To the Editor-in-Chief:
It has been reported that T cells are involved in the process of granuloma formation and may be necessary to induce the formation of granulomas. 1 However, in an issue of The American Journal of Pathology, North and Izzo showed the formation of granulomas in severe combined immunodeficient (SCID) mice, which are not able to generate efficient T and B cells, after infection with Bacillus Calmette-Guerin (BCG). 2 These granulomas were found in spleen and liver. Moreover, the authors described a deficiency of these SCID mice to form granulomas in the lungs after inoculation with BCG. 2 In an attempt to establish a granuloma model to analyze the process of granuloma formation, we intravenously inoculated SCID mice with BCG (strain Connaught) for means of control and examined the animals histomorphologically after 30 days.
Three 10- to 12-week-old female C.B.−17scid/scid mice were housed in filter-top cages in an incubator with a constant horizontal flow of filtered air and supplied with food and water ad libitum. The mice were inoculated intravenously with BCG (10 8 CFU per animal; courtesy of E. Richter, National Reference Center for Mycobacteria, Borstel, Germany). The mice were sacrificed 28 to 30 days after inoculation, and the organs were kept in buffered formaldehyde solution (4% vol/vol). The tissue was paraffin-embedded, sliced, and hematoxylin-and-eosin-stained. Visualization of BCG in the tissues was performed by Ziehl-Neelsen staining.
With regard to the formation of granulomas in spleen and liver, we reproduced the results obtained in the study of North and Izzo. 2 Furthermore, in accordance with previous results, we found isolated alveolar macrophages infected with BCG in the lungs of these mice. 2 However, we also observed the generation of well formed granulomas in the lungs of these mice. These granulomas were not just aggregations of alveolar macrophages, but were morphologically comparable to the granulomas found in spleen and liver (Figure 1a) ▶ . Inside the pulmonary granulomas, acid fast bacilli were detected by Ziehl-Neelsen staining (Figure 1b) ▶ and identified as mycobacteria from the Mycobacterium tuberculosis complex by means of polymerase chain reaction targeting mycobacterial 16S rDNA. 3 This demonstrates that, upon infection with BCG, SCID mice can produce pulmonary granulomas.
Figure 1.
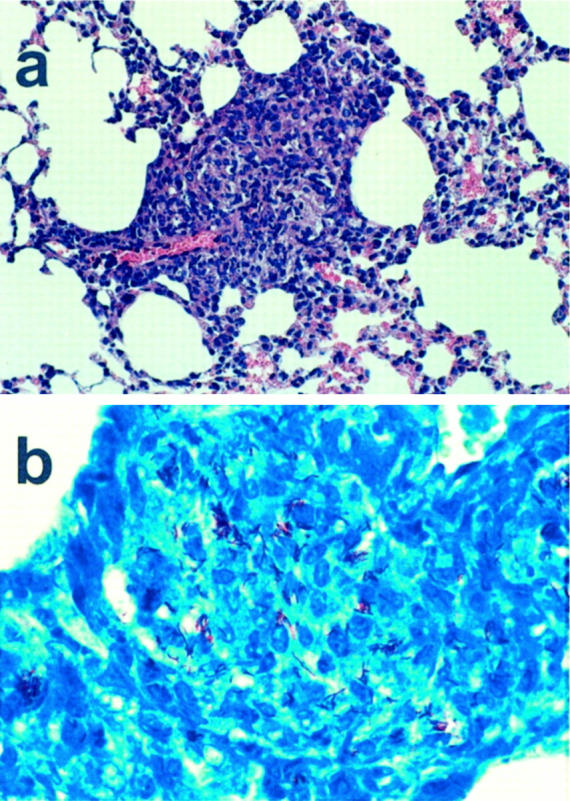
Sections of BCG-induced granulomas in the lungs of a SCID mouse. a: Low-power micrograph (×125) of a hematoxylin-and-eosin-stained section showing a granuloma. b: High-power micrograph (×500) of a Ziehl-Neelsen-stained section showing the infection with BCG inside the granuloma.
Besides tumor necrosis factor-α release, the delivery of interferon-γ (IFN-γ) is an important prerequisite for granuloma formation and the eradication of the bacteria. 4,5 In immunocompetent mice the most important source of IFN-γ is Th1 cells. In a recent publication, it was demonstrated that the development of T-cell-independent granuloma is reliant on the induction of IFN-γ release by natural killer (NK) cells. 6 The induction of NK-cell IFN-γ release is dependent on the release of interleukin-12 and -10 by macrophages. 7,8 In a model of Pneumocystis carinii infection, it was demonstrated that although alveolar macrophages are able to stimulate NK cells to release IFN-γ, they are inferior compared to splenic macrophages. 8
North and Izzo used the BCG strain Pasteur, so it can be speculated that there might be strain-specific differences in the induction of IFN-γ-inducing factors by macrophages in the lung, liver, and spleen, since the number of bacteria injected and the time spans of infection have been comparable.
Our experiments demonstrate that the presence of T cells is not mandatory for the generation of pulmonary granulomas in SCID mice. We conclude that SCID mice infected with BCG are capable of generating granulomas in the lungs.
References
- 1.du Bois RM, Holroyd KJ, Saltini C, Crystal RG: Granulomatous process. The lung-scientific foundations: Edited by Crystal RG, West JB. 1991, :pp. 1925-1938 Raven Press, New York [Google Scholar]
- 2.North RJ, Izzo AA: Granuloma formation in severe combined immunodeficient (SCID) mice in response to progressive BCG infection: tendency not to form granulomas in the lung is associated with faster bacterial growth in this organ. Am J Pathol 1993, 142:1959-1966 [PMC free article] [PubMed] [Google Scholar]
- 3.Richter E, Schlüter C, Duchrow M, Hahn M, Rüsch-Gerdes S, Galle J, Flad HD, Gerdes J: An improved method for the species-specific assessment of mycobacteria in routinely formalin-fixed and paraffin-embedded tissues. J Pathol 1995, 175:85-92 [DOI] [PubMed] [Google Scholar]
- 4.Ehlers S, Richter E: Gamma interferon is essential for clearing Mycobacterium genavense infection. Infect Immun 2000, 68:3720-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL: IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol 1999, 11:346-351 [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Hansch H, Bancroft G, Ehlers S: T-cell-independent granuloma formation in response to Mycobacterium avium: role of tumour necrosis factor-alpha and interferon-gamma. Immunology 1997, 92:413-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata Y, Foster LA, Kurimoto M, Okamura H, Nakamura RM, Kawajiri K, Justice JP, Van Scott MR, Myrvik QN, Metzger WJ: Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK-cell production of IFN-gamma. J Immunol 1998, 161:4283-4288 [PubMed] [Google Scholar]
- 8.Warschkau H, Yu H, Kiderlen AF: Activation and suppression of natural cellular immune functions by Pneumocystis carinii. Immunobiology 1998, 198:343-360 [DOI] [PubMed] [Google Scholar]


