Abstract
Angiotensin II receptors are the specific receptors of angiotensin II of the renin-angiotensin system. The existence and role of the receptors in the skin have not been determined. We immunohistochemically studied the expression of angiotensin receptors in the human skin. The results demonstrated the expression of angiotensin type 1 receptor (AT1) in the normal human suprabasal epidermis. The expression pattern suggests the role of AT1 in association with differentiation. In addition, we studied the expression of AT1 in squamous cell carcinoma (SCC) of the skin, SCC of the lip, and keratoacanthoma (KA). Our experiments showed that high, intermediate, and low levels of AT1 were observed in 37 (74.0%), 7 (14.0%), and 2 (4.0%) of 50 cases of SCC of the skin, respectively, and the negative periphery pattern was observed in 17 (77.3%) of 22 cases of KA. These observations suggest that the immunohistochemical study of AT1 is useful to distinguish SCC from KA. Studying the role and distribution of AT1 may help in understanding the pathophysiology of the skin.
Angiotensin II (AngII) of the renin-angiotensin system is the active biological peptide that regulates blood pressure and salt and water homeostasis. AngII exerts its actions by binding the specific receptors. There are two major subtypes of the angiotensin receptors; type 1 (AT1) and type 2 (AT2). 1 AT1 belongs to the superfamily of seven transmembrane domain, G-protein-coupled receptors. 1 The stimulation of AT1 activates classical second messenger systems, which include rapid production of diacylglycerol and inositol 1,4,5-triphosphate by phospholipase C-mediated hydrolysis of inositol phospholipids and activation of protein kinase C. 2 The rise in intracellular calcium is induced and associated with a variety of responses, such as vasoconstriction, synthesis, and secretion of aldosterone. Protein kinase C as well as elevated intracellular calcium levels can promote the expression of growth-related inducible transcription factors such as c-fos and c-jun. 3-6 The proteins encoded by these genes act as transcription factors for various target genes that may be involved in the stimulation of mitogenesis. Recently, it has been shown that AT1 also stimulates the Jak/STAT pathway. 7 The pathway has been previously identified for cytokine receptors that leads to transcriptional activation of early growth-response genes, and may thus contribute to the proliferative effects of AngII via AT1.
AT2 also belongs to the seven transmembrane receptors. The function of AT2 is a matter of controversy, although it has been suggested that signaling of AT2 is involved in antiproliferative effects, apoptosis, and neuronal differentiation. 8-10
In the human skin, little is known about the expression of the angiotensin receptors. In this report, we studied the expression of angiotensin receptors in the normal human skin. Based on the results, we studied the expression of AT1 in squamous cell carcinoma (SCC) and keratoacantoma (KA). It is sometimes difficult to distinguish SCC of the skin from KA because of the histological resemblance of these tumors. At present, few methods enable it possible to distinguish SCC from KA. Our experiments revealed differences between SCC and KA in AT1 expression.
Materials and Methods
Cases
A total of 50 cases of SCC of the skin and 14 cases of SCC of the lip were selected from a consecutive series of 69 cases diagnosed and treated for SCC at Yamagata University Hospital, Japan between 1978 and 1999 by excluding cases with insufficient tumor material (n = 5). All 22 cases that were diagnosed and treated for KA at the hospital between 1978 and 1999 were also studied. Normal back skin from a 38-year-old Japanese woman, normal lip from a 64-year-old Japanese man, and normal brain tissue from a 33-year-old man were used as normal controls.
Histology
Five-μm-thick paraffin-embedded tissue sections of all tumors were stained with hematoxylin and eosin (H&E). Histological typing and grading of SCCs were re-evaluated for this study according to Lever’s classification. 11
Immunohistochemistry
The specimens were fixed in 10% formalin and embedded in paraffin by routine procedures. Five-μm sections were cut for immunohistochemical investigation using the labeled streptavidin-biotin method as described previously. 12 The primary antibodies used in this study were anti-angiotensin type-1 (affinity-purified rabbit polyclonal anti-human AT1; Santa Cruz, Santa Cruz, CA) and type-2 receptor antibodies (affinity-purified goat polyclonal anti-human AT2; Santa Cruz). Briefly, endogenous peroxidase was blocked by incubating deparaffinized sections in 0.3% hydrogen peroxide in methanol. The sections were washed in phosphate-buffered saline (PBS), pH7.2, then incubated for 15 minutes at room temperature with defatted milk to block nonspecific reactions to the antibodies. The sections were subsequently incubated overnight at 4°C with primary antibodies and washed in PBS. After incubation with a mixture of biotinylated anti-rabbit immunoglobulin goat serum (DAKO, Carpinteria, CA) for anti-AT1 antibody and anti-goat immunoglobulin rabbit serum (DAKO) for anti-AT2 antibody, respectively, for 20 minutes at room temperature, the sections were then rewashed in PBS, and incubated with peroxidase-conjugated streptavidin (DAKO) for 20 minutes at room temperature. Then they were washed again in PBS and the reaction was visualized using 3,3′-diaminobenzidine tetrahydrochloride solution (0.2 mg/ml) containing 0.005% hydrogen peroxide. Sections were subsequently washed in water, counterstained with 1% methyl green, dehydrated, cleared, and mounted. Negative controls using normal rabbit and goat serum instead of the individual primary antisera were stained by the same procedures. Normal skin and brain tissues were used as positive controls for AT1 and AT2, respectively.
Evaluation of the Stainings with AT1
In cases of SCC of the skin, SCC of the lip, and KA, the percentage of AT1-positive tumor cells of all neoplastic cells in the section was estimated and graded into one of three categories: low (<35%), intermediate (35 to 75%), or high (>75%). Among the cases that were graded high, there was a group of cases that showed a distinct staining pattern. In this pattern, 1 to 2 layers of the tumor cells that were located at the periphery of the tumor nest stained negatively. This pattern was termed as “negative periphery” and was graded as one category independent of the other three categories that showed no particular arrangement of AT1-positive cells.
Results
AT1
Normal Human Skin
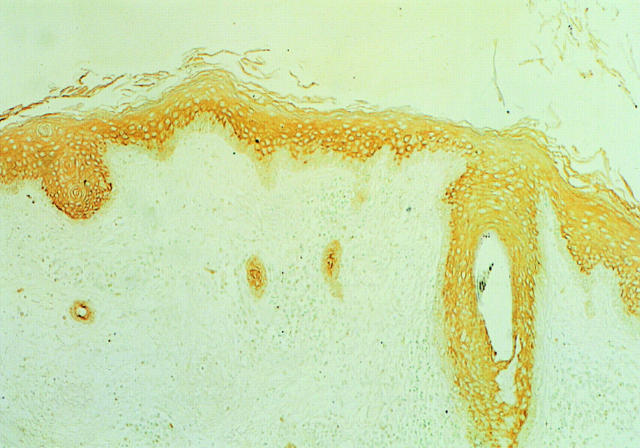
In the interfollicular epidermis, the suprabasal epidermis stained positive with AT1, but the basal layer was negative (Figure 1) ▶ . In the suprabasal epidermis, cell membrane stained positive, but nuclei did not.
Figure 1.
Immunohistochemical staining of AT1 in the normal skin. In the normal interfollicular epidermis, the suprabasal layers were positive, but the basal layer was negative. In the suprabasal epidermis, cell membrane was positive, but nuclei were not. In an infundibulum of a hair follicle, the suprabasal layers of the outer root sheath were all positive, and continued to the interfollicular, suprabasal epidermis. The basal layer of the outer root sheath was negative and continued to the basal layer of the interfollicular epidermis. Original magnification, ×33.
As to the infundibulum of the hair follicle, the suprabasal layers of the outer root sheath were all positive, and continued to the interfollicular, suprabasal epidermis. The basal layer of the outer root sheath was negative and continued to the basal layer of the interfollicular epidermis.
Normal Lip
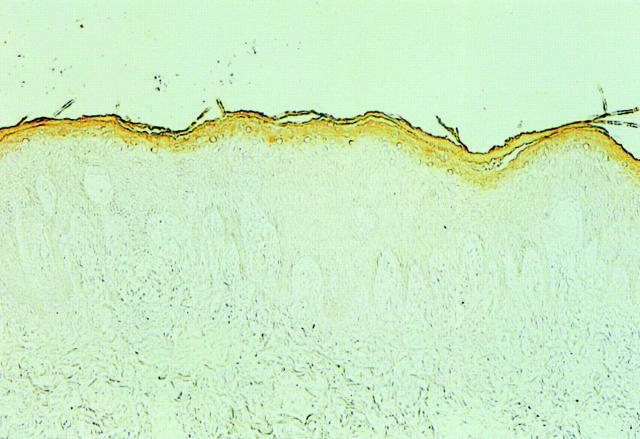
In the vermilion border, unlike the normal epidermis, the upper 2 to 3 layers of the epithelium stained positive with AT1, but the other layers of the epithelium including the basal layer were negative (Figure 2) ▶ .
Figure 2.
Immunohistochemical staining of AT1 in the normal lip. Only the uppermost two to three layers of the epithelium were positive. Original magnification, ×33.
SCC of the Skin
On the whole, high, intermediate, and low levels of AT1 were observed in 37 (74.0%), 7 (14.0%), and 2 (4.0%) of all 50 cases, respectively (Figure 3 ▶ ; A, B, and C and Table 1 ▶ ). Four cases (8.0%) showed negative periphery pattern (Figure 3D) ▶ . In these four cases, the periphery of the tumor nests was composed of less-keratinized tumor cells in H&E stain.
Figure 3.
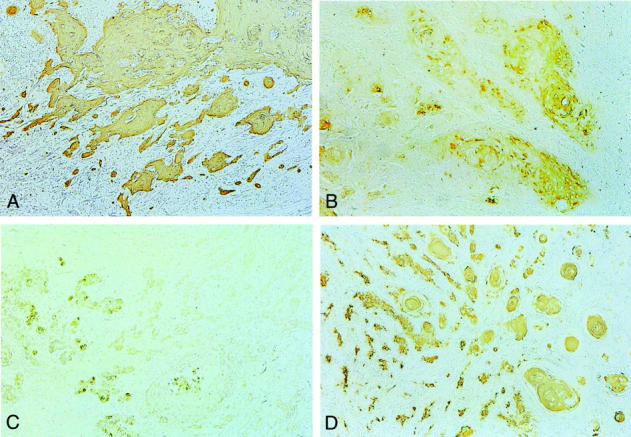
Immunohistochemical staining of AT1 in SCC of the skin. A: Moderately differentiated type graded as “high.” Most of the tumor cells including the tumor nests infiltrating the dermis stained positive. B: Moderately differentiated type graded as “intermediate.” The tumor nests were composed of both AT1-positive and AT1-negative cells. C: Poorly-differentiated type graded as “low.” A few of the tumor cells stained positive, but the majority of the tumor cells were not. D: Well-differentiated type graded as “negative periphery.” The center of the tumor nests was composed of well-keratinized cells that were AT1-positive, but the periphery was composed of AT1-negative cells. Original magnifications: ×20 (A–D).
Table 1.
Summary of Immunohistochemical Staining Patterns of AT1 Receptor in 50 Cases of SCC of the Skin and 22 Cases of Keratoacanthoma
| Histological typing | Number of cases | |||
|---|---|---|---|---|
| High (>75%)* | Intermediate (35–75%)* | Low (<35%)* | Negative periphery | |
| SCC | ||||
| Well differentiated | 17 | 3 | 0 | 1 |
| Moderately differentiated | 18 | 4 | 1 | 2 |
| Poorly differentiated | 0 | 0 | 1 | 1 |
| Acantholytic SCC | 2 | 0 | 0 | 0 |
| Keratoacanthoma | 4 | 1 | 0 | 17 |
* Percentage of AT1-positive cells of tumor cells in the specimen.
KA
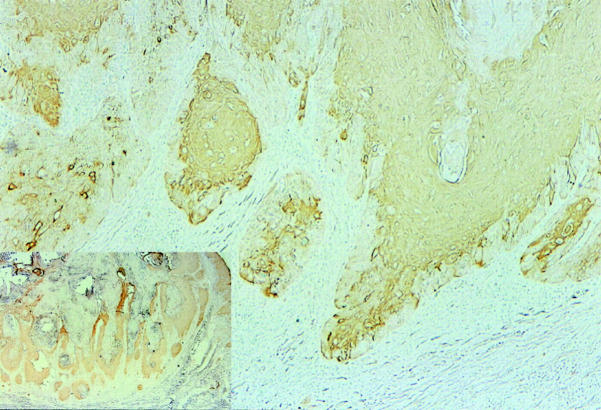
In 17 cases (77.3%), the tumor showed a negative periphery pattern (Figure 4) ▶ . In a typical case, the eosinophilic and keratinizing cells with a glassy appearance at the center of the lobules in H&E stain were AT1-positive. However, the one or two layers of basophilic, nonkeratinized cells at the periphery of the lobules were negative. In some of the nests that were located at the periphery of the tumor, most of the tumor cells were AT1-negative. In five cases (22.7%), tumors did not show a negative periphery pattern.
Figure 4.
Immunohistochemical staining of AT1 in KA. The cells located at the center of the tumor stained positive. However, the cells located at the periphery of the tumor were negative. In some of the tumor nests that were located at the periphery of the tumor, most of the cells were negative. Original magnification, ×20. Insert: The tumor has a keratotic plugging surrounded by AT1-positive cells. Original magnification, ×5.
SCC of the Lip
High, intermediate, and low levels of AT1 were observed in 3 (21.4%), 3 (21.4%), and 6 (42.9%) of all 14 cases, respectively (Table 2) ▶ . Two cases (14.3%) showed a negative periphery pattern.
Table 2.
Summary of Immunohistochemical Staining Patterns of AT1 in 14 Cases of SCC of the Lip
| Histological typing | Number of cases | |||
|---|---|---|---|---|
| High (>75%)* | Intermediate (35–75%)* | Low (<35%)* | Negative periphery | |
| Well differentiated | 2 | 1 | 3 | 1 |
| Moderately differentiated | 1 | 2 | 2 | 1 |
| Poorly differentiated | 0 | 0 | 1 | 0 |
* Percentage of AT1-positive cells of tumor cells in the specimen.
Angiotensin Type 2 Receptor
All specimens were negative with anti-AT2 antibody.
Discussion
AngII exerts the effects through its specific receptors. Currently, two main angiotensin-receptor subtypes, AT1 and AT2, which display a heterogeneous distribution in peripheral tissues and brain, have been characterized. 1 Our immunohistochemical study demonstrated the expression of AT1 in the normal human skin and the skin tumors. The classical role of AngII in regulating blood pressure is mediated by AT1. In addition, the promoting effects of AngII for cell proliferation and extracellular matrix production have been recently attributed to AT1. 1 However, in the epidermis, the notion of a trophic effect of AT1 activation seems difficult to apply. AT1 expression was found in the squamous cell layer and the granular layer but not detected in the basal cell layer. The expression pattern is the same with that of keratin 10. In the normal epidermis, commitment to differentiation of basal cells is accompanied by a switch in keratin gene expression from keratins 5 and 14 to 1 and 10, 13 and keratin 10 is presumed to be a marker of differentiation. Furthermore, keratinocyte proliferation by AngII is mediated through a non-AT1, non-AT2 AngII receptor. 14 Taken together, the possible roles of angiotensin receptors in human keratinocytes are presumed as AT1 in differentiation and a non-AT1, non-AT2 receptor in proliferation.
SCC of the skin is generally believed to originate from the normal squamous cells that are located in the suprabasal epidermis. 11 In our experiments, the suprabasal epidermis stained positive with AT1, and 37 cases (74.0%) of 50 cases were graded as high in terms of immunostaining pattern of AT1. These results seem to reflect the belief of suprabasal origin of SCC. In well-differentiated SCC, high, intermediate, and low levels of AT1 were observed in 17 (81.0%), 3 (14.3%), and 0 (0.0%) of the 21 cases, respectively. In moderately differentiated SCC, high, intermediate, and low levels of AT1 were observed in 18 (72.0%), 4 (16.0%), and 1 (4.0%) of the 25 cases, respectively. In poorly differentiated SCC, high, intermediate, and low levels of AT1 were observed in 0 (0.0%), 0 (0.0%), and 1 (50.0%) of the two cases, respectively. The negative periphery pattern was found only in 4 (8%) of 50 cases. This pattern seems to appear when tumor cells located at the periphery of tumor nests show less keratinization even if the center of the nests is well keratinized.
On the other hand, KA, which is a benign skin tumor, often resembles well-differentiated or moderately differentiated SCC clinically and histologically. In some cases, KA shows a greater degree of nuclear atypia than do some SCCs, and this makes the differentiation of two diseases very difficult. It is generally believed that KA has its origin in the infundibulum of one or several hair follicles. 11 In the infundibulum of the hair follicle, the suprabasal layers stained positive and the basal cell layer stained negative. Our experiments showed that 17 cases (77.3%) of all 22 cases were graded as negative periphery. These observations seem to reflect the infundibular origin of KA. The results of immunostaining of SCC and KA demonstrated a striking contrast. Our experiments indicate that the immunohistochemical study of the expression of AT1 assists one to distinguish SCC from KA.
In the epithelium of normal lip, positive staining of AT1 was found only in the uppermost 2 to 3 layers. This finding may be a result of less keratinization of the lip epithelium, because the normal lip epithelium shows less granular layer and horny layer than the epidermis of the skin does. In SCC of the lip, on the whole, high, intermediate, and low levels of AT1 were observed in 3 (21.4%), 3 (21.4%), and 6 (42.9%) of 14 cases, respectively. The percentage of AT1-positive cells of the tumor cells is low compared to that of SCC of the skin, even in well-differentiated or moderately differentiated types. This relatively low percentage may reflect less immunoreactivity with AT1 of the normal lip epithelium.
Our experiments revealed the expression and distribution of AT1 in the normal epidermis, lip, and the skin tumors. The results of our study suggest that the immunohistochemical study of AT1 is helpful in distinguishing SCC from KA and in understanding the pathophysiology of the skin and skin tumors.
Acknowledgments
We thank Yutaka Hozumi for technical assistance with immunohistochemistry.
Footnotes
Address reprint requests to Hikaru Takeda, M.D., Department of Dermatology, Yamagata University School of Medicine, 2-2-2, Iida-Nishi, Yamagata 990-9585, Japan. E-mail: hitakeda@med.id.yamagata-u.ac.jp.
References
- 1.Helin K, Stoll M, Meffert S, Stroth U, Unger T: The role of angiotensin receptors in cardiovascular diseases. Ann Med 1997, 29:23-29 [DOI] [PubMed] [Google Scholar]
- 2.Csikos T, Chung O, Unger T: Receptors and their classification: focus on angiotensin II and AT2 receptor. J Hum Hypertens 1991, 115:1661-1674 [DOI] [PubMed] [Google Scholar]
- 3.Naftilan AJ, Pratt RE, Eldridge CS, Lin HL, Dzau VJ: Angiotensin II induces c-fos expression in smooth muscle via transcriptional control. Hypertension 1989, 13:706-711 [DOI] [PubMed] [Google Scholar]
- 4.Taubman MB, Berk BC, Izumo S, Tsuda T, Alexander RW, Nadel-Grinard B: Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem 1989, 264:526-530 [PubMed] [Google Scholar]
- 5.Itoh H, Pratt RE, Dzau VJ: Interaction of atrial natriuretic polypeptide and angiotensin II on protooncogene expression and vascular cell growth. Biochem Biophys Res Commun 1991, 176:1601-1609 [DOI] [PubMed] [Google Scholar]
- 6.Lyall F, Dornan ES, McQueen J, Boswell F, Kelly M: Angiotensin II increases proto-oncogene expression and phosphoinositide turnover in vascular smooth muscle cells via the angiotensin II AT1 receptor. J Hypertens 1992, 10:1463-1469 [DOI] [PubMed] [Google Scholar]
- 7.Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE: Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 1995, 375:247-250 [DOI] [PubMed] [Google Scholar]
- 8.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T: Angioten II AT2 receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 1995, 95:651-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T: The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol 1996, 122:59-67 [DOI] [PubMed] [Google Scholar]
- 10.Gallinat S, Csikos T, Meffert S, Herdegen T, Stoll M, Unger T: The angiotensin AT2 receptor downregulates neurofilament M in PC12W cells. Neurosci Lett 1997, 227:29-32 [DOI] [PubMed] [Google Scholar]
- 11.Kirkham N: Tumors and cysts of the epidermis. Elder D Elenitsas R Jaworsky C Johnson B, Jr eds. Lever’s Histopathology of the Skin. 1997, :pp 685-746 Lippincott-Raven, Philadelphia [Google Scholar]
- 12.Giorno R: A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol 1984, 2:161-166 [PubMed] [Google Scholar]
- 13.Fuchs E, Green H: Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 1980, 19:1033-1042 [DOI] [PubMed] [Google Scholar]
- 14.Stecklings UM, Artuc M, Paul M, Stoll M, Henz BM: Angiotensin II stimulates proliferation of primary human keratinocytes via a non-AT1, non-AT2 angiotensin receptor. Biochem Biophys Res Commun 1996, 229:329-333 [DOI] [PubMed] [Google Scholar]