Abstract
During development, the formation and remodeling of primary vascular networks occurs by vasculogenesis and angiogenesis. Recently, the term “vasculogenic mimicry” has been used by our laboratory and collaborators to reflect the embryonic-like ability of aggressive, but not nonaggressive, melanoma tumor cells to form a pattern of matrix-rich networks (containing channels) surrounding spheroids of tumor cells in three-dimensional culture, concomitant with their expression of vascular cell markers. Ovarian cancer is usually diagnosed as advanced stage disease in most patients when widespread metastases have already been established within the peritoneal cavity. In this study, we explored whether invasive ovarian carcinoma cells could engage in molecular vasculogenic mimicry reflected by their plasticity, compared with their normal cell counterparts. The data revealed that the invasive ovarian cancer cells, but not normal ovarian surface epithelial cells, formed patterned networks containing solid and hollow matrix channels when grown in three-dimensional cultures containing Matrigel or type I collagen, in the absence of endothelial cells or fibroblasts. Immunohistochemical analysis showed that matrix metalloproteinases (MMP)-1, -2, and -9, and MT1-MMP were discretely localized to these networks, and the formation of the networks was inhibited by treatment with MMP inhibitors. Furthermore, the RNase protection assay revealed the expression of multiple vascular cell-associated markers by the invasive ovarian cancer cells. In patient tumor sections from high-stage, high-grade ovarian cancers, 7 to 10% of channels containing red blood cells were lined by tumor cells. By comparison, all vascular areas in benign tumors and low-stage cancers were endothelial lined. These results may offer new insights and molecular markers for consideration in ovarian cancer diagnosis and treatment strategies based on molecular vascular mimicry by aggressive tumor cells.
Understanding how blood vessels form in normal physiological processes and malignant tumors has evolved rapidly throughout the last several years. Unraveling these mechanisms has the potential to result in the development of novel therapeutics for a multitude of benign conditions including coronary artery disease and claudication as well as a variety of malignancies. The traditional mechanisms for development of tumor vasculature and perfusion have been thought to be inextricably linked to endothelial lined networks. However, our laboratory and collaborators 1 have recently generated data from a subset of uveal and cutaneous melanomas that demonstrated that the highly aggressive human melanoma tumors, but not nonaggressive tumors, contained patterned, matrix-rich networks containing channels (similar to embryonic vasculogenic-patterned networks) surrounding spheroids of tumor cells—in the absence of tumor necrosis and classical angiogenesis. In addition, the cells lining some of the tumor vessels varied from endothelial cells only to endothelial cells and melanoma cells to only melanoma cells, 2,3 which corresponded with the overexpression of endothelial-specific genes by molecular analysis; hence, vasculogenic mimicry by aggressive melanoma tumor cells. Vasculogenic mimicry has been described in both uveal and cutaneous melanoma, cytotrophoblasts, and Drosophila tumor models; 1-8 however, the physiological significance of these networks is unknown at this time. Although mosaic vessels have recently been described in human colon cancers implanted in mice that also demonstrate vasculature lined by tumor cells and endothelial cells, 9 it is not known whether a similar phenomenon occurs in other epithelial malignancies.
Ovarian cancer has the highest mortality rate of all gynecological malignancies, which reflects the fact that most patients are diagnosed with advanced cancer. Extensive metastatic deposits of tumor have been established within the peritoneal cavity at the time of initial diagnosis in most patients. The growth and spread of neoplasms depend, in part, on the formation of adequate vascular support. 10 As proposed by Folkman, 11 neovascularization of tumors is required to provide essential nutrients to the growing tumor beyond the limit of simple diffusion, and to allow for growth >2 mm. Based on this concept, several different anti-angiogenic therapies have been developed and are now in clinical trials. 12,13 Although angiogenesis and the associated factors have been shown to occur in ovarian cancers 14 and the degree of vascularity in ovarian cancer correlates with poor clinical outcome, 15-19 the mechanisms involved in the generation of this vascularity have not been well defined.
In a recent study, Orre and Rogers 20 found that the endothelial proliferation index in high-vessel density regions of ovarian cancers relative to benign tumors was significantly lower. Thus, we hypothesized that in a manner similar to melanomas, aggressive ovarian cancer cells may have the ability to generate matrix-rich, embryonic-like, patterned networks independent of endothelial cells. In this study, we show experimental and clinicopathological evidence for the presence of molecular vasculogenic mimicry by invasive human ovarian cancer cells. Ovarian cancer plasticity is reflected by their pluripotential ability to express a variety of phenotypes including formation of endothelial-like matrix-rich networks. These findings advance our present thinking concerning the vascularization of ovarian cancers, and identify some new, potential targets for therapeutic intervention.
Materials and Methods
Cell Culture
The five established ovarian cancer cell lines used in this study were OVCAR3, SKOV3, 222, EG, and A2780-PAR. The derivation and sources of these cell lines have been reported previously. 21 These cells were maintained and propagated in vitro by serial passage in RPMI 1640 supplemented with 15% fetal bovine serum and 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA). The immortalized normal human ovarian surface epithelial cell lines (HIO 1120 and HIO 180) were a kind gift from Dr. Andrew Godwin at the Fox Chase Cancer Center, Philadelphia, PA. These lines were maintained in Medium 199/MCDB 105/15% fetal bovine serum with 0.1% gentamicin sulfate. All cell lines are routinely screened for Mycoplasma species (GenProbe detection kit; Fisher, Itasca, IL). All experiments were performed with 70 to 80% confluent cultures.
Invasion Assay
The membrane invasion culture system chamber was used to measure the in vitro invasiveness of all cell lines used in this study. 22 Briefly, a polycarbonate membrane with 10 μmol/L pores (Osmonics, Livermore, CA) was uniformly coated with a defined basement membrane matrix consisting of human laminin/type IV collagen/gelatin and used as the intervening barrier to invasion. Both upper and lower wells of the chamber were filled with serum-free RPMI containing 1× MITO+ (Collaborative Biomedical, Bedford, MA). Single-cell tumor suspensions were seeded into the upper wells at a concentration of 1 × 10 5 cells per well. After a 24-hour incubation in a humidified incubator at 37°C with 5% CO2, cells that had invaded through the basement membrane were collected, stained, and counted by light microscopy. 22 For chemo-invasion assays, conditioned media from normal skin fibroblasts (kindly provided by Dr. Gregory Goldberg, Washington University, St. Louis, MO) was added to the lower wells. Invasiveness was calculated as the percentage of cells that had successfully invaded through the matrix-coated membrane to the lower wells compared to the total number of cells seeded into the upper wells and corrected for cell proliferation.
Three-Dimensional Cultures and in Vitro Network Formation
Fifty μl of Matrigel (Collaborative Biomedical) was dropped onto glass coverslips and allowed to polymerize for 1 hour at room temperature, then 30 minutes at 37°C in a humidified 5% CO2 incubator. Tumor cells (7.5 × 105) were then seeded onto the gels and incubated at 37°C with 5% CO2 and humidity. For experiments on a collagen I matrix, 20 μl of undiluted rat-tail collagen I solution (Collaborative Biomedical) were dropped onto glass coverslips and polymerized for 5 minutes with 100% ethanol. The coverslips were then washed once with 1× phosphate-buffered saline (PBS), rehydrated with culture medium, then seeded with tumor cells. The cultures were maintained in RPMI 1640 supplemented with 15% fetal bovine serum, and 0.1% gentamicin sulfate. The culture medium was changed every 3 days.
Light Microscopy
Five-μm serial sections of paraffin-embedded, formaldehyde-fixed tissues were cut, stained with a hematoxylin and eosin (H&E) stain and examined by light microscopy using a Zeiss Axioskop 2 (Carl Zeiss, Inc., Thornwood, NY), Spot 2 camera (Diagnostic Instruments; Inc., Sterling Heights, MI) and Axiovision 2.0.5 software (Carl Zeiss, Inc.). Companion serial sections were stained with periodic acid-Schiff without a hematoxylin counterstain and viewed through a green filter to visualize any extracellularly derived structures.
Immunohistochemical Staining
Detection of laminin in the in vitro samples was performed using a polyclonal anti-laminin antibody (Life Technologies, Inc., Gaithersburg, MD) at 1:200 dilution in PBS containing 0.3% normal goat serum, and the Vectastain Elite avidin-biotin-peroxidase complex (ABC) kit and 3-amino-9-ethylcarbazole (AEC) substrate kit for peroxidase activity (Vector Laboratories, Inc., Burlingame, CA) with a slight modification of the manufacturer’s protocol. Briefly, after the samples were rinsed with PBS, an additional step of incubating the samples with 0.1% Triton X-100 in PBS for 5 minutes was added. After washing with PBS, these samples were treated with 0.3% H2O2 in PBS containing 0.3% normal goat serum for 5 minutes, washed, incubated for 20 minutes with normal goat blocking serum, then incubated with the anti-laminin antibody for 30 minutes. These samples were then washed with PBS, incubated for 30 minutes with the biotinylated secondary antibody, PBS washed, incubated with the Vectastain Elite ABC reagent for 30 minutes, PBS washed, then incubated with the AEC substrate for 10 to 30 minutes (or until adequate color development). The samples were then washed with PBS, rinsed with tap water, and mounted with an aqueous mounting solution (Aqua-Mount; Lerner Laboratories, Pittsburgh, PA) onto precleaned microscope slides. The AEC substrate turns red in the presence of peroxidase activity.
Immunostaining for laminin in tissue sections was performed using a monoclonal anti-laminin antibody (Sigma Chemical Co., St. Louis, MO) at either 1:100 or 1:250 dilution. Briefly, the samples were treated with proteinase K for 10 to 15 minutes, washed with deionized water, and rinsed with Tris-buffered saline with Tween-20 (0.05%) for 10 minutes. Subsequent steps (3% H2O2 for 5 minutes, avidin for 15 minutes, biotin for 15 minutes, protein block-serum-free (DAKO, Carpinteria, CA) for 7 minutes, laminin antibody for 45 to 52 minutes) were performed in the automated DAKO stainer (DAKO). Tris-buffered saline washes were performed between each step. The Vector Universal Elite ABC ready-to-use kit (Vector Laboratories) was used for secondary and tertiary reagents according to manufacturer’s instructions. For chromagen, VIP substrate (purple) (Vector Laboratories) was used for 7 minutes. Methyl-green counterstaining was performed for 15 to 20 minutes.
Inhibition of Network Formation
Inhibition of the network formation by tumor cells was accomplished using Metastat (CollaGenex Pharmaceuticals, Newton, PA) at 5 μg/ml added to the cultures daily beginning either on day 1, day 7, or day 14. 23
Ribonuclease Protection Assay (RPA)
Total RNA was hybridized with the desired 32P-labeled multitemplate probe (hangio-1; BD Pharmingen, San Diego, CA) for 16 hours. After this, RNase treatment and purification of protected probes was performed according to the manufacturer’s guidelines. The samples were run on a 5% polyacrylamide gel containing urea at 50 W constant power until the leading edge of the bromophenol blue migrated 30 cm. The gel was dried under vacuum for 1 hour and exposed to Kodak Biomax MR X-ray film (Eastman-Kodak, Rochester, NY) at −70°C .
Electron Microscopy
For scanning electron microscopy and transmission electron microscopy, tissue cultures were fixed in cold 2.5% glutaraldehyde in 0.1 mol/L of sodium cacodylate buffer and postfixed in osmium. Specimens were then either embedded, sectioned, and stained by routine means for transmission electron microscopy, or critically point-dried, and sputter-coated with gold/palladium for scanning electron microscopy, as previously described. 24
Results
Invasive Potential
The membrane invasion culture system assay was used to measure the in vitro ability of cells to invade a basement membrane matrix—an important step in the metastatic cascade. The invasion results are summarized in Figure 1 ▶ . Both HIO 180 and HIO 1120 were poorly invasive (2.3%), whereas OVCAR3, EG, A2780-PAR, and SKOV3 were moderately invasive (3.9 to 5.1%), and the 222 cell line was the most invasive (8.3%). With the addition of chemoattractant to the invasion assay, the percent invasive ability of OVCAR3, SKOV3, EG, and 222 increased by 30 to 47%, compared with the poorly invasive cell lines that remained basically unchanged.
Figure 1.
Invasion profile of ovarian cancer and immortalized normal human ovarian surface-epithelial cell lines, based on their ability to invade a basement membrane matrix in vitro in the presence (hatched bars) or absence (solid bars) of a chemoattractant. Error bars represent SE.
In Vitro Evidence of Vasculogenic Mimicry
All of the cell lines were assayed for their ability to form tumor cell-lined, matrix-rich networks on three-dimensional matrices, consisting of Matrigel or collagen I, in the absence of endothelial cells or fibroblasts. The invasive cancer cell lines formed networks on both matrices (representative examples are shown in Figure 2, A–D ▶ ) except A2780-PAR that did not form networks even after 3 weeks in culture. The tumor-formed networks initiated formation within 3 days after seeding the cells onto the matrix with optimal structure formation achieved by 3 weeks. Microscopic analysis at regular intervals revealed that the networks consisted of tubular structures that continued to evolve during the 3-week period with variations of tubular and sinusoidal structures surrounding clusters of tumor cells. There were no significant differences in the efficiency of network formation between the highly invasive and moderately invasive cell lines. During formation, the tubular networks became channelized or hollowed, and were stable through 6 weeks after seeding the cells onto a matrix. However, the HIO 180 and HIO 1120 normal surface epithelial cells did not form networks or channels on either matrix (more than 3 weeks in culture; Figure 2, E and F ▶ ).
Figure 2.

Three-dimensional cultures of ovarian cell lines on Matrigel or collagen I matrix grown for 3 weeks: OVCAR3 (A and B), SKOV3 (C and D), HIO 1120 (E), HIO 180 (F). White arrowheads indicate tumor cell-lined, matrix-rich networks. Original magnifications: ×100 (A–C, E, and F), ×200 (D).
To further define the structure of the tubular networks, scanning electron microscopy was performed on the three-dimensional cultures. This approach confirmed that the structures formed by tumor cells were indeed tubular at 3 weeks and were lined externally by tumor cells (Figure 3, A and B) ▶ . Transmission electron microscopy of the tubular structures revealed that the tubes consisted of a matrix-rich central region (from the collagen gel) lined by tumor cells externally (Figure 3C) ▶ . Polarized tumor cells were arranged with their basal aspects toward the tubular lumen, and their microvillar apical surfaces facing the opposite direction. Tumor cells lining the lumen were connected by electron-dense cellular junctions.
Figure 3.

Scanning (A and B) and transmission (C) electron micrographs of ovarian cancer cell cultures grown on three-dimensional collagen I matrices. At low magnification (A; original magnification, ×150) tubular profiles are evident, which when fractured in preparation, are revealed to be hollow and lined by flattened cancer cells (B; original magnification, ×2,980). In such profiles, it is difficult with scanning electron microscopy to distinguish the limits of cells and matrix contributing to the tubule. However, it is clearly evident from transmission electron microscopy examination of similar structures (C; original magnification, ×7,300) that the lining epithelial cells encompass a matrix-containing central lumen. Note also the polarity of the lining cells: the surface microvilli are directed outwardly, not toward the lumen, as is observed in spheroidal or quasi-acinar cellular aggregates formed occasionally by adenocarcinomas in culture. Electron-dense junctions are evident between tumor cells (arrowheads).
In Vivo Evaluation of Ovarian Tumors
To evaluate the putative existence of tumor-lined networks or channels in ovarian cancer, nine ovarian cancers and four benign ovarian tumors were histologically examined. Representative examples are shown in Figure 4, A–D ▶ , and the results are summarized in Table 1 ▶ . Endothelial-lined blood vessels were identified in all cases of low-stage (stage I) and low-grade (grade I or II) tumors. However, tumor cell-lined channels containing red blood cells were detected in three of five high-grade (III), high-stage (III or IV) tumors. Representative tumor-lined channels are shown in Figure 5, A and B ▶ . Pan-cytokeratin staining of high-grade cancers confirmed the presence of tumor-lined channels (Figure 5C) ▶ . Serial sections at 5 μmol/L were examined to ensure morphological continuity through various thicknesses of the tissue. Among tumors with evidence of tumor cell-lined channels, 10 high-power fields were counted to estimate the proportion of vessels that were nonendothelial-lined. The incidence of these channels ranged from 7 to 10% in the three most aggressive tumors that exhibited this phenomenon.
Figure 4.
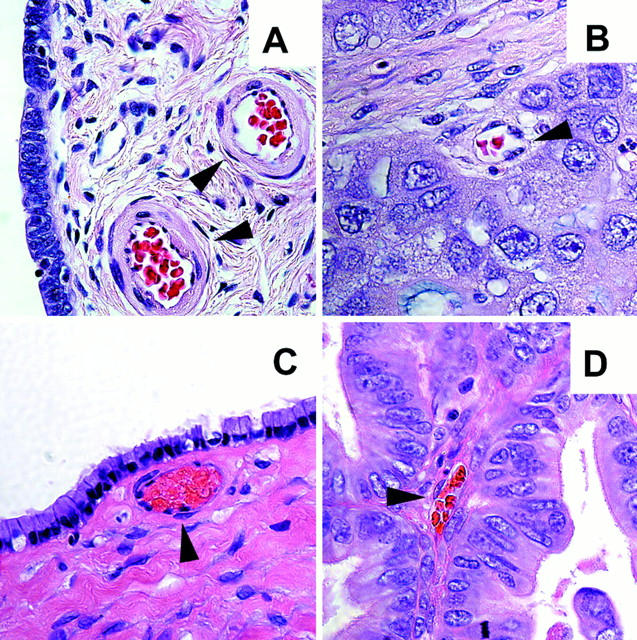
Representative light microscopy pictures of benign ovarian tumors (A and C) low-stage (I/II) ovarian cancer (B and D) from H&E-stained histological sections of representative tumors. Black arrowheads point to endothelial-lined vessels. Original magnifications, ×630.
Table 1.
Pathological Features of Ovarian Tumors Based on Presence of Tumor-Lined Networks and Channels Containing Red Blood Cells
| Tumor no. | Stage | Grade | Histology | Tumor-lined networks and channels |
|---|---|---|---|---|
| Benign | ||||
| 10763 | − | − | Serous | No |
| 7653 | − | − | Serous | No |
| 18597 | − | − | Serous | No |
| 9959 | − | − | Serous | No |
| Low stage | ||||
| 12700 | IA | 2 | Serous | No |
| 1967 | IA | 2 | Mucinous | No |
| 22356 | IB | 2 | Serous | No |
| 2892 | IB | 2 | Serous | No |
| High stage | ||||
| 7369 | IV | 3 | ANOS* | Yes |
| 3022 | IV | 3 | Serous | Yes |
| 3471 | IIIC | 3 | Serous | Yes |
| 18641 | IV | 3 | Serous | No |
| 11252 | IV | 3 | Serous | No |
*ANOS, Adenocarcinoma not otherwise specified.
Figure 5.
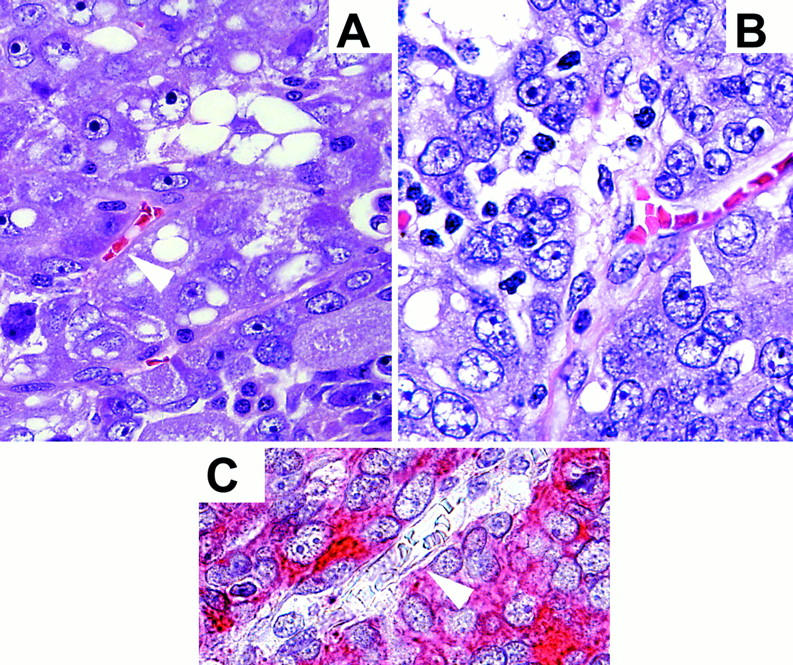
Representative H&E-stained (A and B) histological sections of high-grade, high-stage ovarian cancers showing tumor cell-lined channels (white arrowheads). C: Pan-cytokeratin stain of a high-grade ovarian cancer demonstrating tumor cells lining a vascular channel (white arrowhead). Original magnifications, ×630.
Molecular Determinants of Tumor Cell Plasticity
To explore the potential role(s) of key molecular determinants of ovarian tumor cell plasticity with respect to the formation of tubular networks, we focused on matrix metalloproteinases (MMPs) in the formation and remodeling of the tumor-formed networks in vitro, as well as laminin—an important basement membrane component. Immunohistochemical analysis revealed that MMPs-1, -2, -9, and MT1-MMP were primarily localized to the networks in vitro (Figure 6, B–E) ▶ . The extracellular matrix molecule laminin also localized to these structures (Figure 6F) ▶ . Treatment of the cultures with Metastat, an inhibitor of MMP activity, prevented network formation when added to the culture on day 1 (Figure 6G) ▶ , but did not destroy established networks in separate cultures when added on day 7 (Figure 6H) ▶ .
Figure 6.
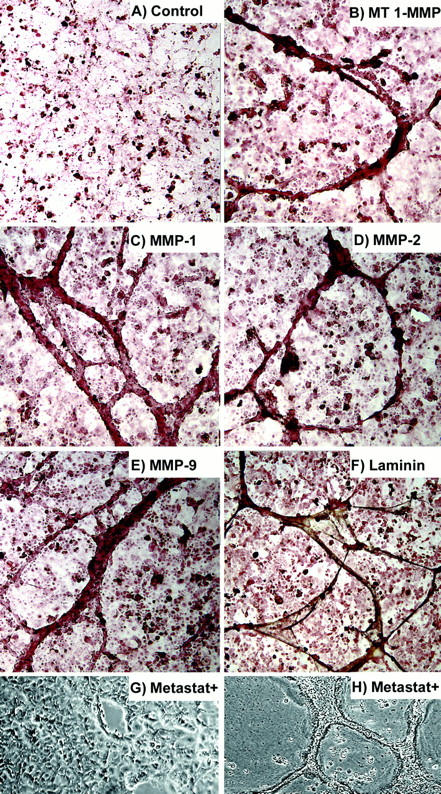
Immunohistochemical peroxidase staining of ovarian cancer cells in three-dimensional cultures (A–F) showing: control (no primary antibody) (A), MT1–MMP (B), MMP-1 (C), MMP-2 (D), MMP-9 (E), and laminin (F). Ovarian cancer cell cultures grown on three-dimensional collagen I matrices were treated with Metastat starting on day 1 (G) or day 7 (H). Network formation did not occur when treatment was started on day 1, but established networks were not destroyed when treatment was started on day 7. Original magnifications, ×100.
Further validation of laminin expression in ovarian cancer tissue sections was performed by immunohistochemistry (Figure 7) ▶ . In benign ovarian cysts, laminin staining was seen in the subepithelial basement membrane and in the walls of the blood vessels (Figure 7A) ▶ . However, in high-grade invasive ovarian cancer, laminin staining was seen in the matrix surrounding spheroids of tumor cells as well as in the cytoplasm of cancer cells (Figure 7B ▶ and inset). The pattern formed by the laminin immunohistochemical stain is reminiscent of a reticular meshwork. Within these areas, in high-grade ovarian cancers, tumor cell-lined channels were seen containing red blood cells (Figure 7C ▶ and inset).
Figure 7.

Immunohistochemical peroxidase staining for laminin demonstrating: benign ovarian cyst with laminin staining the subepithelial basement membrane (A, arrowhead; original magnification, ×630); high-grade ovarian cancer (B; original magnification, ×630) with laminin staining the matrix-surrounding tumor cells (cells were not counterstained). Inset shows ovarian cancer cells with laminin staining counterstained with methyl-green (original magnification, ×630). C: H&E-stained invasive ovarian cancer tissue section visualized under DAPI filter (original magnification, ×200), highlighting the autofluorescent red blood cells. The boxed area is shown at higher magnification in the inset (original magnification, ×630) demonstrating a column of red blood cells within a tumor cell-lined channel. D: Benign ovarian cyst used as control (no primary antibody, counterstained with methyl-green; original magnification, ×630).
An RNase protection assay was used to evaluate the expression of a series of well-characterized vascular-associated markers by the ovarian cancer cells. All of the tumor cell lines that formed tubular networks in vitro expressed several of the traditional endothelial cell markers (Figure 8) ▶ . The human umbilical vein endothelial cell line was used as an internal positive control. All of the ovarian cancer cell lines that formed matrix-rich networks expressed the thrombin receptor and three lines expressed TIE2. EG and 222 showed strong expression of CD31 (PECAM) whereas SKOV3 showed weaker expression. In addition, EG and 222 expressed other vascular-associated markers including endoglin and angiopoietin 1. All of the cancer cell lines expressed vascular endothelial growth factor (VEGF). The most invasive cell line, 222, also expressed the VEGF receptor, FLT1 (VEGFR-1), and TIE-1. Although A2780-PAR and HIO-180 both express VEGF, neither showed significant expression of the other vascular-associated markers.
Figure 8.
RNase protection assay (RPA) analysis for vascular-associated markers expressed by human ovarian cancer cell lines (OVCAR3, SKOV3, EG, 222, and A2780-PAR), immortalized normal ovarian surface epithelial cells (HIO 1120 and HIO 180), and normal endothelial cells (human umbilical vein endothelial cells) as an internal positive control. Equal loading was assessed by L32 and GAPDH expression.
Discussion
It is generally recognized that tumor growth and metastasis requires a blood supply for survival and that invasive cancers use several mechanisms to increase tumor perfusion. In general, the formation of a microcirculation (blood supply) can occur via the traditionally recognized mechanisms of angiogenesis (the formation or sprouting of endothelium-lined vessels from pre-existing vessels), and vasculogenesis (the differentiation of precursor cells to endothelial cells that develop de novo vascular networks). 25 The earliest stages of vascular development during embryogenesis include the differentiation, expansion, and coalescence of vascular endothelial cell precursors into an initial vascular network that results in the formation of interconnected vessels consisting of differentiated endothelial cells. 26 This primary plexus is then remodeled, which results in the sprouting, branching, and differential growth of blood vessels to form the mature vascular patterns. During embryonic and fetal development, both angiogenesis and vasculogenesis occur concomitantly. In the vascularization of tumors, traditional angiogenesis is observed in addition to vascular channels, cords, and sinuses (without endothelial cell lining), and mosaic vessels (endothelial and tumor-lined). 25
Recent findings from our laboratory have shown that aggressive melanomas, but not nonaggressive tumors, contain patterns of matrix-rich networks surrounding spheroids of tumors cells in the absence of tumor necrosis and classical angiogenesis. 1,27 The presence of matrix-rich networks in aggressive primary and metastatic melanoma tumors from patients has been shown to correlate with a poor clinical outcome. 28 In vitro, aggressive melanoma cells capable of generating similar matrix-rich networks with channels express inappropriate vascular molecular markers that include TIE-1 (an endothelial receptor kinase), and more than a dozen other endothelial- and vascular-associated genes, in addition to fibroblast- and epithelial-related genes. This molecular profile suggests a genetic reversion to an embryonic-like phenotype, which may be capable of forming a primitive network in aggressive tumors, as demonstrated experimentally in three-dimensional cultures in vitro-– referred to as vasculogenic mimicry. 1 However, the physiological significance of these networks has yet to be elucidated.
Before this study, it was not known whether epithelial tumors such as ovarian cancer were capable of engaging in vasculogenic mimicry. However, it has recently been shown that human breast cancer metastasis occurs via angiogenic- and nonangiogenic-characterized pathways, suggesting an important role for alternative vascularization and dissemination of epithelial tumors. 29 It has also been shown that aggressive breast cancer cells express vascular-associated markers. 30 Most epithelial ovarian cancers are thought to metastasize through the process of exfoliation from the primary tumor followed by dissemination of cells throughout the peritoneal cavity, implantation, and subsequent growth. The majority of patients succumb to their disease, ultimately dying from bowel obstruction resulting from multiple metastatic tumor deposits. For this mode of tumor spread, the tumor cells must develop a vascular supply at the sites of metastasis and tumors capable of rapid vascularization would theoretically be able to rapidly increase their volume. In addition, we have previously shown that at least 22% of the epithelial ovarian cancer patients either present with or develop distant metastases, most likely through hematogenous spread. 31 Our in vitro studies indicate that invasive ovarian cancer cells are able to develop matrix-rich tubular networks in a three-dimensional matrix without the presence of fibroblasts or endothelial cells. This pattern of tubular networks was not observed with the normal, immortalized human ovarian surface epithelial cells, but may represent a primitive microcirculatory network for tumors to support blood flow similar to embryonic microcirculatory vasculogenic networks. Alternatively, these networks may represent receptacles for vascular leakage previously reported in certain animal models. 32 We are actively pursuing this question in appropriate ovarian cancer animal models.
In recent years, the vascular bed of human tumors has been characterized extensively by performing microvessel density counts. 15-19,33,34 These studies have revealed that high microvessel density counts within vascular hot spots of tumors correspond with a poor prognosis for patients. Microvessel density studies using endothelial markers may reflect the vascular status of a tumor, but they may not be indicative of the nature of the vascularity. In a recent study, Eberhard and colleagues 35 demonstrated that counting microvessels in tumor vascular hot spots resulted in a relatively uniform high microvessel density in the different tumor types, but showed marked differences in the endothelial proliferation index among the various tumor types. This suggested that there is marked heterogeneity of vasculature in human tumors. Similarly, Orre and Rogers 20 found that the endothelial proliferation index in high-vessel density regions of ovarian cancers relative to benign tumors was significantly lower. Thus, it is tempting to speculate that these findings may be partially explained by the phenomenon of tumor cell vasculogenic mimicry in the paucity of endothelial cells.
Tumor vessels seem to differ from normal vessels in many ways. Human colon tumors in mice were found to contain mosaic vessels that are lined by tumor cells in many areas and endothelial cells in other regions. 9 In the present study, ∼10% of tumor vessels seemed to be nonendothelial-lined. Interestingly, mosaic vessels comprised ∼15% of colon tumor vessels in a mouse model. 9 Another consideration with respect to red blood cells in areas surrounded by tumor cells might be explained by a recent report that tumor vessels are leaky because of disorganized and loosely interconnected endothelial cells. 32
There is also strong evidence that even in normal developmental processes, nonendothelial cells can adopt a vascular-related phenotype. 6,8 For example, it has been shown that human cytotrophoblasts can adopt an endothelial cell phenotype as they actively participate in the dynamics of establishing the placenta and primordial microcirculation, which has been designated “trophoblast pseudo-vasculogenesis.” 6-8 Cytotrophoblasts were able to mimic the molecular phenotype of cells of the vascular system by expressing adhesion molecules characteristic of endothelial cells. 6 In our study, we have demonstrated that ovarian cancer cells forming networks in vitro also display molecular vasculogenic mimicry. Our findings show that pure populations of ovarian cancer cells can express aberrant endothelial- and other vascular-associated markers. As shown in Figure 8 ▶ , all of the invasive ovarian cancer cells except A2780-PAR express several of the traditional endothelial markers. These include several members of the VEGF family that mediate angiogenic signals to the vascular endothelium via high-affinity receptors that have been thought to be specific for endothelial cells. 36 Endoglin (CD105) is a receptor for transforming growth factor-β and has been used as an endothelial cell-specific marker. It has been shown to be important for developmental angiogenesis and plays a significant role in capillary-like tube formation by endothelial cells in three-dimensional collagen matrix cultures. 37 The endothelial cell-cell adhesion molecule, CD31, has also been shown to play a critical role in migration and the process of tube formation by endothelial cells. 38,39 Thus, our findings suggest that invasive ovarian cancer cells may express a deregulated, embryonic-like phenotype and are capable of forming primitive tubular networks in vitro, thereby exhibiting tumor plasticity.
The ability to invade extracellular matrices plays an important role in metastasis and in development of blood flow to tumors. Matrix metalloproteinases have been shown to play an active role in the neovascularization of tumors through their ability to degrade the extracellular matrix. 40,41 Human cytotrophoblasts have also been shown to up-regulate the expression of MMP-9 in the process of invasion and when adopting a vascular phenotype. 7,42 The thrombin receptor has been shown to mediate an increase in matrix metalloproteinases in human endothelial cells during the processes of tissue remodeling and vessel development. 43 Thrombin receptor has also been shown to be expressed in human cancers and play a role in increasing breast cancer invasiveness. 44,45 Interestingly, the ovarian cancer cells capable of generating tubular networks in vitro expressed both thrombin receptor and MMPs in our study. These findings indicate that secretion of MMPs may facilitate matrix-rich network formation, which was inhibited by treatment with an MMP inhibitor. Thus, MMP inhibitors might play an important role in the treatment of ovarian cancer in conjunction with other therapies. Our study also examined laminin, a major component of blood vessels that has been shown to be a critical glycoprotein in tube formation by endothelial cells in three-dimensional collagen gels. 46,47 Laminin was expressed in the tubular structures formed by pure ovarian cancer cells.
Based on our cellular, molecular, and clinical findings, we speculate that the tumor-generated, laminin-containing networks may represent either a primitive microcirculatory-like network or a remodeled vascularized portion of a tumor and/or discrete areas of vascular leakage, which is strictly associated with the aggressive tumors, but not the low-stage/grade and benign tumors. Further experiments and imaging data are required to elucidate issues related to the physiological significance of these networks. The invasive ovarian cancer cells expressing a molecular vasculogenic phenotype may serve as an important indication of tumor plasticity in a growing tumor mass, signifying aggressive treatment management. Additionally, these data may offer alternative targets for therapeutic intervention.
Footnotes
Address reprint requests to Anil K. Sood, M.D., Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of Iowa Hospitals and Clinics, 4630 JCP, 200 Hawkins Dr., Iowa City, IA 52242-1109. E-mail: anil-sood@uiowa.edu.
Supported by grant IN-1227 from the American Cancer Society administered through the Holden Comprehensive Cancer Center at the University of Iowa (to A. K. S.), the Reproductive Scientist Development Program through National Institutes of Health grant 5K12HD00849 and the American Board of Obstetrics and Gynecology (to A. K. S.); National Institutes of Health grants CA83137 (to R. E. B. S.) and CA59702 (to M. J. C. H.), and the Kate Daum Research Endowment and the H. B. Wallace Foundation Award (to M. J.C. H.).
References
- 1.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LMG, Pe’er J, Trent JM, Meltzer PS, Hendrix MJC: Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999, 155:739-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timár J, Tóth J: Tumor sinuses—vascular channels. Pathol Oncol Res 2000, 6:122-126 [DOI] [PubMed] [Google Scholar]
- 3.Folberg R, Hendrix MJC, Maniotis AJ: Vasculogenic mimicry and tumor angiogenesis. Am J Pathol 2000, 156:361-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu T, Wei W, Zhang S, Stewart RA, Yu W: Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 1995, 121:1053-1063 [DOI] [PubMed] [Google Scholar]
- 5.Potter CJ, Rurenchall GS, Xu T: Drosophila in cancer research: an expanding role. Trends Genet 2000, 16:33-39 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH: Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest 1997, 99:2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Damsky CH, Fisher SJ: Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype: one cause of defective endovascular invasion in this syndrome? J Clin Invest 1997, 99:2152-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]
- 9.Chang YS, diTomaso E, McDonald DM, Jones R, Jain RK, Munn LL: Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA 2000, 97:14608-14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman J, D’Amore PA: Blood vessel formation: what is its molecular basis? Cell 1996, 87:1153-1155 [DOI] [PubMed] [Google Scholar]
- 11.Folkman J: What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990, 82:4-6 [DOI] [PubMed] [Google Scholar]
- 12.Kerbel RS: Tumor angiogenesis: past, present and the near future. Carcinogenesis 2000, 21:505-515 [DOI] [PubMed] [Google Scholar]
- 13.Folkman J: Clinical applications of research on angiogenesis. Seminars in medicine of the Beth Israel Hospital, Boston. N Engl J Med 1995, 333:1757-1763 [DOI] [PubMed] [Google Scholar]
- 14.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, Twentyman PR, Smith SK: Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst 1995, 87:506-516 [DOI] [PubMed] [Google Scholar]
- 15.Gasparini G, Bonoldi E, Viale G, Verderiio P, Boracchi P, Panizzoni GA, Radaelli V, Di Bacco A, Guglielmi RB, Bevilacqua P: Prognostic and predictive value of tumor angiogenesis in ovarian carcinomas. Int J Cancer 1996, 69:205-211 [DOI] [PubMed] [Google Scholar]
- 16.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219:983-985 [DOI] [PubMed] [Google Scholar]
- 17.Paley PJ, Staskus KA, Gebhard K, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S: Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 1997, 80:98-106 [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ: Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995, 147:33-41 [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC: The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res 1999, 5:587-591 [PubMed] [Google Scholar]
- 20.Orre M, Rogers PAW: VEGF, VEGF-1, and VEGF-2, microvessel density and endothelial cell proliferation in tumors of the ovary. Int J Cancer 1999, 84:101-108 [DOI] [PubMed] [Google Scholar]
- 21.Skilling J, Squatrito RC, Connor JP, Niemann T, Buller RE: p53 gene mutation analysis and antisense-mediated growth inhibition of human ovarian carcinoma cell lines. Gynecol Oncol 1996, 60:72-80 [DOI] [PubMed] [Google Scholar]
- 22.Hendrix MJ, Seftor EA, Seftor RE, Fidler IJ: A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett 1987, 38:137-147 [DOI] [PubMed] [Google Scholar]
- 23.Seftor REB, Seftor EA, De Larco JE, Kleiner DE, Leferson J, Stetler-Stevenson WG, McNamara TF, Golub LM, Hendrix MJC: Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin Exp Metastasis 1998, 16:217-225 [DOI] [PubMed] [Google Scholar]
- 24.Thompson SA, Johnson MP, Heidger PM, Lubaroff DM: Characterization of the heterogeneity of the R-3327 rat prostatic tumors derived from single-cell clones. Prostate 1985, 6:369-387 [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000, 6:389-395 [DOI] [PubMed] [Google Scholar]
- 26.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 27.Bissell MJ: Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch: a rose by any other name? [Commentary.] Am J Pathol 1999, 155:675-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe’er J, Gruman LM: The prognostic value of tumor blood vessels morphology in primary uveal melanoma. Ophthalmology 1993, 100:1389-1398 [DOI] [PubMed] [Google Scholar]
- 29.: Breast Cancer Progression Working Party: Evidence for novel non-angiogenic pathway in breast-cancer metastasis. Lancet 2000, 355:1787-1788 [PubMed] [Google Scholar]
- 30.Hendrix MJC, Seftor EA, Kirschmann DA, Seftor REB: Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res 2000, 2:417-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sood AK, Sorosky JI, Dolan M, Anderson B, Buller RE: Distant metastases in ovarian cancer: association with p53 mutations. Clin Cancer Res 1999, 5:2485-2490 [PubMed] [Google Scholar]
- 32.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM: Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000, 156:1363-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidner N: Tumoral vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol 1998, 184:119-122 [DOI] [PubMed] [Google Scholar]
- 34.Fox SB: Tumour angiogenesis and prognosis. Histopathology 1997, 30:294-301 [DOI] [PubMed] [Google Scholar]
- 35.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG: Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 2000, 60:1388-1393 [PubMed] [Google Scholar]
- 36.Veikkola T, Karkkainen M, Claesson-Welsh l, Alitalo K: Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 2000, 60:203-212 [PubMed] [Google Scholar]
- 37.Li C, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S: CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. FASEB J 2000, 14:55-64 [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Graham J, Kahn JW, Schwartz EA, Gerritsen ME: Functional roles for PECAM-1(CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol 1999, 155:887-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM: Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 1997, 151:671-677 [PMC free article] [PubMed] [Google Scholar]
- 40.Zetter BR: Cell motility in angiogenesis and tumor metastasis. Cancer Invest 1990, 8:669-671 [DOI] [PubMed] [Google Scholar]
- 41.Liotta LA, Thorgeirrson UP, Garbisa S: Role of collagenases in tumor cell invasion. Cancer Metastasis Rev 1982, 1:277-288 [DOI] [PubMed] [Google Scholar]
- 42.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ: 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991, 113:437-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duhamel-Clerin E, Orvain C, Lanza F, Cazenave JP, Klein-Soyer C: Thrombin receptor-mediated increase of two matrix metalloproteinases, MMP-1 and MMP-3, in human endothelial cells. Arterioscler Thromb Vasc Biol 1997, 17:1931-1938 [DOI] [PubMed] [Google Scholar]
- 44.Henrikson SP, Salazar SL, Fenton JW, Pentecost BT: Role of thrombin receptor in breast cancer invasiveness. Br J Cancer 1999, 79:401-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R: Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998, 4:909-914 [DOI] [PubMed] [Google Scholar]
- 46.Kanda S, Tomasini-Johansson B, Klint P, Dixelius J, Rubin K, Claesson-Welsh L: Signaling via fibroblast growth factor receptor-1 is dependent on extracellular matrix in capillary endothelial cell differentiation. Exp Cell Res 1999, 248:203-213 [DOI] [PubMed] [Google Scholar]
- 47.Ponce ML, Nomizu M, Delgado MC, Kuratomi Y, Hoffman MP, Powell S, Yamada Y, Kleinman HK, Malinda KM: Identification of endothelial cell binding sites on the laminin gamma 1 chain. Circ Res 1999, 84:688-694 [DOI] [PubMed] [Google Scholar]




