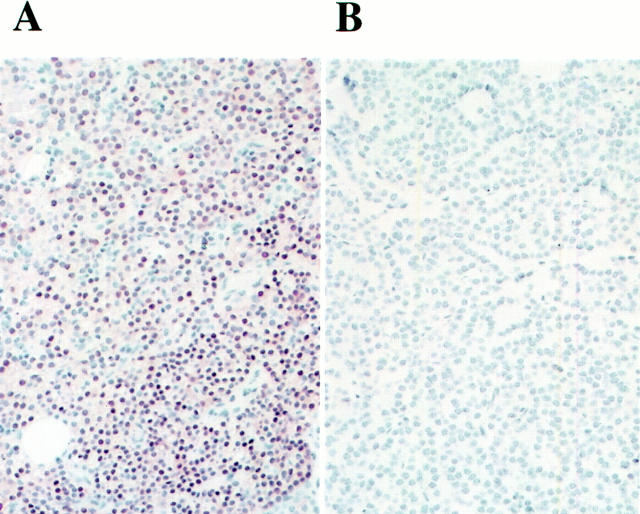
Forty-seven glands were immunostained for cyclin D1 (Table 1) ▶ . None of the 27 hyperplasias studied overexpressed cyclin D1, whereas cyclin D1 immunostaining was positive in six (40%) out of the 15 adenomas studied (P = 0.0010, Figure 3 ▶ ). The samples that contained histologically normal parathyroid tissue (n = 5) did not overexpress cyclin D1. Either chromosomal deletion or cyclin D1 overexpression was present in 13 (81%) of the 16 adenomas, and either deletion of 11q23 or cyclin D1 overexpression was present in 10 (63%) adenomas (Table 1) ▶ .
Figure 3.
Immunostaining for cyclin D1. A: Adenoma overexpressing cyclin D1 (case 26, Table 1 ▶ ). B: Cyclin D1-negative hyperplasia (case 7a). Original magnification, ×300.
Only one of the five adenomas with 11q deletion showed positive staining for cyclin D1 (case 29). However, the cyclin D1/PRAD1 oncogene is located on 11q13 and the 11q deletion in case 29 was located at 11q22-q23, so none of the cyclin D1-overexpressing adenomas showed loss of 11q13. There was no difference in serum calcium or parathyroid hormone concentrations between patients with cyclin D1 overexpressing adenoma and those with cyclin D1-negative parathyroid adenoma (P = 0.34 and 0.69, respectively), and no difference in parathyroid gland weight was found between these two groups (P = 0.75).
Discussion
In the present series DNA copy number changes were relatively infrequent in primary parathyroid hyperplasias (17%) as compared with parathyroid adenomas (63%). However, DNA copy number changes were seen in same chromosomes in adenomas and hyperplasias, notably in chromosomes 11, 6, and 13. We found a clear-cut difference between hyperplasias and adenomas in cyclin D overexpression that was present in 40% of adenomas but in none of the hyperplasias. Frequent overexpression of cyclin D1 in adenomas suggests that pericentromeric inversion of chromosome 11, which places the PTH gene transcriptional regulatory sequences adjacent to the cyclin D1 proto-oncogene resulting in inappropriate cyclin D1 overexpression and enhanced cell proliferation, is common in parathyroid adenomas unlike hyperplasias. Of note, either DNA copy number deletion or cyclin D1 overexpression was present in as many as 81% of adenomas, suggesting that the pericentromeric inversion of chromosome 11, which cannot be detected by CGH, and suppressor gene loss are important mechanisms in the molecular pathogenesis of parathyroid adenomas. Although pericentromeric inversion and suppressor gene loss are different molecular genetic mechanisms, they might both favor cell proliferation over apoptosis, and result in genesis of morphologically similar parathyroid adenomas. Based on the present results these two mechanisms might explain the molecular pathogenesis of the majority of parathyroid adenomas, but these findings need to be confirmed in other series.
Pathogenesis of nonfamilial parathyroid hyperplasia is poorly understood. It has been assumed that the disease results from polyclonal nonneoplastic proliferations involving multiple glands, but recent studies focusing on clonality of cell proliferation suggest that this view may be oversimplified. In one study on renal failure-associated hyperparathyroidism, all four specimens from diffuse parathyroid gland hyperplasia were found to be polyclonal, whereas all seven specimens from nodules in nodular hyperplasia were monoclonal based on restriction fragment length polymorphism analysis of the X-chromosome-linked phosphoglycerokinase gene, and random inactivation of the gene by methylation, suggesting that nodular hyperplasia may represent monoclonal parathyroid neoplasia. 32 In another study in which clonality of hyperplastic parathyroid lesions was assessed with X-chromosome inactivation analysis and by searching for monoclonal allelic losses, seven of 11 (64%) informative patients with uremic refractory hyperparathyroidism harbored at least one monoclonal parathyroid tumor, and tumor monoclonality was demonstrated in six of 16 (38%) patients with primary parathyroid hyperplasia. 4
Large changes in the cellular DNA content are common in adenomas of endocrine organs. 33 Most changes in the present study were losses of the genetic material, which is in line with previous studies on parathyroid adenomas. 19,21,22 The CGH profiles of parathyroid adenomas are clearly different from those of thyroid adenomas, where losses of genetic material are seldom found, but DNA copy number gains of several chromosomes are frequent. 34 Moreover, parathyroid hyperplasias and adenomas showed more DNA copy number changes in CGH analysis than papillary thyroid carcinomas, suggesting that the molecular genetic mechanisms that lead to benign endocrine tumors may vary in different endocrine organs, and that the association between the amount of DNA lost or gained has poor association with the tumor malignancy potential.
Deletion of the entire chromosome 11 or a part of it was present in 8% of primary hyperplasias and 31% of adenomas with a common minimum deleted region in 11q22-q23. The number of secondary, tertiary, and MEN-related hyperplasias studied was too small to allow for meaningful conclusions, but a loss of whole chromosome 11 was present in all four MEN1-related cases, where somatic loss of the remaining MEN1 allele probably resulted in parathyroid hyperplasia. These CGH findings were in line with those obtained with FISH. Importantly, all three cases that had 11q23 deletion by CGH and FISH and that were further investigated with FISH to determine the MEN1 allele copy number, had both MEN1 alleles located at 11q13 present, suggesting the possibility that 11q23 might contain an important suppressor gene that is distinct from MEN1. 35 This hypothesis is supported by the frequent deletion of 11q22-q24 in several human cancers, such as ovarian and colorectal carcinoma, and mantle cell lymphoma. 36-39 One candidate tumor suppressor gene located at 11q23 is PPP2R1B that encodes the β isoform of the A subunit of the serine/threonine protein phosphatase 2A (PP2A), and deletion of PPP2R1B may lead to colon or lung cancer. 40,41 The ATM (ataxia-telangiectasia mutated) suppressor gene located at 11q22.3 has a role in the cell-cycle check-point control, genome surveillance, and cellular defense against oxidative stress. 42
Apart from loss of 11q and 11p many other losses or gains may also be important in the pathogenesis of parathyroid adenomas and hyperplasias. We found DNA loss in chromosome 13 in four hyperplasias and three adenomas with a minimal common deleted region at 13q21.3.-q31.3. Loss of chromosome 13q has been detected more often in parathyroid carcinoma than in parathyroid adenoma, 15-18,43 and it contains several candidate suppressor genes such as RB1 (13q14) and ING1 (13q34). We also found DNA loss in chromosome 6 in two hyperplasias and three adenomas with a minimal deleted region at 6q22-q24. Loss of chromosome 6q is frequent in human cancers, 39 and studies on breast and ovarian cancer have implicated the chromosomal regions 6q23-q25 and 6q24-q25 as locations for putative tumor suppressor genes. 44,45
Although gains of genetic material were less common than losses, high-level amplifications were found at 7q21-q35, 8p12-p22, and 8q21.2-qter in one adenoma. These amplifications have not been described previously in parathyroid neoplasms. Amplifications of 8q are frequently seen in many other tumor types, and one of the most important target genes in this amplicon is MYC at 8q24. 46 However, in this oxyphilic adenoma tumor blood vessel invasion was seen in one small vein, which might have been interpreted as a sign of carcinoma. As the tumor did not fulfill other criterias of malignancy, it was diagnosed as atypical adenoma. The association between this amplification and genesis of parathyroid carcinoma requires further study.
In conclusion, both DNA copy number loss and cyclin D1 overexpression are common in parathyroid adenomas unlike in primary parathyroid hyperplasias. This suggests that pericentromeric inversion of chromosome 11 and suppressor gene loss are important underlying mechanisms in the molecular pathogenesis of parathyroid adenomas. The region 11q23 is frequently lost in parathyroid adenomas and occasionally in parathyroid hyperplasias, and this finding suggests the possibility that this region may contain a tumor suppressor gene that is important in their pathogenesis.
Acknowledgments
We thank Anna-Maija Pylvänäinen for skillful technical assistance.
Footnotes
Address reprint requests to Heikki Joensuu, MD, Department of Oncology, Helsinki University Central Hospital, Haartmaninkatu 4, P.O.Box 180, FIN-00029 HYKS, Finland. E-mail: heikki.joensuu@hus.fi.
Supported by grants from the Cancer Society of Finland, Academy of Finland, Helsinki University Central Hospital Research Fund, Finnish Cultural Foundation, Emil Aaltonen Foundation, Maud Kuistila Foundation, and the Clinical Research Institute of Helsinki University Central Hospital.
References
- 1.Berger AC, Libutti SK, Bartlett DL, Skarulis MG, Marx SJ, Spiegel AM, Doppman JL, Alexander HR: Heterogeneous gland size in sporadic multiple gland parathyroid hyperplasia. J Am Coll Surg 1999, 188:382-389 [DOI] [PubMed] [Google Scholar]
- 2.Yong JL, Vrga L, Warren BA: A study of parathyroid hyperplasia in chronic renal failure. Pathology 1994, 26:99-109 [DOI] [PubMed] [Google Scholar]
- 3.Tominaga Y: Mechanism of parathyroid tumourigenesis in uraemia. Nephrol Dial Transplant 1999, 14(suppl 1):63-65 [DOI] [PubMed] [Google Scholar]
- 4.Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB: Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest 1995, 95:2047-2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A: A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 1991, 350:512-515 [DOI] [PubMed] [Google Scholar]
- 6.Arnold A: The cyclin D1/PRAD1 oncogene in human neoplasia. J Invest Med 1995, 43:543-549 [PubMed] [Google Scholar]
- 7.Hsi ED, Zukerberg LR, Yang WI, Arnold A: Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study. J Clin Endocrinol Metab 1996, 81:1736-1739 [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buch MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim Ys, Heppner C, Dong Q, Spiegel Am, Burns AL, Marx SJ: Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997, 276:404-407 [DOI] [PubMed] [Google Scholar]
- 9.Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, Manickam P, Olufemi SE, Skarulis MC, Doppman JL, Alexander RH, Kim YS, Saggar SK, Lubensky IA, Zhuang Z, Liotta LA, Chandrasekharappa SC, Collins FS, Spiegel AM, Burns AL, Marx SJ: Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet 1997, 16:375-378 [DOI] [PubMed] [Google Scholar]
- 10.Friedman E, Sakaguchi K, Bale AE, Falchetti A, Streeten E, Zimering MB, Weinstein LS, McBride WO, Nakamura Y, Brandi ML, Norton JA, Aurbach GD, Spiegel AM, Marx SJ: Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med 1989, 321:213-218 [DOI] [PubMed] [Google Scholar]
- 11.Friedman E, De Marco L, Gejman PV, Norton JA, Bale AE, Aurbach GD, Spiegel AM, Marx SJ: Allelic loss from chromosome 11 in parathyroid tumors. Cancer Res 1992, 52:6804-6809 [PubMed] [Google Scholar]
- 12.Cryns VL, Yi SM, Tahara H, Gaz RD, Arnold A: Frequent loss of chromosome arm 1p DNA in parathyroid adenomas. Genes Chromosom Cancer 1995, 13:9-17 [DOI] [PubMed] [Google Scholar]
- 13.Thompson DB, Samowitz WS, Odelberg S, Davis RK, Szabo J, Heath H, III: Genetic abnormalities in sporadic parathyroid adenomas: loss of heterozygosity for chromosome 3q markers flanking the calcium receptor locus. J Clin Endocrinol Metab 1995, 80:3377-3380 [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki H: A possible tumor suppressor gene for parathyroid adenomas. Int Surg 1996, 81:71-76 [PubMed] [Google Scholar]
- 15.Tahara H, Smith AP, Gaz RD, Cryns VL, Arnold A: Genomic localization of novel candidate tumor suppressor gene loci in human parathyroid adenomas. Cancer Res 1996, 56:599-605 [PubMed] [Google Scholar]
- 16.Cryns VL, Thor A, Xu H-J, Hu SX, Wierman ME, Vickery AL, Jr, Benedict WF, Arnold A: Loss of the retinoblastoma tumor suppressor gene in parathyroid carcinoma. N Engl J Med 1994, 330:757-761 [DOI] [PubMed] [Google Scholar]
- 17.Dotzenrath C, Teh BT, Farnebo F, Cupisti K, Svensson A, Toell A, Goretzki P, Larsson C: Allelic loss of the retinoblastoma tumor suppressor gene: a marker for aggressive parathyroid tumors? J Clin Endocrinol Metab 1996, 81:3194-3196 [DOI] [PubMed] [Google Scholar]
- 18.Pearce SH, Trump D, Wooding C, Sheppard MN, Clayton RN, Thakker RV: Loss of heterozygosity studies at the retinoblastoma and breast cancer susceptibility (BRCA2) loci in pituitary, parathyroid, pancreatic, and carcinoid tumours. Clin Endocrinol 1996, 45:195-200 [DOI] [PubMed] [Google Scholar]
- 19.Palanisamy N, Imanishi Y, Rao PH, Tahara H, Chaganti RSK, Arnold A: Novel chromosomal abnormalities identified by comparative genomic hybridization in parathyroid adenomas. J Clin Endocrinol Metab 1998, 83:1766-1770 [DOI] [PubMed] [Google Scholar]
- 20.Arnold A, Staunton CE, Kim HG, Gaz RD, Kronenberg HM: Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med 1988, 318:658-662 [DOI] [PubMed] [Google Scholar]
- 21.Agarwal SK, Schröck E, Kester MB, Burns AL, Heffess CS, Ried T, Marx SJ: Comparative genomic hybridization analysis of human parathyroid tumors. Cancer Genet Cytogenet 1998, 106:30-36 [DOI] [PubMed] [Google Scholar]
- 22.Farnebo F, Kytölä S, Teh BT, Dwight T, Wong FK, Höög A, Elvius M, Wassif WS, Thompson NW, Farnebo L-O, Sandelin K, Larsson C: Alternative genetic pathways in parathyroid tumorigenesis. J Clin Endocrinol Metab 1999, 84:3775-3780 [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, ed 2. Cold Spring Harbor, Cold Spring Harbor Laboratory, 1989
- 24.Isola JJ, de Vries S, Chu LW, Ghazvini S, Waldman FM: Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol 1994, 145:1301-1308 [PMC free article] [PubMed] [Google Scholar]
- 25.Kallioniemi O-P, Kallioniemi A, Piper J, Isola J, Waldman FM, Gray JW, Pinkel D: Optimizing comparative genomic hybridization for analysis of DNA sequence copy number changes in solid tumor. Genes Chromosom Cancer 1994, 10:231-243 [DOI] [PubMed] [Google Scholar]
- 26.El-Rifai W, Larramendy ML, Björqkvist A-M, Hemmer S, Knuutila S: Optimization of comparative genomic hybridization by fluorochrome conjugated to dCTP and dUTP nucleotides. Lab Invest 1997, 77:699-700 [PubMed] [Google Scholar]
- 27.Hemmer S, Wasenius V-M, Knuutila S, Franssila F, Joensuu H: DNA copy number changes in thyroid carcinoma. Am J Pathol 1999, 154:1539-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larramendy ML, El-Rifai W, Knuutila S: Comparison of fluorescein isothiocyanate- and Texas red-conjugated nucleotides for direct labeling in comparative genomic hybridization. Cytometry 1998, 31:174-179 [PubMed] [Google Scholar]
- 29.Heiden T, Wang N, Bernhard T: An improved Hedley method for preparation of paraffin-embedded tissues for flow-cytometric analysis of ploidy and S-phase. Cytometry 1991, 12:614-621 [DOI] [PubMed] [Google Scholar]
- 30.Hyytinen E, Visakorpi T, Kallioniemi A, Kallioniemi O, Isola J: Improved technique for analysis of formalin-fixed, paraffin-embedded tumors by fluorescence in situ hybridization. Cytometry 1994, 16:93-99 [DOI] [PubMed] [Google Scholar]
- 31.Guru SC, Olufemi S-E, Manickam P, Cummings C, Gieser LM, Pike BL, Bittner ML, Jiang Y, Chinault AC, Nowak NJ, Brzozowska A, Crabtree JS, Wang Y, Roe BA, Weisemann JM, Boguski MS, Agarwal SK, Burns AL, Spiegel AM, Marx SJ, Flejter WL, de Jong PJ, Collins FS, Chandrasekharappa SC: A 2.8-Mb clone contig of the multiple endocrine neoplasia type 1 (MEN1) region at 11q13. Genomics 1997, 42:436-445 [DOI] [PubMed] [Google Scholar]
- 32.Tominaga Y, Kohara S, Namii Y, Nagasaka T, Haba T, Uchida K, Numano M, Tanaka Y, Takagi H: Clonal analysis of nodular parathyroid hyperplasia in renal hyperparathyroidism. World J Surg 1996, 20:744-752 [DOI] [PubMed] [Google Scholar]
- 33.Joensuu H, Klemi PJ: DNA aneuploidy in adenomas of endocrine organs. Am J Pathol 1988, 132:145-151 [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmer S, Wasenius V-M, Knuutila S, Joensuu H, Franssila K: Comparison of benign and malignant follicular thyroid tumours by comparative genomic hybridization. Br J Cancer 1998, 78:1012-1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans MF, Koreth J, Bakkenist CJ, Herrington CS, McGee JO’D: Allelic deletion at 11q23.3-q25 is an early event in cervical neoplasia. Oncogene 1998, 16:2557-2564 [DOI] [PubMed] [Google Scholar]
- 36.Foulkes WD, Campbell IG, Stamp GWH, Trowsdale J: Loss of heterozygosity and amplification chromosome 11q in human ovarian cancer. Br J Cancer 1993, 67:268-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keldysh PL, Dragani TA, Fleischman EW, Konstantinova LN, Perevoschikov AG, Pierotti MA, Della Porta G, Kopnin BP: 11q deletions in human colorectal carcinomas: cytogenetics and restriction fragment length polymorphism analysis. Genes Chromosom Cancer 1993, 6:45-50 [DOI] [PubMed] [Google Scholar]
- 38.Monni O, Oinonen R, Elonen E, Franssila K, Teerenhovi L, Joensuu H, Knuutila S: Gain of 3q and deletion of 11q22 are frequent aberrations in mantle cell lymphoma. Genes Chromosom Cancer 1998, 21:298-307 [DOI] [PubMed] [Google Scholar]
- 39.Knuutila S, Aalto Y, Autio K, Björkqvist A-M, El-Rifai W, Hemmer S, Huhta T, Kettunen E, Kiuru-Kuhlefelt S, Larramendy ML, Lushnikova T, Monni O, Pere H, Tapper J, Tarkkanen M, Varis A, Wasenius V-M, Wolf M, Zhu Y: DNA copy number losses in human neoplasms. Am J Pathol 1999, 155:683-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA: Alterations of the PPP2R1B gene in human lung and colon cancer. Science 1998, 282:284-287 [DOI] [PubMed] [Google Scholar]
- 41.Wang SS, Virmani A, Gazdar AF, Minna JD, Evans GA: Refined mapping of two regions of loss of heterozygosity on chromosome band 11q23 in lung cancer. Genes Chromosom Cancer 1999, 25:154-159 [PubMed] [Google Scholar]
- 42.Rotman G, Siloh Y: The ATM gene and protein: possible roles in genome surveillance, checkpoint controls and cellular defence against oxidative stress. Cancer Surv 1997, 29:285-304 [PubMed] [Google Scholar]
- 43.Kytölä S, Farnebo F, Obara T, Isola J, Grimelius L, Farnebo L-O, Sandelin K, Larsson K: Patterns of chromosomal imbalances in parathyroid carcinomas. Am J Pathol 2000, 157:579-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theile M, Seitz S, Arnold W, Jandrig B, Frege R, Schlag PM, Haensch W, Guski H, Winzer K-J, Barrett JC, Scherneck SA: A defined chromosome 6 fragment (at D6S310) harbors a putative tumor suppressor gene for breast cancer. Oncogene 1996, 13:677-685 [PubMed] [Google Scholar]
- 45.Wan M, Sun T, Vyas R, Zheng J, Granada E, Dubeau L: Suppression of tumorigenicity in human cancer cell lines is controlled by a 2 cM fragment in chromosomal region 6q24–q25. Oncogene 1999, 18:1545-1551 [DOI] [PubMed] [Google Scholar]
- 46.Knuutila S, Björkqvist A-M, Autio K, Tarkkanen M, Wolf M, Monni O, Szymanska J, Larramendy ML, Tapper J, Pere H, El-Rifai W, Hemmer S, Wasenius V-M, Vidgren V, Zhu Y: DNA copy number amplifications in human neoplasms—a review of comparative genomic hybridization studies. Am J Pathol 1998, 152:1107-1123 [PMC free article] [PubMed] [Google Scholar]