Abstract
RA301/Tra2β, a sequence-specific RNA-binding protein, was first cloned as a stress molecule in re-oxygenated astrocytes. In human vascular tissues, we have found enhanced RA301/Tra2β expression in coronary artery with intimal thickening, and atherosclerotic aorta. Balloon injury to the rat carotid artery induced RA301/Tra2β transcripts followed by expression of the antigen, which was detected in medial and neointimal vascular smooth muscle cells (VSMCs). In cultured VSMCs, hypoxia/re-oxygenation caused induction of RA301/Tra2β and was accompanied by cell proliferation, both of which were blocked by the addition of either diphenyl iodonium, a NADPH oxidase inhibitor, PD98059, a mitogen-activated protein kinase kinase inhibitor, or antisense oligonucleotide for RA301/Tra2β. Consistent with a link between RA301/Tra2β and cell proliferation, platelet-derived growth factor also induced expression of RA301/Tra2β in cultured VSMCs. These data suggest a possible role for RA301/Tra2β in the regulation of VSMC proliferation, especially in the setting of hypoxia/re-oxygenation-induced cell stress.
Exposure of cells to hypoxia results in the expression of a set of stress proteins, termed “oxygen-regulated proteins.” 1 These stress-induced proteins contribute to the adaptive response, at least in part, by protecting biosynthetic pathways. 2,3 In a complementary manner, restitution of normal oxygen tensions after hypoxia/ischemia triggers another series of cellular stress responses that are quite distinct from those observed in hypoxia, even though these two environmental perturbations are closely linked in vivo. De novo protein synthesis occurring in re-oxygenation is essential for re-establishment of homeostasis, as suggested by our previous observation that inhibition of protein synthesis early in re-oxygenation caused death of cultured astrocytes. 4 This finding led us to clone proteins rapidly expressed in astrocytes consequent to hypoxia/re-oxygenation, including RA301/Tra2β 5 and RA410. 6 Both of these polypeptides are also expressed in response to central and peripheral nervous system injury in vivo. 5-7 Furthermore, RA301/Tra2β, which was later identified as a sequence-specific RNA-binding protein, 8 participates in the elaboration of neurotrophic mediators by re-oxygenated astrocytes, 5 suggesting a functional role for this molecule in controlling protein expression.
Ischemia-induced perturbation of vascular function is associated with a range of blood vessel disorders. For example, decreased arterial wall oxygen tension occurs in atherosclerotic lesions. 9,10 In this context, we have demonstrated expression of a 150-kd oxygen-regulated protein (ORP150) in atherosclerotic lesions, as well as its capacity to buttress cellular viability under hypoxic conditions, especially in the presence of otherwise toxic levels of modified low density lipoprotein. 11 In atherosclerotic lesions, re-oxygenation may also trigger a range of mechanisms. For example, cycles of ischemic stress and re-oxygenation in atherosclerotic lesions may promote macrophage generation of pro-inflammatory mediators, such as interleukin-1, thereby contributing to remodeling of the vessel wall. 12
These considerations led us to examine the expression of RA301/Tra2β in human vascular tissues, balloon-injured rat carotid arteries, and cultured vascular cells. Our results demonstrate expression of RA301/Tra2β in vascular smooth muscle cells (VSMCs) of neointimal lesions. Analysis of cultured VSMCs showed that hypoxia/re-oxygenation stimulated expression of RA301/Tra2β and proliferation, both of which seemed to be related based on their parallel inhibition by diphenyl iodonium (DPI), PD98059, and antisense oligonucleotides for RA301/Tra2β. Because RA301/Tra2β functions as a sequence-specific RNA-binding protein, its expression in re-oxygenated vessels might be important for posttranscriptional regulation of multiple genes important in the vascular stress response.
Materials and Methods
Preparation of Human and Animal Tissues
Twenty specimens of human tissues were obtained from autopsy cases within 1 to 4 hours of death (see text for the description of patients). Tissue samples for RNA extraction were stored at −80°C until use, and those for in situ hybridization and immunohistochemistry were fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.0, and embedded in paraffin. Certain samples for immunohistochemical analysis were fixed in 10% buffered formaldehyde and embedded in paraffin. Tissue samples from the rat arterial injury model were excised and fixed in 10% buffered formaldehyde and embedded in paraffin. The rat carotid balloon angioplasty model was performed as described. 13 Serial sections (3 μm) were cut from either rat or human vascular tissues for further study.
In Situ Hybridization
A RA301/Tra2β BglII fragment corresponding to 292 to 531 (240 bp) 5 was subcloned into the BamHI site of the pBluescript SK(−) vector (Stratagene Inc., La Jolla, CA) and sequencing was performed. 14 After linearization of the plasmid, digoxigenin-labeled single-stranded RNA was synthesized by T7 and T3 RNA polymerase with digoxigenin-UTP and unlabeled ATP, GTP, and CTP. Details of the in situ hybridization protocol have been described. 15 Hybridization was performed at 50°C for 16 hours, and signals were detected using a Nucleic Acid Detection kit (Boehringer Mannheim Biochemicals, Mannheim, Germany). Controls included hybridization with sense probe, RNase treatment before hybridization, and omission of either the antisense RNA probe or the antibody to digoxigenin.
Immunohistochemistry
Proliferating cells were identified with a mouse monoclonal antibody to proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Smooth muscle cells and macrophages were identified with mouse monoclonal antibodies 1A4 and PG-M1 (anti-CD68) (DAKO SA, Glostrup, Denmark). Endothelial cells were identified with goat polyclonal antibody to mouse PECAM-1 (Santa Cruz Biotechnology Inc.) as described. 16 For detection of RA301/Tra2β and PCNA antigens, sections were microwaved for 10 minutes and incubated in H2O2 (0.3%) in methanol for 30 minutes, followed by washing in phosphate-buffered saline (PBS) (0.1 mol/L) and incubation with normal mouse serum (1%) for 30 minutes at room temperature (to block nonspecific binding of the antibody). Slides were incubated with rabbit anti-RA301/Tra2β polyclonal antibody (40 μg/ml) or mouse anti-PCNA monoclonal antibody (5 μg/ml) for 18 hours at 4°C. Sites of primary antibody binding were detected by incubating slides with horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG followed by reaction with the substrate, 3,3′-diaminobenzidine tetrahydrochloride. As a negative control, either preimmune rabbit or mouse serum was used instead of the antiserum. Antibody specificity was confirmed as described previously. 5 For the detection of α-smooth muscle actin or CD68 or PECAM-1, sections were treated as the same method as described above except for the microwave treatment. Co-expression of RA301/Tra2β and PCNA was confirmed with confocal laser-scanning microscopy (Olympus LSM-GB200; Olympus, Tokyo, Japan) as described. 17 Fluorescein isothiocyanate-conjugated swine anti-rabbit IgG and R-phycoerythrin-conjugated goat anti-mouse IgG (DAKO SA) were used as the secondary antibodies. The excitation wavelength is 488 nm for fluorescein isothiocyanate and 515 nm for R-phycoerythrin.
Cell Culture and Conditions for Hypoxia/Re-Oxygenation
Rat VSMCs were isolated by enzyme digestion from thoracic aortae of 12-week-old Sprague-Dawley rats as described. 18 VSMC populations obtained by this method were grown to the fifth passage in Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Grand Island, NY) supplemented with 10% fetal calf serum. Human aortic endothelial cells were purchased from Kurabo (Osaka, Japan) and cultured according to previously described methods. 19 Human mononuclear phagocytes were cultured as described. 11 Cells were plated at a density of 2 × 10 4 cells/cm2, and exposed to hypoxia using an incubator attached to a hypoxia chamber that maintained a humidified atmosphere with low oxygen tension (pO2 = 12 to 14 torr; Coy Laboratory Products, Ann Arbor, MI) as described. 19 After exposure to hypoxia, cells were returned to ambient atmosphere. Oxygen tension in the medium was measured using a blood gas analyzer (ABL-2; Radiometer, Sweden). Cell viability was assessed by morphological criteria, trypan blue exclusion, lactate dehydrogenase (LDH) release, and general protein synthesis was measured by the incorporation of 3H-leucine to trichloroacetic acid-precipitable material. 4 Cell proliferation was evaluated by measuring the change in cell number using a Coulter counter (Coulter Electronic, Hialeah, FL) and by the dimethylthiazoldiphenyl tetrazolium bromide (MTT) assay.
Northern Blot Analysis
Total RNA was extracted from the indicated tissue of human autopsy cases as described. 20 After purification of total RNA, its quality was judged by Northern analysis and hybridization with a human β-actin probe. RNA samples that failed to show a distinct β-actin band were excluded from further experiments. For Northern blotting, total RNA (10 μg) was fractionated by agarose gel (1%) electrophoresis, transferred to Hybond N+ membrane (Amersham International, Buckinghamshire, UK) and then hybridized with a 32P-dCTP-labeled rat RA301/Tra2β cDNA probe. After hybridization, the membrane was washed and signals were detected by autoradiography. Total RNA was processed from cultured cells using the same procedure.
Western Blot Analysis
Detection of RA301/Tra2β antigen in cultured vascular cells was performed as described previously. 5 In brief, either cultured rat VSMCs, human aortic endothelial cells, or human mononuclear phagocytes were exposed to hypoxia for 36 hours, followed by re-oxygenation. At the indicated time points, cells were harvested and lysed in PBS (pH 7.4) containing Nonidet P-40 (1%), sodium dodecyl sulfate (0.5%), and ethylenediaminetetraacetic acid (5 mmol/L). After measurement of protein content, 21 ∼5 μg of protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%) under reducing condition, transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore Corporation, Bedford, MA), and incubated with either rabbit anti-RA301/Tra2β polyclonal antibody (5 μg/ml) 5 or mouse anti-PCNA monoclonal antibody (0.5 μg/ml; Santa Cruz Biotechnology Inc.). Sites of binding of primary antibody were detected with either anti-rabbit IgG or anti-mouse IgG, both conjugated to alkaline phosphatase (Sigma, St. Louis, MO). Where indicated, cultured rat VSMCs were maintained under serum-free conditions for 48 hours. After the addition of either platelet-derived growth factor-BB (PDGF-BB) (10 ng/ml; Pepro Tech EC, London, UK) or angiotensin II (100 μg/ml; Peptide Institute, Osaka, Japan), cultures were further maintained in normoxia for up to 48 hours. Cells were then harvested at the indicated time points, followed by Western blot analysis as described above.
Detection of Superoxide Anion (O2−) in Re-Oxygenated VSMCs
O2− was measured by lucigenin-based chemiluminescence by adding bis N-methyl acridinium nitrate (lucigenin, 0.2 mg/ml; Sigma)/CaCl2 (10 mmol/L) to hypoxic VSMCs (∼10 7 cells) suspended in PBS at the time of re-oxygenation. Chemiluminescence was detected using a luminometer (BRL-301; Aloka, Tokyo, Japan), after equilibration of the cell suspension with 5% CO2/room air. 22
Effects of DPI, PD98059, and RA301/Tra2β Antisense Oligonucleotide on Re-Oxygenated VSMCs
Rat VSMCs (∼5 × 10 4 cells/well) were exposed to hypoxia for 36 hours, followed by re-oxygenation. Either DPI, PD98059, or sense/antisense oligonucleotide for RA301/tra2β 5 was added to culture medium 30 minutes before re-oxygenation. After re-oxygenation, cells were maintained in normoxia for 4 hours and then lysed in PBS containing Nonidet P-40 (1%) and sodium dodecyl sulfate (0.5%), as described above. Protein extracts (∼10 μg/lane) were then subjected to Western blot analysis using either rabbit anti-RA301/Tra2β polyclonal antibody or mouse anti-human inducible 70-kd heat-shock protein (HSP70i) monoclonal antibody (0.2 μg/ml; Amersham International, Buckinghamshire, UK) as described previously. 23 Where indicated, protein extracts were subjected to Western blot analysis using either rabbit anti-ERK1/2 polyclonal antibody for the detection of total p42/44 mitogen-activated protein kinase, or anti-active MAPK polyclonal antibody or anti-active c-Jun N-terminal kinase (JNK) antibody for the detection of phosphorylated p42/44 MAP kinase or phosphorylated JNK (Promega, Madison, WI).
Cellular proliferation and viability were also assessed in response to the above agents. VSMCs were plated in 24-well plates (∼10 4 cells/well) and exposed to hypoxia for 36 hours. Cells were then re-oxygenated in the presence of DPI, PD98059, or sense/antisense oligonucleotide for RA301/Tra2β. Cultures were maintained for an additional 48 hours in normoxia and cell proliferation was quantified using the MTT (Sigma Chemical Co.) rapid colorimetric assay, as described. 24 In brief, MTT (100 μl of 5 mg/ml solution of MTT in PBS) was added to all wells for the final 4 hours of re-oxygenation. Cells were lysed with 0.02 mol/L HCl/isobutanol, and optical density at 520 nm was determined. Where indicated, DPI was added to the culture at the indicated time points before or after re-oxygenation.
Cell viability was assessed by the release of LDH activity into culture supernatants using a commercially available kit (Wako Chemicals, Japan). Cultured rat VSMCs plated in 24-well plates (∼10 4 cells/well) were exposed to hypoxia for 36 hours and re-oxygenated in the presence of either DPI or PD98059 at the indicated concentrations. Cells were further maintained for 48 hours under normoxic condition, followed by the measurement of LDH activity in the supernatant as described in the manual of the kit.
In the above experiments, sense and antisense oligonucleotides for RA301/Tra2β were used: 5 sense, 3′-CGA CAG CGA GTA CTG AGG CC-5′ and antisense, 5′-GCT GTC GCT CAT GAC TCC GG-3′.
Statistical Analysis
Where indicated, statistical analysis was performed by multiple comparison analysis (Neuman-Keul’s analysis) after analysis of variance.
Results
Expression of RA301/Tra2β in Human Vascular Tissues
Human arteries harvested postmortem from 20 cases were analyzed by Northern blotting for expression of RA301/Tra2β. Among them, representative cases are shown in Figure 1 ▶ . Human spleen was used as a positive control. Transcripts for RA301/Tra2β were obviously observed in an aorta with atheromatous plaques from a 58-year-old male who died of cerebral hemorrhage because of hypertension and had a past history of hypercholesterolemia (lane 8), in an atherosclerotic coronary artery of a 63-year-old male who died of gastric cancer/hepatocellular carcinoma and had a past history of hypertension and angina pectoris (lane 9), and in an atherosclerotic aorta of a 69-year-old male who died of cardioembolic brain infarction (lane 10).
Figure 1.
Northern blotting for RA301/Tra2β mRNA in human arteries. Total RNA was extracted from human vascular tissues, subjected to electrophoresis (10 μg/lane) and transferred to nylon membranes. Northern blotting was performed using either a 32P-dCTP-labeled human RA301/Tra2β probe (top) or human β-actin probe (bottom). The numbers above lanes represent samples prepared from human spleen (lane 1, positive control), aorta from a 0-year-old male who died of congenital heart disease (lane 2), aorta of a 1-year-old male who died of congenital heart disease (lane 3), aorta of a 13-year-old male who died of congestive heart failure because of dilated cardiomyopathy (lane 4), aorta of a 31-year-old female who died of congestive heart failure because of cardiac sarcoma (lane 5), aorta of a 44-year-old male who died of congestive heart failure because of dilated cardiomyopathy (lane 6), aorta of a 55-year-old female who died of congestive heart failure because of dilated cardiomyopathy (lane 7), aorta from a 58-year-old male who died of cerebral hemorrhage because of hypertension and had a past history of hypercholesterolemia (lane 8), coronary artery from a 63-year-old male who died of gastric cancer and hepatocellular carcinoma, and had past history of hypertension and angina pectoris (lane 9), aorta of a 69-year-old male who died of cardioembolic brain infarction (lane 10), aorta of an 82-year-old male who died of congestive heart failure because of combined valvular disease (lane 11), and aorta of an 81-year-old female who died of cardioembolic brain infarction. The migration of ribosomal RNA is indicated on the left. Arrowheads indicate the splicing variants of RA301/RA301/Tra2β transcripts.
Note that in each case, several bands because of alternative splicing/editing of RA301/Tra2β message are visualized (demonstrated by the arrowheads). 25,26 In contrast, mRNA for RA301/Tra2β was at low levels in either almost normal aortae from patients (0 to 55 years old) who died of congestive heart failure because of congenital heart disease or cardiomyopathy (lanes 2 to 7) or advanced atherosclerotic lesions with extensive fibrosis and scarring from aortae of an 82-year-old male who died with congestive heart failure because of valvular heart disease (lane 11) and an 81-year-old female who died of a cardioembolic brain infarction (lane 12).
In situ hybridization and immunohistological studies were performed to further analyze the expression of RA301/Tra2β in the above vascular samples. A coronary artery from a 63-year-old male (Figure 1 ▶ , lane 10) who had a past history of hypertension and angina pectoris was studied first (Figure 2A ▶ displays hematoxylin and eosin (H&E) staining for orientation). In situ hybridization with the antisense RA301/Tra2β probe showed the presence of RA301/Tra2β transcripts (Figure 2, B and E) ▶ in proliferating smooth muscle cells based on co-localization with PCNA (Figure 2C) ▶ and α-smooth muscle actin (Figure 2D) ▶ . To ensure the color reaction of known positive cells for anti-PCNA antibody, sections of normal human colon were stained with anti-PCNA antibody or preimmune serum (Figure 2, H and I) ▶ (Figure 2G ▶ displays H&E stain for orientation.) In addition, transcripts for RA301/Tra2β in an aorta from a 58-year-old patient with hypertension and hypercholesterolemia (above; Figure 1 ▶ , lane 8) were shown to be present in the intima, most likely in smooth muscle cells (data not shown). And plasma cells, visualized in another portion of this atherosclerotic aorta near the vasa vasorum, showed RA301/Tra2β mRNA, consistent with its function as an RNA splicing factor (data not shown).
Figure 2.
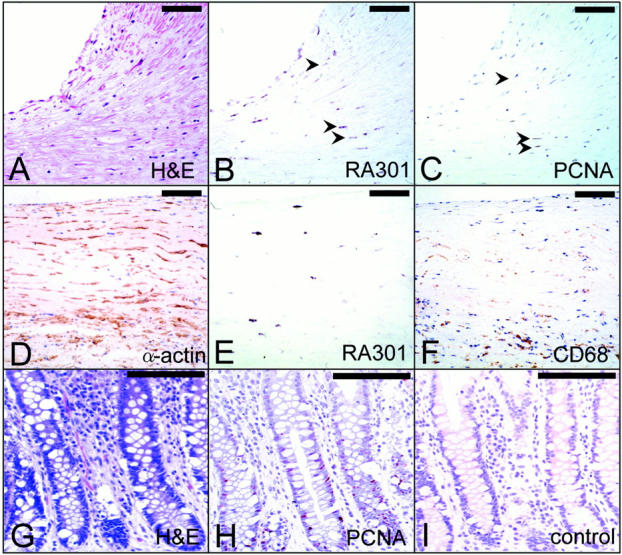
Expression of human RA301/Tra2β message in human coronary artery. A coronary artery was obtained from the same patient as described in Figure 1 ▶ , lane 9. A to C and D to F are adjacent sections, respectively. Sections of coronary artery were subjected to in situ hybridization using UTP-digoxigenin-labeled RA301/Tra2β riboprobes (B and E), and immunohistochemistry using anti-PCNA antibody (C, counterstained with hematoxylin), anti-α-smooth muscle actin antibody (1A4) (D) and anti-CD68 antibody (PG-M1) (F). A was shown as an H&E staining for orientation. Arrowheads in B and C are same cells positive for both RA301 message and PCNA antigen. RA301 mRNA-positive cells in E are not stained with anti-CD68 antibody but stained with anti-α-smooth muscle actin in adjacent sections (F and D). Sections of human colon from surgical specimen were immunostained with anti-PCNA antibody or preimmune serum, respectively, as positive and negative controls (H and I). An adjacent section was stained with H&E for orientation (G). Scale bars, 100 μm.
Expression of RA301/Tra2β in Rat Arterial Injury Model
The effect of acute arterial injury on RA301/Tra2β expression was studied using the rat carotid-artery balloon injury model. Transcripts for RA301/Tra2β were up-regulated by day 2, and remained increased through day 7 (Figure 3A ▶ ; arrowheads indicate the splicing variants observed in arterial injury). Immunohistochemical analysis of instrumented rat carotid arteries showed increased expression of RA301/Tra2β by day 3 (Figure 3B ▶ , panel F; note that uninjured rat carotid artery showed no RA301/Tra2β staining, Figure 3B ▶ , panel E), reaching a maximum by day 7 (Figure 3B ▶ , panel G), and thereafter falling off (Figure 3B ▶ , panel H).
Figure 3.
Expression of RA301/Tra2β in the rat carotid artery balloon injury model. A: Northern blot analysis. At the indicated times after balloon injury of rat carotid arteries, injured and uninjured (designated day 0) carotid arteries were dissected out, total RNA was extracted and subjected to electrophoresis on agarose (1%) gels (5 μg/lane). RNA was then transferred to Hybond N+ membrane and hybridized using a 32P-dCTP-labeled cDNA probe for rat RA301/Tra2β (top) or human β-actin (bottom). After hybridization, the membrane was washed and signals were detected by autoradiography. Arrowheads indicate the splicing variants of RA301/Tra2β transcripts. Migration of ribosomal RNA is indicated on the right of the autoradiogram. Experiments were repeated four times and a representative autoradiogram is shown. B: Localization of RA301/Tra2β antigen in rat carotid-artery injury model. Injured (B–D, F–H, and J–P) and uninjured (A, E, and I) rat carotid arteries at various time points were analyzed as follows. A to D show sections stained with H&E. E to H show sections immunostained with rabbit anti-RA301/Tra2β polyclonal antibody. I to L show sections immunostained with mouse anti-PCNA monoclonal antibody. In E, RA301/Tra2β antigens are not detected in rat carotid artery. In F, RA301/Tra2β antigens are scattered throughout the media, whereas in G and H, RA301/Tra2β antigens are shown mainly in neointima, but partly in media. In N to P, sections of rat carotid artery 7 days after the injury were immunostained with either RA301/Tra2β antibody (N) or anti-PCNA antibody (O). Both images are digitally overlapped in P. M shows H&E staining for orientation. Corresponding areas marked by a rectangle in M are magnified in N to P. Note the overlapping distribution of RA301 and PCNA antigens. 3d, 7d, and 14d indicates days after balloon injury, respectively. Control indicates uninjured carotid artery. E to L are counterstained with hematoxylin. Scale bars, 100 μm (D, M, and N in Figure 3 ▶ B). A to L or N to P in Figure 3 ▶ B are at the same magnification.
The distribution of RA301/Tra2β approximately coincided with staining of the adjacent sections to detect PCNA-positive cells (Figure 3B ▶ ; panels J, K, and L). The localization of RA301/Tra2β antigen within cells appeared confined to nuclei, consistent with the results of previous studies. 8,25,26 Double staining of injured artery (day 7) showed the overlapping of RA301 and PCNA signals (Figure 3B ▶ , panels N to P; H&E staining is shown in panel M for orientation). Furthermore, the expression pattern of RA301/Tra2β antigen paralleled the distribution reported for cells displaying either thymidine incorporation or BrdU uptake or PCNA immunogenicity after arterial injury in animals. 13,27-29 On day 3 after the injury (panel F), RA301/Tra2β antigens were scattered throughout the media and adventitia, whereas in panels G and H (on days 7 & 14), RA301/Tra2β antigens were shown mainly in neointima, but partly in media. Cells expressing RA301/Tra2β antigens mainly consisted of smooth muscle cells, but partly of endothelial cells on the luminal surface and adventitial cells. In Figure 3B ▶ , we showed intimal and medial components of uninjured and injured arteries.
Effect of Re-Oxygenation on the Induction of RA301/Tra2β in Vascular Cells
Subconfluent VSMCs subjected to hypoxia displayed diminished cell growth (not shown), consistent with our previous results in cultured endothelial cells. 30 However, on subsequent re-oxygenation, there was a burst of cell proliferation (Figure 4A) ▶ accompanied by induction of PCNA antigen (Figure 4B) ▶ , a well-known marker of cell proliferation. 31 In cultured VSMCs, re-oxygenation also triggered a marked increase in RA301/Tra2β message within 1 hour that was sustained for up to 24 hours (Figure 4D) ▶ and followed by an increase in RA301/Tra2β antigen (Figure 4C) ▶ . In contrast to the results shown in the injured artery, VSMCs in cultured condition basically expressed the lower Mr variant and re-oxygenation mainly increased the lower Mr variant, whereas the higher Mr variant was observed 1 to 4 hours after the re-oxygenation (Figure 4D ▶ , indicated by arrowheads). The expression of RA301/Tra2β was also observed when subconfluent cultured endothelial cells were exposed to hypoxia/re-oxygenation, accompanied by accelerated proliferation (data not shown). In contrast, exposure of blood-derived mononuclear phagocytes to hypoxia/re-oxygenation failed to induce RA301/Tra2β antigen and had no effect on cell proliferation (not shown). These data provided further support for a link between proliferation of vascular cells and induction of RA301/Tra2β. Consistent with this concept, cultured VSMCs incubated with platelet-derived growth factor isoform BB (PDGF-BB) under serum-free conditions demonstrated increased cell growth (not shown) 32 and induction of RA301/Tra2β (Figure 5) ▶ . Addition of angiotensin II, which causes hypertrophy of VSMCs but not proliferation, 33 failed to induce RA301 antigen in cultured VSMCs (Figure 5) ▶ .
Figure 4.
Effects of hypoxia/re-oxygenation on the proliferation of vascular cells and the expression of RA301/Tra2β. In A, rat VSMCs plated in 6-well plates (∼2 × 10 5 cells) were exposed to hypoxia for 36 hours, followed by re-oxygenation. At the indicated time points, cell number was determined by Coulter counter. In B and C, subconfluent rat VSMCs (∼2 × 10 6 cells) plated on 10-cm dishes were exposed to hypoxia for 36 hours, followed by re-oxygenation. At the indicated time points, cellular proteins were extracted, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%; ∼5 μg/lane) and detected by immunoblotting using either mouse monoclonal anti-PCNA antibody (B; 0.5 μg/ml) or rabbit anti-RA301/Tra2β antibody (C; 5 μg/ml). The migration of molecular weight markers is shown on the right of the gel. In D, total RNA prepared from re-oxygenated VSMCs (∼10 μg/lane) was subjected to Northern analysis using either a 32P-dCTP-labeled rat RA301/Tra2β cDNA (top) or human β-actin probe (bottom). The migration of ribosomal RNA is shown on the right of the gel. Splicing variants of RA301/Tra2β transcripts are indicated by arrowheads.
Figure 5.
Expression of RA301/Tra2β in VSMCs: effect of PDGF-BB and angiotensin II. Subconfluent rat VSMCs plated in 10-cm dishes (∼2 × 10 6 cells) were maintained under normoxic conditions in serum-free medium for 48 hours and either PDGF-BB (top; 10 ng/ml) or angiotensin II (bottom; 100 μg/ml) was then added. At the indicated times, proteins were extracted and subjected to immunoblotting with rabbit anti-RA301/Tra2β polyclonal antibody (5 μg/ml).
RA301/Tra2β Induction in Re-Oxygenated Cultured VSMCs: Role of O2−
To investigate the mechanism of RA301 induction in cultured VSMCs after re-oxygenation, the generation of superoxide anion (O2−) was assessed by chemiluminescence. Exposure of cultured VSMCs to hypoxia followed by re-oxygenation caused the production of O2−, which was completely inhibited by pre-incubation of hypoxic VSMCs with DPI, an inhibitor of NADPH oxidase (Figure 6A) ▶ . Because DPI also blocks nitric oxide synthase, experiments were performed in an arginine-free condition. However, production of O2− by re-oxygenated VSMCs was not altered in the absence of arginine (data not shown), suggesting involvement of an NAPDH oxidase-like enzyme. Suppression of O2− in re-oxygenated VSMCs with DPI also inhibited expression of RA301/Tra2β antigen in a dose-dependent manner (Figure 6B ▶ , i). In contrast, the level of HSP70i antigens was not altered by hypoxia/re-oxygenation, and DPI had no effect on HSP70i expression (Figure 6B ▶ , i). These data indicate that generation of O2− in cultured VSMCs could serve as a trigger for expression of RA301, similar to what we observed previously in cultured astrocytes. 5
Figure 6.
Generation of superoxide anion (O2−) in the re-oxygenated VSMCs and the effect of RA301/Tra2β suppression on VSMC proliferation. In A, subconfluent VSMCs plated in 150-mm dishes (∼5 × 10 6 cells) were exposed to hypoxia for 36 hours. Cells were then pelleted and resuspended in PBS (1 ml) inside the hypoxic chamber. Re-oxygenation was then performed either in the presence of DPI (50 μmol/L), PD98059 (50 μmol/L), or their absence (no addition). Generation of O2− by re-oxygenated cultures was then measured in a luminometer as described in the text (n = 4, mean ± SD is shown.). The horizontal bar means time after re-oxygenation. In B, subconfluent cultured VSMCs plated in 6-well plates (∼2 × 10 5 cells) were re-oxygenated in the presence of either DPI (i), PD98059 (ii), sense RA301/Tra2β oligonucleotide (iii), or antisense oligonucleotide (iv). Cells were harvested 6 hours after re-oxygenation, lysed, and their lysates were subjected to Western blot analysis using either rabbit anti-RA301/Tra2β polyclonal antibody (5 μg/ml) or mouse anti-human HSP70i monoclonal antibody (0.2 μg/ml). Experiments were repeated four times and a representative blot is shown in the figure. In C, subconfluent cultured VSMCs plated in 24-well plates (∼5 × 10 4 cells) were re-oxygenated in the presence of either DPI or PD98059. Twenty-four hours after the re-oxygenation, either MTT uptake (C, i) or release of LDH into the culture supernatant (C, ii) was assessed as described in the text (n = 6, mean ± SD is shown). **, P < 0.01 by multiple comparison analysis. In D, cultured VSMCs plated in 24-well plates (∼5 × 10 4 cells) were exposed to hypoxia (36 hours), followed by re-oxygenation. DPI (50 μmol/L) was added to cultures at the indicated times. D, i; −30 indicates 30 minutes before re-oxygenation. In D, ii, either sense or antisense oligonucleotide (10 to 50 μmol/L) was added to cultures 30 minutes before re-oxygenation. MTT incorporation was then assessed 48 hours after re-oxygenation as described in the text (n = 6, mean ± SD is shown). **, P < 0.01 by multiple comparison analysis.
To further assess the mechanisms underlying RA301 induction in re-oxygenated VSMCs, cultures were re-oxygenated in the presence of PD98059, a potent and relatively selective inhibitor of MAPKK. 34 Even though addition of PD98059 was without effect on O2− generation in cultured VSMCs (Figure 6A) ▶ , it completely blocked the expression of RA301/Tra2β (Figure 6B ▶ , ii). These data suggested that the pathway regulating expression of RA301/Tra2β in re-oxygenated VSMCs involved activation of the MAP kinase cascade.
Suppression of VSMC Proliferation by the Inhibition of RA301/Tra2β Expression
To determine whether RA301/Tra2β had a causative role in cellular proliferation associated with hypoxia/re-oxygenation, we further investigated whether its expression was suppressed by the addition of a specific RA301/Tra2β antisense oligonucleotide.
Addition of the antisense oligonucleotide to hypoxic/re-oxygenated VSMC cultures demonstrated dose-dependent inhibition of the RA301/Tra2β antigen (Figure 6B ▶ , iv), whereas the sense oligonucleotide was without effect at the same concentrations (Figure 6B ▶ , iii). The apparent specificity of antisense RA301/Tra2β oligonucleotide was suggested by its lack of effect on HSP70i expression (Figure 6B ▶ , iv).
Next, we examined the relationship between proliferation of VSMCs subject to hypoxia/re-oxygenation and generation of O2− and activation of the MAP kinase pathway. The same approximate concentration range of DPI and PD98059 that suppressed RA301/Tra2β expression in hypoxic/re-oxygenated VSMCs also inhibited proliferation (Figure 6C ▶ , i). Furthermore, the effect of DPI was only observed when it was added to hypoxic VSMC cultures before or simultaneously with re-oxygenation, but not 60 minutes thereafter (Figure 6D ▶ , i). Thus, for DPI to inhibit cell proliferation, it had to be present before generation of O2− occurred early during the re-oxygenation period (Figure 6A) ▶ . It is important to note that cell lysis with extrusion of cytosolic contents, such as LDH, into the medium did not occur during these experiments performed in the presence of DPI and PD98059.
To specifically examine the contribution of RA301/Tra2β expression to VSMC proliferation in this setting, cultures subject to hypoxia/re-oxygenation were exposed to either antisense or sense RA301Tra2β oligonucleotides (Figure 6D ▶ , ii). In a dose-dependent manner, only the antisense oligonucleotide inhibited cellular proliferation with a maximal effect of ∼70% by a concentration of 50 μmol/L. To further understand the role of RA301/Tra2β, Western blot analysis was performed in cultured VSMCs re-oxygenated in the presence of antisense oligonucleotide. The suppression of RA301/Tra2β was without effect on either cellular p42/44 MAP kinase content or activation of c-Jun N-terminal kinase (Figure 7, A and C) ▶ . In contrast, the amount of activated p42/44 MAP kinase remain elevated in the presence of antisense oligonucleotide 6 hours after re-oxygenation, although activated p42/44 MAP kinase was not detected in the presence of sense oligonucleotide (Figure 7B) ▶ . Further, suppression of RA301 in culture suppressed the re-oxygenation-mediated increase of PCNA antigen, compared with cultures treated with sense oligonucleotide (Figure 7D) ▶ . These data suggest that RA301/Tra2β participates in the proliferation of VSMCs in the downstream of the MAP kinase pathway.
Figure 7.
Contribution of RA301/Tra2β on ERK signaling pathway. VSMCs in 35-mm dish (∼2 × 10 5 cells) were exposed to hypoxia (36 hours) and re-oxygenated in the presence of either sense or antisense oligonucleotide of RA301/Tra2β (25 μmol/L). At the indicated time points, protein extracts (∼20 μg/lane) were subjected to Western blot using either anti-ERK 1/2 antibody (A; 2 μg/ml), which recognizes total p42/44 MAP kinase, anti-phosphorylated p42/44 MAP kinase (B; 2 μg/ml), anti-phosphorylated c-Jun N-terminal kinase (JNK) antibody (C; 2 μg/ml), or anti-PCNA antibody (D; 0.5 μg/ml). Note that larger amount (∼4×) of protein extracts were loaded on the gel compared with Figure 4B ▶ .
Discussion
De novo proteins induced in cells subjected to hypoxia/re-oxygenation are likely to have a pivotal role in promoting cell survival. In this context, RA301 was first identified based on its prominent induction in cultured rat astrocytes exposed to hypoxia followed by a brief period of re-oxygenation. 5 This molecule possesses an RNP-type RNA binding domain (RBD), together with two RS domains rich in arginine/serine dipeptide repeats, and has highest homology to transformer-2 (Tra2). Tra2 is a Drosophila splicing factor that functions in concert with Tra (transformer) in events regulating sex determination by its involvement in sex-specific alternative splicing of transcripts from the double-sex gene. 35,36
Recently, two human homologs of Tra2, Tra2α 37 and Tra2β, 25,26 have been identified. Surprisingly, RA301 is completely identical to human Tra2β. Furthermore, both Tra2α and Tra2β splice pre-mRNA in a sequence-specific manner. 8 Because mechanisms of sexual differentiation are not conserved between mammals and flies, these data suggest that human Tra2 proteins have more general functions in RNA processing than their Drosophila homologs. In fact, RA301/Tra2β is associated with the stress response to hypoxia/re-oxygenation 5 and has also been shown to be up-regulated in response to nerve injury. 7
Our current study using human vascular tissues extends these observations by indicating enhanced expression of RA301/Tra2β transcripts and antigens in VSMCs at lesional sites, especially in those cells likely to be in a proliferative mode. In human lesions, however, proliferation in VSMCs occurs infrequently. 38 To further examine the role of RA301/Tra2β in VSMC replication, we therefore applied a balloon injury model of rat carotid artery, where proliferation of VSMCs are more prominent, although this extrapolation has limitations because other mechanisms are proposed in this model distinct from those operating in human lesions. 39 Although there is the growing importance of the adventitial compartment in neointimal formation of arterial injury model, we could not show the precise effect of adventitial cells on the neointimal expression of RA301/Tra2β. But our data showing the up-regulation of RA301/Tra2β by PDGF in cultured VSMCs might pose some suggestions as described. 40
Consistent with the increase in RA301/Tra2β antigen in injured vascular lesions, the results obtained from cultured VSMCs support this association of RA301/Tra2β with cell growth. First, the pathway mediating increased expression of RA301/Tra2β in hypoxia/re-oxygenation seems to result from generation of O2− and, most likely, their subsequent activation of the MAP kinase cascade. Thus, addition of DPI to hypoxic cultures before re-oxygenation (when the burst of O2− formation was observed) suppressed, in parallel, RA301/Tra2β expression and cell proliferation. However, PD98059 acted more distally, although it was without effect on O2− production, it also blocked proliferation and RA301/Tra2β expression. Finally, RA301/Tra2β antisense oligonucleotide selectively suppressed expression of RA301/Tra2β in VSMCs subject to hypoxia/re-oxygenation, and decreased proliferation and induction of PCNA antigen. Although experiments with antisense can be difficult to interpret, putting together these different lines of evidence, including the lack of effect of RA301/Tra2β sense oligonucleotide, it seems logical to conclude that RA301/Tra2β is likely to contribute to mechanisms leading to increased cellular proliferation during the re-oxygenation period. That RA301/Tra2β might be more generally involved in growth of VSMCs was suggested by its up-regulation in the presence of the potent smooth muscle cell mitogen PDGF, but not by angiotensin II that induces hypertrophy not proliferation. Furthermore, increased expression of RA301/Tra2β was observed in cultured aortic endothelial cells, which also demonstrate a proliferative response to hypoxia/re-oxygenation, but not in mononuclear phagocytes, which do not proliferate under these conditions.
Taken together, these data lead us to propose that RA301/Tra2β may be a common denominator of cellular proliferation in a variety of settings, especially those associated with vascular disease. Although considerable additional studies will be required to gain insight into underlying mechanisms and to extrapolate these data in vivo, it is possible that RA301/Tra2β represents a new target for control of vascular cell proliferation in vivo.
Acknowledgments
We thank Akinori Fukuyama and Kenji Morihana, Department of Pathology Osaka University Graduate School of Medicine, for their excellent technical support; and Professor Kentaro Shimokado, Division of Geriatrics and Vascular Medicine Tokyo Medical and Dental University Graduate School, for his valuable advice and encouragement during the course of these studies.
Footnotes
Address reprint requests to Yoshitane Tsukamoto, M. D., Department of Pathology, National Cardiovascular Center, 5-7-1 Fujishiro-dai, Suita City, 565-8565 Japan. E-mail: ytukamot@hsp.ncvc.go.jp.
Supported in part by the Research Grants for Cardiovascular diseases (10C-1) from the Ministry of Health and Welfare, Japan.
References
- 1.Heacock CS, Sutherland RM: Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys 1986, 12:1287-1290 [DOI] [PubMed] [Google Scholar]
- 2.Lee AS: Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol 1992, 4:267-273 [DOI] [PubMed] [Google Scholar]
- 3.Ozawa K, Kuwabara K, Tamatani M, Takatsuji K, Tsukamoto Y, Kaneda S, Yanagi H, Stern DM, Eguchi Y, Tsujimoto Y, Ogawa S, Tohyama M: 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J Biol Chem 1999, 274:6397-6404 [DOI] [PubMed] [Google Scholar]
- 4.Hori O, Matsumoto M, Maeda Y, Ueda H, Ohtsuki T, Stern DM, Kinoshita T, Ogawa S, Kamada T: Metabolic and biosynthetic alterations in cultured astrocytes exposed to hypoxia/reoxygenation. J Neurochem 1994, 62:1489-1495 [DOI] [PubMed] [Google Scholar]
- 5.Matsuo N, Ogawa S, Imai Y, Takagi T, Tohyama M, Stern D, Wanaka A: Cloning of a novel RNA binding polypeptide (RA301) induced by hypoxia/reoxygenation. J Biol Chem 1995, 270:28216-28222 [DOI] [PubMed] [Google Scholar]
- 6.Matsuo N, Ogawa S, Takagi T, Wanaka A, Mori T, Matsuyama T, Pinsky DJ, Stern DM, Tohyama M: Cloning of a putative vesicle transport-related protein, RA410, from cultured rat astrocytes and its expression in ischemic rat brain. J Biol Chem 1997, 272:16438-16444 [DOI] [PubMed] [Google Scholar]
- 7.Kiryu-Seo S, Matsuo N, Wanaka A, Ogawa S, Tohyama M, Kiyama H: A sequence-specific splicing activator, Tra2β, is up-regulated in response to nerve injury. Mol Brain Res 1998, 62:220-223 [DOI] [PubMed] [Google Scholar]
- 8.Tacke R, Tohyama M, Ogawa S, Manley JL: Human Tra2 proteins are sequence-specific activators of pre-mRNA splicing. Cell 1998, 93:139-148 [DOI] [PubMed] [Google Scholar]
- 9.Nakata Y, Shionoya S: Vascular lesions due to obstruction of vasa vasorum. Nature 1966, 212:1258-1259 [DOI] [PubMed] [Google Scholar]
- 10.Crawford DW, Blackenhorn DH: Arterial wall oxygenation, oxyradicals, and atherosclerosis. Atherosclerosis 1991, 89:97-108 [DOI] [PubMed] [Google Scholar]
- 11.Tsukamoto Y, Kuwabara K, Hirota S, Ikeda J, Stern DM, Yanagi H, Matsumoto M, Ogawa S, Kitamura Y: 150-kD oxygen-regulated protein is expressed in human atherosclerotic plaques and allows mononuclear phagocytes to withstand cellular stress on exposure to hypoxia and modified low density lipoprotein. J Clin Invest 1996, 98:1930-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga S, Ogawa S, Kuwabara K, Brett J, Leavy JA, Ryan J, Koga Y, Plocinski J, Benjamin W, Burns DK, Stern DM: Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest 1992, 90:1007-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clowes AW, Reidy MA, Clowes MM: Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 1983, 49:327-333 [PubMed] [Google Scholar]
- 14.Sanger A, Nicklen S, Coulson A: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 1977, 74:5463-5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota S, Imakita M, Kohri K, Ito A, Morii E, Adachi S, Kim HM, Kitamura Y, Yutani C, Nomura S: Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. Am J Pathol 1993, 143:1003-1008 [PMC free article] [PubMed] [Google Scholar]
- 16.Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, Symes J, Isner JM: Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic arteries. Am J Pathol 1997, 150:1673-1685 [PMC free article] [PubMed] [Google Scholar]
- 17.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y: Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279:577-580 [DOI] [PubMed] [Google Scholar]
- 18.Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J, Furie MB, Torcia G, Cozzolino F, Kamada T, Stern DM: Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci USA 1995, 92:4606-4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa S, Gerlach H, Esposito C, Macaulay AP, Brett J, Stern DM: Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest 1990, 85:1090-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18:5294-5299 [DOI] [PubMed] [Google Scholar]
- 21.Lowry O, Rosenbrough NJ, Farr LA, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 1951, 193:265-275 [PubMed] [Google Scholar]
- 22.Maeda Y, Matsumoto M, Hori O, Kuwabara K, Ogawa S, Yan SD, Ohtsuki T, Kinoshita T, Kamada T, Stern DM: Hypoxia/reoxygenation-mediated induction of astrocyte interleukin 6: a paracrine mechanism potentially enhancing neuron survival. J Exp Med 1994, 180:2297-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imuta N, Ogawa S, Maeda Y, Kuwabara K, Hori O, Ueda H, Yanagihara T, Tohyama M: Induction of 72-kDa inducible heat shock protein (HSP72) in cultured rat astrocytes after energy depletion. J Neurochem 1998, 70:550-557 [DOI] [PubMed] [Google Scholar]
- 24.Monner DA: An assay for growth of mouse bone marrow cells in microtiter liquid culture using tetrazolium salt MTT, and its application to studies of myelopoiesis. Immunol Lett 1988, 19:261-269 [DOI] [PubMed] [Google Scholar]
- 25.Beil B, Screaton G, Stamm S: Molecular cloning of hTra-2beta-1 and hTra-2beta-2, two human homologs of tra-2 generated by alternative splicing. DNA Cell Biol 1997, 16:679-690 [DOI] [PubMed] [Google Scholar]
- 26.Nayler O, Cap C, Stamm S: Human transformer-2-beta gene (SFRS10): complete nucleotide sequence, chromosomal localization, and generation of a tissue-specific isoform. Genomics 1998, 53:191-202 [DOI] [PubMed] [Google Scholar]
- 27.Zeymer U, Fishbein MC, Forrester JS, Cercek B: Proliferating cell nuclear antigen immunohistochemistry in rat aorta after balloon denudation. Comparison with thymidine and bromodeoxyuridine labeling. Am J Pathol 1992, 141:685-690 [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y, Pieniek M, Fard A, O’Brien J, Mannion JD, Zalewski A: Adventitial remodeling after coronary arterial injury. Circulation 1996, 93:340-348 [DOI] [PubMed] [Google Scholar]
- 29.Igura T, Kawata S, Miyagawa J, Inui Y, Tamura S, Fukuda K, Isozaki K, Yamamori K, Taniguchi N, Higashiyama S, Matsuzawa Y: Expression of heparin-binding epidermal growth factor-like growth factor in neointimal cells induced by balloon injury in rat carotid arteries. Arterioscler Thromb Vasc Biol 1996, 16:1524-1531 [DOI] [PubMed] [Google Scholar]
- 30.Shreeniwas R, Ogawa S, Cozzolino F, Torcia G, Braunstein N, Butura C, Brett J, Lieberman HB, Furie MB, Joseph-Silverstein J, Stern D: Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol 1991, 146:8-17 [DOI] [PubMed] [Google Scholar]
- 31.Jaskulski D, deRiel JK, Mercer WE, Calabretta B, Baserga R: Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science 1988, 240:1544-1546 [DOI] [PubMed] [Google Scholar]
- 32.Heldin CH, Westermark B: Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999, 79:1283-1316 [DOI] [PubMed] [Google Scholar]
- 33.Walsh K, Shiojima I, Gualberto A: DNA replication and smooth muscle cell hypertrophy. J Clin Invest 1999, 104:673-674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR: PD098059 is a specific inhibitor of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 1995, 270:27489-27494 [DOI] [PubMed] [Google Scholar]
- 35.Hoshijima K, Inoue K, Higuchi I, Sakamoto H, Shimura Y: Control of double-sex alternative splicing by transformer and transformer-2 in Drosophila. Science 1991, 252:833-836 [DOI] [PubMed] [Google Scholar]
- 36.Mattox W, Palmer MJ, Baker BS: Alternative splicing of the sex determination gene transformer-2 is sex-specific in the germ line but not in the soma. Genes Dev 1990, 4:789-805 [DOI] [PubMed] [Google Scholar]
- 37.Dauwalder B, Amaya-Manzanares F, Mattox W: A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc Natl Acad Sci USA 1996, 93:9004-9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon D, Reidy MA, Benditt EP, Schwartz SM: Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA 1990, 87:4600-4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien ER, Alpers CE, Stewart DK, Ferguson M, Tran N, Gordon D, Benditt EP, Hinohara T, Simpson JB, Schwartz SM: Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res 1993, 73:223-231 [DOI] [PubMed] [Google Scholar]
- 40.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K, Takeshita A: Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest 1996, 97:769-776 [DOI] [PMC free article] [PubMed] [Google Scholar]








