Abstract
The Rab small G protein family participates in intracellular vesicle transport, including exocytosis and endocytosis. The cDNA encoding a novel Rab-related small G protein (Rab38) has been cloned from rat lung cDNA library and recorded in GenBank (accession no. M94043). However, the expression and localization of the protein in the lung remains primarily unknown. We produced polyhistidine-tagged recombinant Rab38 and a polyclonal antibody with a synthetic peptide. Immunohistochemistry demonstrated that the protein is specifically localized in alveolar type II cells and in bronchial epithelial cells. In situ hybridization using a digoxygenin-labeled RNA riboprobe clearly showed that the mRNA of the protein is localized in alveolar type II cells and bronchial epithelial cells, especially terminal airway epithelial cells. Western blot and reverse transcriptase-polymerase chain reaction showed distinct expression of the protein and mRNA in isolated alveolar type II cells, but not in alveolar macrophages. The native protein was predominantly hydrophobic and was enriched in a high-density vesicle fraction but was barely detectable in nuclear and lamellar body fractions in alveolar type II cells. Immunofluorescence cytochemistry performed on cultured alveolar type II cells showed that Rab38 distributed extensively in the cytoplasm with a distribution pattern similar to endoplasmic reticulum rather than other subcellular organelles. These results suggest that this novel rab small G protein (Rab38) mediates vesicular transport in terminal airway epithelium.
Small GTP-binding proteins are a group of monomeric intracellular proteins that have GTP/GDP binding and GTPase activities, and mediate essential cell functions, including cell growth/differentiation, cytoskeletal configuration (cell movement, change of shape, and contraction and relaxation), and intracellular vesicle transport, including exocytosis and endocytosis. 1,2 The ras p21 was found first and was soon followed by several other proteins. Now, more than 50 members form a ras superfamily. They have highly conserved domains, contributing to interaction with guanine nucleotides, in organisms from yeast through mammals. Most of these proteins are prenylated at their carboxy termini and specific subsets are also modified by palmitoylation. The ras-related superfamily is now recognized as consisting of three major gene families (ras, rho, and rab) and other minor families.
The rab family has more than 30 members, the largest number among small G proteins. The Rab proteins are known to localize to specific cell organelles, and are found in both membrane-bound and cytosolic forms. 2,3 Thus, they are believed to mediate intracellular vesicle transport among restricted intracellular compartments. Although current information in the sequence database indicates more than 30 members, there are few Rab proteins for which intracellular localization and function have been clarified.
A novel cDNA has been cloned from the rat lung cDNA library encoding a rab-related small G protein (GenBank accession no. M94043). Analysis of the cDNA analysis revealed that this protein shares large similarity with other Rab proteins and the predicted molecular weight from the deduced amino acid sequence is 24 kd. Recently, a cDNA encoding the same protein was cloned from a human melanoma cDNA library and was numbered as Rab38. 4 Although the cDNA sequence of the protein has been elucidated, the cells expressing this protein and its intracellular distribution and function remain primarily unknown. In this study, we sought to clarify the expressions of the native protein and mRNA of rab38 in the lung, and examine the localization of the protein in specific lung cells and subcellular organelles.
Materials and Methods
Chemicals and Reagents
Common chemicals and reagents were purchased from Sigma (St. Louis, MO) or Wako Chemicals (Osaka, Japan). Cell culture plasticware was from Falcon (Becton Dickinson, Tokyo, Japan). Metrizamide was from Sigma. Fetal calf serum and culture media were from Life Technologies, Inc. (Rockville, MD). Porcine pancreatic elastase was from Worthington (Freehold, NJ). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were from Novex (San Diego, CA). Nitrocellulose membranes were from Bio-Rad (Hercules, CA). ABC method-based histochemical staining kit [Histofine SAB-PO (R)] was from Nichirei (Tokyo, Japan). Restriction enzymes, BamHI, HindIII, and DNA molecular weight markers were from WAKO Chemicals. Designated DNA primers were from Funakoshi Life Science (Tokyo, Japan).
Cells
Alveolar type II cells were isolated from specific pathogen-free adult male Sprague-Dawley rats (Sankyo Labo Service, Tokyo, Japan) by pancreatic elastase digestion and metrizamide density-gradient centrifugation, according to the method described by Dobbs and colleagues. 5 Alveolar macrophages were isolated by bronchoalveolar lavage. In SPF rats, >98% of the lavaged cells were macrophages and these were used without further purification. Spodoptera frugiperda cells (Sf9 cells) (Invitrogen, Carlsbad, CA) were cultured in TNM-FH medium in 25-cm plastic culture dishes.
Expression Strategy of rab38
The original cDNA clone was constructed in a pET-3 vector. The rab38 cDNA (Figure 1) ▶ was amplified by polymerase chain reaction (PCR) using specific primers. The 5′ primer was 5′-TCCCGGATCCATGCAGACACCGCACAAG-3′. The primer was designed to have the BamHI restriction sequence at its 5′ site upstream of the initiation codon in the cDNA sequence. The 3′ primer was 5′-TTAAAAGCTTGTAAACACTGTGCTGAC-3′. The primer originally contained the HindIII restriction sequence present in downstream site of the termination codon. The PCR product was inserted into the pBlueBacHis 2A vector. The DNA sequencing of the recombinant vector was performed with the ABI BigDye terminator cycle sequencing ready reaction kit (PE Applied Biosystems, Foster City, CA). Co-transfection of the recombinant plasmid and lethal-depleted baculovirus (Bac-N-Blue DNA, Invitrogen) was performed with Sf9 cells using cationic liposome (InsectinPlus, Invitrogen) according to the instructions in the manufacturer’s manual. The resultant culture supernatant was used for plaque purification of recombinant virus. The presence of rab38 containing recombinant virus was verified by PCR. The PCR-positive virus was amplified for large-scale culture. Virus titers were determined by plaque assay.
Figure 1.

Nucleic acid sequence of cDNA and predicted amino acid sequence of rab38. The nucleic acid sequence was downloaded from GenBank (accession no. M94043). The cDNA consists of 633 bp, and the predicted molecular weight of the translated protein is 24 kd. Four consensus amino acid sequences of a small G protein superfamily are boxed. GTPase activity: GDLGVGK and DIAG. GTP/GDP-binding activity: NKCD and ETSAK. Nucleic acid sequences of primers, which were used for PCR after reverse transcription of total extracted RNA leading to the subsequent synthesis of the cRNA probe for in situ hybridization, are underlined.
Protein Production and Purification
Protein production was performed using Sf9 cells cultured in 25-cm plastic dishes. Cells at 80 to 90% confluency were infected with the recombinant virus at a multiplicity of 10. Four days after infection, the cells were harvested, washed twice with cold phosphate-buffered saline (PBS), rapidly frozen with dry-ice in ethanol, and stored at −80°C until use. The frozen cells were rapidly thawed and suspended in lysis buffer (1% Triton X-114/50 mmol/L Hepes at pH 7.4, 150 mmol/L NaCl, 1.5 mmol/L EGTA, 10% glycerol) containing protease inhibitors (1 mmol/L phenylmethyl sulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin) on ice for 30 minutes. The cell suspension was centrifuged at low speed (360 × g, 10 minutes), and the supernatant was collected. The supernatant was extracted using Triton X-114 partitioning. 6,7 Briefly, the supernatant with 1% Triton X-114 was layered on 6% sucrose/50 mmol/L Hepes at pH 7.4/150 mmol/L NaCl/0.06% Triton X-114. It was then incubated at 30°C in a water bath for 30 minutes and centrifuged at a low speed for 10 minutes. The upper layer was recovered, adjusted to 0.5% Triton X-114, incubated at 30°C for 30 minutes, again layered on the previous sucrose cushion, and centrifuged at a low speed. The detergent pellet in the bottom of the tube was redissolved in PBS and used for further processing. The Triton X-114-extracted cell lysate was loaded on a Ni++-charged affinity column (Probond, Invitrogen) under native conditions, according to the manufacturer’s manual. Elution was performed with imidazole gradients. The purified fraction was monitored with SDS-PAGE using 8 to 16% gradient gels under reducing conditions and subsequent Coomassie Blue staining.
Polyclonal Antibody Production
Based on the amino acid sequence predicted for rab38, a polypeptide consisting of 20 amino acid residues of the C-terminal domain was synthesized. Keyhole limpet hemocyanin (Calbiochem, San Diego, CA) was coupled with m-maleimidobenzoyl-N-hydroxysuccinimide ester (MBS) (Calbiochem) and fractionated with Sephadex G-25 column chromatography (Pharmacia, Tokyo, Japan). The keyhole limpet hemocyanin-m-maleimidobenzoyl-N-hydroxysuccinimide ester conjugate was allowed to couple with the synthesized polypeptide and was stored at −20°C until use. An emulsion of the polypeptide-m-maleimidobenzoyl-N-hydroxysuccinimide-keyhole limpet hemocyanin preparation and a complete Freund’s adjuvant was prepared and injected into the subcutaneum of two female New Zealand White rabbits (body weight, ∼2.5 kg). A booster injection with an incomplete Freund’s adjuvant was added 3 weeks after the first injection, and the third injection was given 2 weeks later. The titer of rabbit blood was monitored by the dot blot method using an immobilized Sf9-cell lysate infected with the recombinant virus. Serum was collected and an IgG fraction was purified with protein A-conjugated Sepharose CL4B beads (Sigma). The IgG fraction was further purified with the synthesized polypeptide-coupled 2-fluoro-1-methylpyridinium toluene-4-sulfonate (FMP) activated Cellulofine (Seikagakukogyo, Tokyo, Japan).
Immunohistochemistry
Excised rat lungs were slowly infused endotracheally with an OCT-compound (Sakura Finetechnical, Tokyo, Japan) and were frozen rapidly with ethanol/dry ice. Lung slices were prepared and fixed with 4% paraformaldehyde for 20 minutes, followed by acetone for 30 seconds. Immunostaining was performed based on the ABC method using a commercial kit [Histofine SAB-PO(R) Kit, Nichirei, Tokyo]. The polyclonal antibody for Rab38 was layered onto the fixed lung slices using a concentration of 8 μg/ml. Control staining was performed in two ways by using the same concentration of nonimmune rabbit IgG, and by using anti-Rab38 plus the synthesized polypeptide in a 100-fold molar excess. A biotin-labeled goat anti-rabbit IgG antibody was added as the second antibody followed by horseradish peroxidase-conjugated streptavidin. Color development was performed for 3 minutes in the presence of diaminobenzidine and H2O2.
Reverse Transcriptase (RT)-PCR
Total RNA was extracted from perfused rat lung, isolated alveolar type II cells, and isolated alveolar macrophages using an RNA isolation kit (Trizol, Life Technologies, Inc.). DNase I (a RQ1 RNase-free DNase, Promega) was added to the total RNA sample and incubated at 37°C for 15 minutes. The reaction mixture was extracted with phenol:chloroform, and RNA was precipitated with methanol. First-strand DNA was synthesized with random hexamer primers using a reverse transcriptase (Superscript RT, Life Technologies, Inc.). RNase H (Life Technologies, Inc.) was next added. Designated primers, Taq DNA polymerase (Takara Ex Taq; Takara, Shiga, Japan), and other reaction components for PCR were subsequently added. PCR was started at 94°C for 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 90 seconds using a Thermal Cycler (Gene Amp PCR System 2400, Perkin Elmer). Aliquots of the PCR product were electrophoresed on a 2% agarose gel and stained with ethidium bromide. The primers for rab38 were 5′-ATGCAGACACCGCACAAG-3′ and 5′-AGGGAGAGTTAACTTTGAGTC-3′. The primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′.
Western Blot
Total protein was extracted from perfused rat lungs, isolated alveolar type II cells, alveolar macrophages, and Sf9 cells using a lysis buffer containing protease inhibitors. The protein concentration was determined by a deoxycholate-trichloroacetic acid precipitation and a BCA microprotein assay kit (Pierce, Rockford, IL). SDS-PAGE was performed under reducing conditions with 1-mm-thick, 8 to 16%-gradient precast minigels (IWAKI, Tokyo, Japan). The nitrocellulose membranes were blocked with 3% skim milk/1% Triton X-100/PBS. The membranes were reacted with a rabbit anti-rat Rab38 polyclonal antibody at 8 μg/ml/3% skim milk/1% Triton X-100/PBS overnight at 4°C. Subsequently the membranes were reacted with a horseradish peroxidase-conjugated anti-rabbit IgG antibody (Bio-Rad) at 1:1000 dilution. Color development was performed in the presence of H2O2 and diaminobenzidine as the chromogen for ∼3 minutes.
In Situ Hybridization
Perfused rat lungs were fixed with 4% paraformaldehyde, embedded in paraffin, and sliced into 5- to 7-μm-thick sections. PCR amplification of rab38 using recombinant pBlueBacHis2A as the template was performed using the same primers used in RT-PCR. The PCR products were isolated from the gels with Geneclean (BIO101, Vista, CA) and directly inserted into the pGEM-T Easy vector (Promega, Madison, WI). Competent E. coli cells (One Shot Cells, Invitrogen) were transformed with the recombinant pGEM-T Easy plasmid. E. coli was grown and plasmid DNA was isolated with a plasmid purification kit (Plasmid Mini; Qiagen, Valencia, CA). Digoxigenin-labeled RNA riboprobes were synthesized using a DIG RNA labeling kit (SP6/T7) (Boehringer Mannheim, Indianapolis, IN). The rab38 cDNA-pGEM-T EASY plasmid was linearized with BamHI or HindIII. The linearized plasmids were used as DNA templates to synthesize RNA riboprobes (antisense and sense riboprobes) with SP6 or T7 RNA polymerase in the presence of digoxygenin-labeled UTP. An antisense or sense digoxygenin-labeled RNA probe was warmed to 85°C for 3 minutes, quickly put on ice, and hybridized with the lung-tissue section at 42°C for 16 hours. Concentrations of the RNA probes in the hybridization buffer were 0.1 to 0.5 μg/ml. The sections were treated with RNase, blocked with 10% normal sheep serum, and hybridized for 30 minutes at room temperature with sheep polyclonal anti-digoxygenin Fab fragments conjugated with alkaline phosphatase (750 U/ml), that was diluted to 1:500 before use. Color development was performed in the nitroblue tetrazolium salt / 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) solution overnight in the dark.
Cell Fractionation
Freshly isolated alveolar type II cells (60 × 10 6 cells) were disintegrated with a Dounce glass homogenizer. The homogenate was divided into two samples. One homogenate was centrifuged at 360 × g for 10 minutes. The resulting pellet was referred to as the nucleus fraction. The supernatant was centrifuged at 15,000 × g for 10 minutes, and this pellet was referred to as the heavy vesicle fraction. The supernatant was centrifuged at 100,000 × g for 1 hour, and this pellet was referred to as the light vesicle fraction. The supernatant was referred to as the cytosol fraction. Another homogenate was quickly adjusted to 0.9 mol/L sucrose concentration and 3 ml of the homogenate in a 0.9 mol/L sucrose was layered above 3 ml of 1.48 mol/L sucrose in a centrifuge tube for an SW 28 rotor (Beckman). Next, 1.5-ml steps of sucrose (in 0.1 mol/L increments) from 0.8 mol/L to 0.2 mol/L were layered above the 0.9 mol/L sucrose, and the gradient was centrifuged at 100,000 × g for 3 hours. Under these conditions, low-density lamellar bodies, characteristic secretory granules of lung surfactant in alveolar type II cells, migrate against the direction of the gravitational field from the 0.9 mol/L sucrose zone to the 0.4 to 0.5 mol/L sucrose zone. 8,9 The sucrose gradient was fractionated from low- to high-density sucrose gradients by controlled vacuum aspiration of fractions.
Confocal Laser Microscopy
Freshy isolated rat alveolar type II cells were seeded in Chamber Slide (Lab-Tek 177437; Nalgen Nunc International Corp., Naperville, IL) and cultured for 24 hours. The adherent cells were washed with cold PBS and fixed with 4% paraformaldehyde/PBS for 10 minutes, and acetone for 30 seconds. The slides were sealed and stored at −80°C until use. The slides were blocked with 10% normal goat serum/5% BSA/PBS and were reacted with a rabbit anti-rat Rab38 polyclonal antibody (8 μg/ml), and one of the following mouse monoclonal antibodies: BiP/GRP78 at 1:100, GM130 at 1:100, TGN38 at 1:100, EEA1 at 1:100 (Transduction Laboratories, Lexington, KY), or an affinity-purified goat polyclonal antibody: Lamp-1 at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA). The slides were reacted for 30 minutes with second antibodies: Alexa 488-labeled goat anti-rabbit IgG antibody at 1:400 dilution and Alexa 594-labeled goat anti-mouse IgG antibody at 1:400 dilution (Molecular Probes, Eugene, OR). For the Lamp-1, fluorescein isothiocyanate-conjugated affinity-purified donkey anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA) at 1:100 dilution followed by Alexa 488-labeled rabbit anti-fluorescein IgG (Molecular Probes) at 1:200 and Cy3.5-conjugated affinity-purified donkey anti-goat IgG antibody at 1:200 dilution (Rockland, Gilbertsville, PA). Confocal microscopic images were obtained using a computer-interfaced, laser-scanning microscope (Leica TCS-4D). Immunolabeled slides (n = 3 to 4 representative fields per slide), were sectioned optically through the cell monolayer to obtain the appropriate focal depth. The representative image chosen contained the nucleus and relevant organelle of interest (ER, Golgi, trans-Golgi network, endosomes, and lamellar bodies). Simultaneous wavelength scanning allowed superimposition of fluorescent labeling with Alexa 488 and Alexa 594 (or Cy 3.5) fluorophores. Confocal images were obtained using the following parameters for Alexa 488-labeled secondary antibody: voltage 700 to 800, offset −1, and for Alexa 594-labeled secondary antibody: voltage 600 to 700, offset −1. Laser power was adjusted between 75 to 90% to obtain the best images. The pinhole setting, which was identical for both Alexa 488 and Alexa 594 images because of simultaneous scanning, was fixed to 100. Image sizes (Zoom) were X: 100.00 and Y: 100.00. Image output was at 512 × 512 pixels.
Results
Expression of Native Rab38 in the Lung
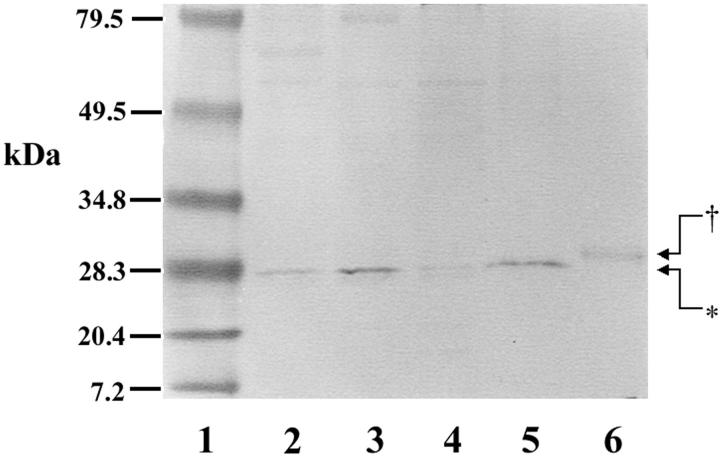
The affinity-purified rabbit anti-rat rab38 polyclonal antibody reacted with a protein of ∼26-kd in both total lung homogenate and the alveolar type II cell lysate, by Western blot (Figure 2) ▶ . The reaction seemed to be specific and appeared as one band. The 26-kd band still was found in the detergent phase after Triton X-114 extraction, suggesting that the 26-kd protein had significant hydrophobic character. Only a faint signal of the 26-kd protein was recovered in the water-soluble fraction after Triton X-114 partitioning of the alveolar type II cell lysate. Thus, the majority of native Rab38 exists as a hydrophobic protein in the cell. The recombinant baculovirus-infected Sf9 cell lysate showed abundant immunoreactive protein with a molecular weight of ∼27 kd. However, Sf9 cells contained approximately equal amounts of soluble Rab38 and Triton X 114-partitioning Rab38 (data not shown). The difference in the molecular weights of native Rab38 and the recombinant Rab38 seemed to be because of an N-terminal fusion peptide (37 amino acids) that had been constructed in the pBlueBacHis2A, including the six histidine-tag and the enterokinase restriction site.
Figure 2.
Expression of native Rab38 protein in total lung and freshly isolated alveolar type II cells and of the recombinant protein in transfected baculovirus-infected Sf9 cells analyzed by Western blot. Total proteins were extracted with 1% Triton X-114 directly from isolated cells or homogenized rat lungs. Triton X-114 phase separation was performed for an aliquot of alveolar type II cell lysate. Equal amounts of 50 μg protein, except for lane 6 (5 μg protein), were loaded on SDS-PAGE under reducing conditions and transferred to a nitrocellulose membrane. The membrane was immunoblotted with a rabbit anti-rat Rab38 polyclonal antibody and a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin antibody. Color development was performed in the presence of diaminobenzidine and H2O2 for less than 3 minutes. Native Rab38 appeared as a 26-kd band (*) and recombinant Rab38 as a 27-kd band (†). Lane 1: Prestained protein marker. Lane 2: Total lung homogenate. Lane 3: Total type II cell lysate. Lane 4: Water-soluble protein in Triton X-114-partitioning of type II cell lysate. Lane 5: Detergent-extracted protein in Triton X-114-partitioning of type II cell lysate. Lane 6: Recombinant baculovirus-infected Sf 9 cell lysate.
Isolated Alveolar Type II Cells Express rab38 mRNA and Protein, but Alveolar Macrophages Do Not
The RT-PCR for rab38 showed one distinct band corresponding to the molecular size predicted from the chosen primers. The PCR product was specifically observed in a total RNA sample derived from whole lungs or freshly isolated alveolar type II cells, but it was not detected in a total RNA sample from alveolar macrophages (Figure 3B) ▶ . In contrast, the PCR products for GAPDH were observed in similar amounts, both in alveolar type II cells and in alveolar macrophages. Western blotting of the Rab38 using the rabbit anti-rat Rab38 antibody clearly showed the existence of the Rab38 protein in isolated alveolar type II cells, but not in alveolar macrophages (Figure 3A) ▶ . Thus, rab38 is selectively expressed in alveolar type II cells but not in alveolar macrophages.
Figure 3.
Expression of rab38 in alveolar type II cells and alveolar macrophages. A: Western blot of cell lysates. Cell lysis and Western blot were performed as described in Figure 2 ▶ . Lane 1: Prestained molecular marker. Lane 2: Alveolar type II cell lysate (35 μg protein). Lane 3: Alveolar macrophage lysate (35 μg protein). B: RT-PCR of rab38 for total RNA extracted from whole lung (10 μg RNA) and the two cell preparations (1 μg RNA). Reverse transcription of first strand DNA was performed with random hexamer primers. Then specific primers for a partial length of rab38 cDNA (top) and for GAPDH (bottom) as a control were used for PCR. The PCR products were electrophoresed in 2% agarose and visualized with ethidium bromide stain. Lane 1: Molecular marker (only for top panel). Lane 2: Whole lung. Lane 3: Alveolar macrophages. Lane 4: Alveolar type II cells.
Localization of rab38 in the Lung Tissue
Native Rab38 Protein
Immunostaining of rapidly frozen and paraformaldehyde-fixed lung tissue showed specific immunoreactivity of the anti-rat rab38 antibody with a number of alveolar corner cells (Figure 4A) ▶ and some bronchial epithelial cells (Figure 4B) ▶ . This immunoreactivity was abolished when the antibody was added together with the synthesized polypeptide for Rab38. Moreover, when the same amount of nonimmune rabbit IgG was used as the primary antibody, these cells showed no positive signal (data not shown). The alveolar corner cells seemed to be exclusively alveolar type II cells. Some, but not all, of the airway epithelial cells showed an immunoreactive signal, and most of the terminal airway epithelial cells were immunoreactive. Alveolar epithelial cells not existing in alveolar corners, alveolar macrophages, pulmonary vessels, airway smooth muscle cells, connective tissue cells, and other lung cells were not immunoreactive.
Figure 4.
a: Immunohistochemistry of native Rab38 protein for rat alveolar tissue. Perfused and excised rat lungs were rapidly frozen in an OCT compound and cut into 5-μm-thick sections. The sections were fixed with 4% paraformaldehyde for 20 minutes and then with acetone for 30 seconds. A polyclonal anti-rat Rab38 antibody (8 μg/ml) was added as the first antibody. For controls, the same concentration of the antibody supplemented with the synthesized polypeptide in 100-fold molar excess was used. Biotin-labeled goat anti-rabbit IgG antibody was added, followed by horseradish peroxidase-conjugated streptavidin. Color development was performed for ∼3 minutes in the presence of diaminobenzidine and H2O2. The slides were counterstained with hematoxylin stain. A and B: Anti-Rab38 antibody. C and D: Control (antibody plus synthetic peptides). Original magnification: ×400 (A and C). ×1,000 (B and D). b: Immunohistochemisty of native Rab38 protein for rat bronchial tissue (original magnification, ×1,000). Experimental procedures are same as described in a. A: Anti-Rab38 antibody. B: Control (antibody plus synthetic peptides).
rab38 mRNA
In situ hybridization of rab38 using a digoxigenin-labeled antisense RNA probe clearly demonstrated localization of rab38 mRNA in airway epithelial cells and alveolar corner cells (Figure 5) ▶ ; this was consistent with the results of immunostaining. Most of the terminal airway epithelial cells showed positive signals (Figure 5 ▶ , inset). No other lung cells showed positive signals. The sense RNA probe did not produce a significant signal. Because the in situ hybridization used a paraffin-embedded lung section, preservation of the structure of the lung tissue was better than that of the rapidly frozen immunostained sections.
Figure 5.
In situ hybridization of rab38 in rat lung tissue. Perfused and excised rat lungs were fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 5- to 7-μm-thick slices. The sections were hybridized with digoxigenin-labeled cRNA probes and then treated with RNase. The sections were treated with sheep anti-digoxigenin Fab fragments conjugated with alkaline phosphatase. Color development was performed in 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt. A: Antisense RNA probe (original magnification, ×40). Some of the positive airway cells are indicated by arrowheads. B: Sense RNA probe (original magnification, ×40). Insets are original magnification of ×200 of the boxed areas. C: Antisense RNA probe (original magnification, ×200). Positive alveolar corner cells are indicated by arrows. D: Sense RNA probe (original magnification, ×200).
Subcellular Localization of Rab38 in Alveolar Type II Cells
Freshly isolated alveolar type II cells were homogenized with a Dounce homogenizer. The cell homogenate was fractionated by differential centrifugation and sucrose density gradient ultracentrifugation. Fifty μg of total protein from each cell fraction was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. An affinity-purified anti-rat Rab38 antibody (8 μg/ml) was used as the primary antibody for Western blotting. The total alveolar type II cell lysate, heavy vesicles (15,000 × g, for 10 minutes), light vesicles (100,000 × g, for 1 hour), and cytosol showed immunoreactivity, whereas the nuclear fraction was negative (Figure 6A) ▶ . The heavy vesicles showed the strongest signal. There was a prominent positive signal in the 0.9 mol/L sucrose fraction where the homogenized sample was loaded (Figure 6B) ▶ . The 0.4 to 0.6 mol/L sucrose fraction containing the lung surfactant-rich lamellar bodies did not give specific immunoreactive signal.
Figure 6.
Localization of Rab38 protein in subcellular organelle fractions from freshly isolated alveolar type II cells. Isolated alveolar type II cells were disrupted with a Dounce glass homogenizer. The cell homogenate was divided into two parts. The first was centrifuged with a series of differential centrifugal forces (A). The second portion of homogenate was adjusted to a 0.9 mol/L sucrose solution and loaded on a discontinuous sucrose gradient and centrifuged at 100,000 × g. Each sucrose gradient was grouped into fractions, diluted, and centrifuged (B). Equal 50-μg proteins were loaded on SDS-PAGE, and Western blotting was performed as described in Figure 2 ▶ . A: Lane 1, Total alveolar type II cells; lane 2, nucleus; lane 3, heavy vesicles (15,000 × g, 10 minutes); lane 4, light vesicles (100,000 × g, 3 hours); lane 5, cytosol. B: Lane 1, Total alveolar type II cells; lane 2, 1.48 mol/L sucrose; lane 3, 0.9 mol/L sucrose; lane 4, 0.7 to 0.8 mol/L sucrose; lane 5, 0.4 to 0.6 mol/L sucrose. Native Rab38 is indicated by arrows. Note that 0.4 to 0.6 mol/L sucrose (lane 5) is a lamellar body-enriched fraction.
Confocal laser-scanning microscopy using double-fluorescence immunostaining showed that Rab38 distributed extensively in the cytoplasm of alveolar type II cells (Figure 7 ▶ ; a, b, and c). Major subcellular organelles were simultaneously stained with Rab38 on the same slide, and the same planar section of the cells was imaged with simultaneous two wavelength scanning. The immunostained organelles included ER, Golgi, trans-Golgi network (Figure 7a) ▶ , and early and late endosomes (Figure 7b) ▶ . Surfactant apoproteins, SP-A and SP-B, were also stained (Figure 7c) ▶ . Among these organelles, Rab38 showed a distribution pattern similar to resident ER protein, BiP/GRP78. Rab38 seemed to show no co-localization with the Golgi and TGN, which had a tubular distribution pattern with juxtanuclear location. The endosomal marker proteins, EEA1 and Lamp-1, showed a granular distribution pattern. Although EEA1 showed minor co-localization with Rab38, Lamp-1 did not show any co-localization. Surfactant apoproteins, SP-A and SP-B, showed granular distribution in the cytoplasm. Although SP-A did not seem to co-localize with Rab38, SP-B showed partial co-localization. Thus, Rab38 seemed to distribute with a pattern most like the ER among major subcellular organelles examined in this study.
Figure 7.
a: Double-fluorescence immunocytochemistry by confocal laser-scanning microscopy on isolated rat alveolar type II cells. Adherent cells were fixed and reacted with a rabbit anti-Rab38 polyclonal antibody and a mouse anti-subcellular organelle protein. Second antibodies were conjugated with Alexa 488 fluorophore (green) and Alexa 594 (red). Left: Images are stained with Rab38 with green fluorescence (Alexa 488). Middle: Images are stained of subcellular organelle marker proteins (Alexa 594). Right: Merged images of left and middle. General distribution pattern of Rab38 resembles that of ER. Middle: Images are BiP/GRP78 (B, ER resident protein), GM130 (E, Golgi matrix protein), and TGN38 (H, trans-Golgi network resident protein). b: Double-fluorescence immunocytochemistry by confocal laser-scanning microscopy on isolated rat alveolar type II cells. Experimental procedures are identical to those described in a. Middle: Images are EEA1 (B, early endosome resident protein) and Lamp-1 (E, lysosome-associated transmembrane protein-1). Rab38 seems to show minor co-localization with EEA1. c: Double-fluorescence immunocytochemistry by confocal laser scanning microscopy on isolated rat alveolar type II cells. Experimental procedures are identical to those described in a. Middle: Images are SP-A (B) and SP-B (E). Rab38 seems to show partial co-localization with SP-B.
Discussion
A rab38 has been cloned from a rat lung cDNA library (GenBank accession no. M94043). The predicted amino acid sequence contains the four conserved motifs characteristic of small G proteins (Figure 1) ▶ . Comparison of the primary structure shows the greatest similarity between this protein and members of the rab family. The Rab family is the largest among the small G protein superfamily that contains more than 50 members. Rab family members are strictly localized to defined intracellular organelles and play prominent roles in intracellular trafficking 2,4,10-13 . No single cell expresses all of the rab family members. However, there are many Rab proteins whose expressions are ubiquitous over a variety of tissues and cells, such as Rab 1, 2, 4, 5, 6–14, 18, 20, 22, 24, 28, and 30. 2 Expression of some Rab proteins are highly regulated depending on cell type (epithelial, mesenchymal, or endothelial) and specific phenotype (differentiation, activation, or polarization). These proteins include Rab 3, 15, 17, 19, 23, 25, 26, and 27. 2 Few reports mention expression of small numbers of particular rab small G proteins in the lung. Although the number of rab family members exceeds more than 30, there are only a few members for which the molecular mechanism of vesicular transport has been clarified. The molecular mechanism for Rab3A have been best elucidated. Rab3A is exclusively expressed in neural tissues, especially in presynaptic vesicles. 14 There are several regulator proteins for the rab protein, 1,2,4,15,16 including rab GDP dissociation inhibitors (rab GDI), the rab GDP dissociation stimulator (rab GDS), the rab guanine nucleotide exchange protein (rab GEP), and the rab GTPase activating protein (rab GAP). Moreover, there are target proteins that interfere with rab proteins. In the case of rab 3A, Rabphillin3A is a target protein whose cDNA has been cloned and whose recombinant protein has been made. 17,18
Although many Rab proteins are expressed ubiquitously, some Rab proteins are restricted to specific tissues and cells. Distribution of a novel Rab protein in defined tissues and cells can provide unique information about the role of the protein. Indeed, there are some Rab proteins whose roles were initially identified in this way, including Rab3 10,14 and Rab17. 12,13 Examination of the distribution of a novel Rab protein requires specific antibody with relatively high affinity. In this study, we made a rabbit anti-rat Rab38 polyclonal antibody using a C-terminal amino acid sequence. The C-terminal sequence of small G proteins is the hypervariable region containing the most prominent structural differences in primary structure. It is customary to choose this region to synthesize polypeptides for specific immunogens. 19 Although there are several bands in the Western blot, the most prominent band at 26 to 27 kd seemed to have reacted with the native and recombinant Rab38 (Figure 2) ▶ . The native Rab38 was expressed in whole lung and alveolar type II cells but not in alveolar macrophages by Western blot. Most Rab38 in alveolar type II cells was present in the Triton X-114-extracted phase, and only a trace amount was present in the water-soluble phase. This strongly suggests that most of the native Rab38 present in alveolar type II cells has significant hydrophobic character, most likely attributable to prenylation.
Western blotting of subcellular fractions derived from isolated alveolar type II cells revealed that the Rab38 protein was present in both vesicle and cytosol fractions (Figure 6) ▶ . The Rab38 was most prominent in the heavy vesicle fractions. This result suggests that the Rab protein is present both in a vesicle membrane-bound form and in a cytosol-soluble form. Rab proteins cycle between GDP- and the GTP-bound forms. GTP-bound Rab proteins are most often membrane-associated whereas the GDP-bound proteins are soluble. 20 The Rab38 was predominantly found in the heavy vesicles rather than in the light vesicles, and was absent from lamellar body-enriched fraction. Lamellar bodies, characteristic structures of alveolar type II cells, are known to store lung surfactant components, including phospholipids, neutral lipids, and surfactant apoproteins and undergo regulated secretion. 9,21 Although this Rab38 is selectively expressed in the surfactant-producing alveolar type II cells, it does not seem to be involved in the exocytosis of lamellar bodies.
Confocal laser microscopic analysis of immunofluorescence cytochemistry of isolated and cultured rat alveolar type II cells showed that the distribution pattern of the Rab38 matched that of ER but not Golgi, TGN, or endosomes. However, care must be taken to conclude that the Rab protein co-localizes with ER, because the broad distribution pattern is not specific only for the ER. As already discussed, Rab proteins exhibit both membrane and soluble forms. This property was confirmed for Rab38 in Figure 6 ▶ , in which the protein was found in the cytosol fraction as well as membrane fractions. Rab38 did not seem to co-localize with SP-A and SP-B. But, this does not necessarily exclude the possibility that the Rab38 participates in specific steps in the transport of surfactant components. Because SP-A and SP-B are highly enriched in LB and also found throughout the secretory pathway, 22 the co-localization of these proteins with Rab38 is unlikely to be extensive.
The Rab38 was exclusively expressed in alveolar corner cells and bronchial epithelial cells in immunohistochemistry and in situ hybridization (Figures 4 and 5) ▶ ▶ . A number of alveolar corner cells were positively stained for Rab38, and most of these cells were identified as alveolar type II cells based on their characteristic alveolar position. Among bronchial cells the Rab protein and mRNA was predominantly expressed in terminal bronchial cells, although some large bronchial airway epithelial cells also showed positive staining. In rat lungs, Clara cells are bronchial epithelial cells accounting for >80% of the cells in the terminal airway. 23 Therefore, most of the terminal airway epithelial cells staining for Rab38 are likely to be Clara cells. It is noteworthy that Clara cells synthesize many components of the lung surfactant, but do not contain lamellar bodies, the characteristic lipid-containing secretory granules present in alveolar type II cells. 21,24-26 Clara cells do not express surfactant protein C (SP-C), an extraordinarily hydrophobic protein that is a distinct marker of alveolar type II cells. 27 Because Rab38 is not present in lamellar body fractions and is present in Clara cells that have no lamellar bodies, it is likely that this protein is not required for lamellar body secretion. Many Rab proteins mediate very restricted steps in vesicle trafficking and it is possible that Rab38 plays a role with respect to the surfactant system. Comparison of protein and lipid sorting in alveolar type II cells demonstrates that surfactant components have different and independent trafficking routes in alveolar type II cells. 20
Although the precise role of Rab38 still remains to be elucidated, this study clearly demonstrates the restricted expression of a novel Rab protein (Rab38) to alveolar type II cells and terminal bronchial epithelial cells in the lung. This restricted expression of Rab38 strongly implies a unique role for the protein related to the surfactant system.
Figure 7B.

Acknowledgments
We thank Dr. Yoshino Yoshitake (Department of Biochemistry, Kanazawa Medical University) for preparing the synthetic peptides for rab38; Dr. Yoshimichi Ueda and Dr. Takahide Ota (Department of Pathology, Kanazawa Medical University) for help in in situ hybridization and confocal laser microscopy; Dr. Takayasu Date (Department of Biochemistry, Kanazawa Medical University) for discussing this paper; and Dr. Chiharu Tsukano for her technical assistance.
Footnotes
Address reprint requests to Kazuhiro Osanai, M.D., Kanazawa Medical University, Dept. of Internal Medicine, Div. of Respiratory Disease, 1-1 Daigaku-Uchinada, Kahoku-Gun, Ishikawa, Japan 920-0293. E-mail: k-osanai@kanazawa-med.ac.jp.
Supported by a Grant-in-Aid for Scientific Research (C) (2) 08670685 and 10670566, and (A) (2) 08557042, The Ministry of Education, Science, Sports, and Culture, Japan.
References
- 1.Takai Y, Kaibuchi K, Kikuchi A, Kawata M: Small GTP-binding proteins. Int Rev Cytol 1992, 133:187-230 [DOI] [PubMed] [Google Scholar]
- 2.Olkkonen VM, Stenmark H: Role of Rab GTPases in membrane traffic. Int Rev Cytol 1997, 176:1-85 [DOI] [PubMed] [Google Scholar]
- 3.Novick P, Brennwald P: Friends and family: the role of the Rab GTPases in vesicular traffic. Cell 1993, 75:597-601 [DOI] [PubMed] [Google Scholar]
- 4.Jager D, Stockert E, Jager E, Gure AO, Scanlan MJ, Knuth A, Old LJ, Chen YT: Serological cloning of a melanocyte rab guanosine 5′-triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res 2000, 60:3584-3591 [PubMed] [Google Scholar]
- 5.Dobbs LG, Geppert EF, Williams MC, Greenleaf RD, Mason RJ: Metabolic properties and ultrastructure of alveolar type II cells isolated with elastase. Biochim Biophys Acta 1980, 618:510-523 [DOI] [PubMed] [Google Scholar]
- 6.Page MJ, Hall A, Rhodes S, Skinner RH, Murphy V, Sydenham M, Lowe PN: Expression and characterization of the Ha-ras p21 protein produced at high levels in the insect/baculovirus system. J Biol Chem 1989, 264:7-54 [PubMed] [Google Scholar]
- 7.Bordier C: Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 1981, 256:1604-1607 [PubMed] [Google Scholar]
- 8.Suwabe A, Mason RJ, Voelker DR: Temporal segregation of surfactant secretion and lamellar body biogenesis in primary cultures of rat alveolar type II cells. Am J Respir Cell Mol Biol 1991, 5:80-86 [DOI] [PubMed] [Google Scholar]
- 9.Osanai K, Mason RJ, Voelker DR: Trafficking of newly synthesized surfactant protein A in isolated rat alveolar type II cells. Am J Respir Cell Mol Biol 1998, 19:929-935 [DOI] [PubMed] [Google Scholar]
- 10.Fischer von Mollard G, Mignery GA, Baumert M, Perin MS, Hanson TJ, Burger PM, Jahn R, Sudhof TC: rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci USA 1990, 87:1988-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldenring JR, Shen KR, Vaughan HD, Modlin IM: Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 1993, 268:18419-18422 [PubMed] [Google Scholar]
- 12.Lutcke A, Jansson S, Parton RG, Chavrier P, Valencia A, Huber LA, Lehtonen E, Zerial M: Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J Cell Biol 1993, 121:553-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunziker W, Peters PJ: Rab17 localizes to recycling endosomes and regulates receptor-mediated transcytosis in epithelial cells. J Biol Chem 1998, 273:15734-15741 [DOI] [PubMed] [Google Scholar]
- 14.Martin G, Yukiko G, Charles FS, Thomas CS: The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 1997, 387:810-814 [DOI] [PubMed] [Google Scholar]
- 15.Martinez O, Goud B: Rab proteins. Biochim Biophys Acta 1998, 1404:101-112 [DOI] [PubMed] [Google Scholar]
- 16.Boguski MS, McCormick F: Proteins regulating Ras and its relatives. Nature 1993, 366:643-654 [DOI] [PubMed] [Google Scholar]
- 17.Shirataki H, Kaibuchi K, Yamaguchi T, Wada K, Horiuchi H, Takai Y: A possible target protein for smg 25A/rab3A small GTP-binding protein. J Biol Chem 1992, 267:10946-10949 [PubMed] [Google Scholar]
- 18.Kishida S, Shirataki H, Sasaki T, Kato M, Kaibuchi K, Takai Y: Rab3A GTPase-activating protein-inhibiting activity of Rabphilin-3A, a putative Rab3A target protein. J Biol Chem 1993, 268:22259-22261 [PubMed] [Google Scholar]
- 19.Zerial M, Parton R, Chavrier P, Frank R: Localization of Rab family members in animal cells. Methods Enzymol 1992, 219:398-407 [DOI] [PubMed] [Google Scholar]
- 20.Novick P, Zerial M: The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol 1997, 9:496-504 [DOI] [PubMed] [Google Scholar]
- 21.Hawgood S: Pulmonary surfactant apoproteins: a review of protein and genomic structure. Am J Physiol 1989, 257:L13-L22 [DOI] [PubMed] [Google Scholar]
- 22.Korimilli A, Gonzales LW, Guttentag SH: Intracellular localization of processing events in human surfactant protein B biosynthesis. J Biol Chem 2000, 275:8672-8679 [DOI] [PubMed] [Google Scholar]
- 23.Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR: Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest 1992, 67:553-565 [PubMed] [Google Scholar]
- 24.O’Reilly MA, Weaver TE, Pilot MTJ, Sarin VK, Gazdar AF, Whitsett JA: In vitro translation, post-translational processing and secretion of pulmonary surfactant protein B precursors. Biochim Biophys Acta 1989, 1011:140-148 [DOI] [PubMed] [Google Scholar]
- 25.Walker SR, Williams MC, Benson B: Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 1986, 34:1137-1148 [DOI] [PubMed] [Google Scholar]
- 26.Balis JU, Paterson JF, Paciga JE, Haller EM, Shelley SA: Distribution and subcellular localization of surfactant-associated glycoproteins in human lung. Lab Invest 1985, 52:657-669 [PubMed] [Google Scholar]
- 27.Kalina M, Mason RJ, Shannon JM: Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol 1992, 6:594-600 [DOI] [PubMed] [Google Scholar]