Abstract
Apert syndrome is an autosomal dominant disorder characterized by premature cranial ossification resulting from fibroblast growth factor receptor-2 (FGFR-2)-activating mutations. We have studied the effects of the prominent S252W FGFR-2 Apert mutation on apoptosis and the underlying mechanisms in human mutant osteoblasts. In vivo analysis of terminal deoxynucleotidyl transferase-mediated nick-end labeling revealed premature apoptosis of mature osteoblasts and osteocytes in the Apert suture compared to normal coronal suture. In vitro, mutant osteoblasts showed increased apoptosis, as demonstrated by terminal deoxynucleotidyl transferase-mediated nick-end labeling analysis, trypan blue staining, and DNA fragmentation. Mutant osteoblasts also showed increased activity of caspase-8 and effector caspases (-3, -6, -7) constitutively. This was related to protein kinase C activation because the selective protein kinase C inhibitor calphostin C inhibited caspase-8, effector caspases, and apoptosis in mutant osteoblasts. Apert osteoblasts also showed increased expression of interleukin (IL)-1α, IL-1β, Fas, and Bax, and decreased Bcl-2 levels. Specific neutralizing anti-IL-1 antibody reduced Fas levels, Bax expression, effector caspases activity, and apoptosis in mutant cells. Thus, the Apert S252W FGFR-2 mutation promotes apoptosis in human osteoblasts through activation of protein kinase C, overexpression of IL-1 and Fas, activation of caspase-8, and increased Bax/Bcl-2 levels, leading to increased effector caspases and DNA fragmentation. This identifies a complex FGFR-2 signaling pathway involved in the premature apoptosis induced by the Apert S252W FGFR-2 mutation in human calvaria osteoblasts.
Several missense mutations in the extracellular domains of fibroblast growth factor receptor (FGFR)-1 and -2 have been identified in patients with Pfeiffer, Jackson-Weiss, Crouzon, and Apert syndromes that are characterized by premature fusion of cranial sutures (craniosynostosis) and other abnormalities. 1-4 Apert syndrome, an autosomal-dominant disorder characterized by severe bicoronal craniosynostosis, is associated with S252W or P253R point mutations in the linker region between the second and third extracellular Ig domains. These FGFR-2 mutations lead to gain-of-function by constitutive receptor activation. 5-7 Activating Apert FGFR-2 mutations were found to induce variable alterations in cell proliferation and differentiation in human and mouse osteoblasts. Mutant osteoblasts from syndromic patients show a low (P253R mutation) or normal (S252W mutation) proliferation rate compared to control cells, and a marked differentiated phenotype characterized by increased expression of bone matrix proteins and mineralization 8,9 associated with activation of protein kinase C (PKC). 10,11 On the other hand, introduction of FGFR-2 carrying the C342Y (Crouzon syndrome) or the Apert S252W FGFR-2 mutations in murine calvaria cells inhibit differentiation and induce apoptosis. 12 In addition, FGF induces apoptosis in murine differentiating calvaria osteoblasts and mice overexpressing FGF-2 have increased apoptosis in the calvaria suture, 12 suggesting that FGF/FGFR signaling plays a major role in controlling the balance between cell growth, differentiation, and apoptosis in the cranial suture. 12-16
Apoptosis is an important component involved in normal and pathological osteogenesis. 17 Pathological abnormalities in cell death may result from alteration of diverse targets. In mammalian cells, apoptosis is a multiple step process implicating upstream induction phases and downstream execution stages. 18-20 Upstream events involve inducing signal transduction cascades and activation of intracellular molecules. Downstream events in the apoptotic cascade involve release of proteins from mitochondria and activation of proteases and nucleases leading to DNA degradation and ultimately to cell death. 21 One known upstream apoptotic pathway implicates Fas receptor (APO-1/CD95), a member of the tumor necrosis factor receptor family characterized by a death domain in the cytoplasmic region. 22 Activation of Fas by Fas ligand results in receptor aggregation and triggers recruitment of Fas-associated death domain protein, allowing recruitment of caspase-8 pro-enzyme, activation of caspase-8, and subsequent downstream caspases, including caspase-3, leading to cell death. 23,24 Caspases are a family of cysteine proteases that are activated in proteolytic cascades during cell death. 25,26 Initiator caspases (caspases-2, -8, -9, -10) either directly or indirectly activate downstream effectors (caspases-3, -6, -7) that cleave intracellular substrates during the execution phase of apoptosis. Another upstream pathway that plays a central role in controlling cell death involves the apoptotic promoter Bax family and the inhibitory protein family Bcl-2. 27,28 The heterodimerization of these molecules leads to balance apoptotic signals through activation of caspases. In osteoblasts, apoptosis was recently found to involve both Fas 29,30 and Bax/Bcl-2 levels. 12,31,32
The molecular mechanisms that are downstream of FGF/FGFR interactions and that cause apoptosis in osteoblasts remain primarily unknown. In differentiating murine calvaria osteoblasts, the apoptotic effect of FGF is associated with increased Bax level and delayed Bcl-2 accumulation. 12 In other cell types, FGF-2 promotes apoptosis 33,34 in part by down-regulating Bcl-2. 34 Nothing is known about the signaling cascades that are involved in apoptosis in pathological cranial osteogenesis in humans. In this study, we have determined the effect of the activating Apert S252W FGFR-2 mutation on apoptotic cell death in human osteoblasts in vivo and in vitro, and we examined the mechanisms and signaling pathways involved in apoptosis in mutant cells. Our data indicate that the FGFR-2 Apert mutation triggers premature apoptosis in human mutant osteoblasts through PKC-dependent pathways involving interleukin (IL)-1, Fas, caspase-8, and Bax/Bcl-2 that converge to activate effector caspases.
Materials and Methods
Bone Samples and Specimens
Calvaria samples were obtained from two aborted normal and two Apert 26-week-old fetuses in accordance with the French Ethical Committee recommendations. Mutation analyses performed by single-strand conformation polymorphism and restriction analyses of the coding sequence of the FGFR-2 gene revealed the S252W mutation, the most frequent mutation in Apert syndrome, in the two Apert fetuses. 9 To examine apoptosis in situ, coronal sutures obtained from one Apert and one control fetus were fixed in 10% formaldehyde, decalcified in 4.1% ethylenediaminetetraacetic acid (EDTA) for 3 weeks at 37°C, and embedded in paraffin as described previously. 35
Cell Cultures
To examine apoptosis and the underlying cellular mechanisms in vitro, normal and mutant calvaria cells, obtained by collagenase digestion from the coronal suture in one Apert and one control fetus as described previously, 35 were immortalized by transfection with the original defective large T antigen of the SV-40 oncogene and called Apert (Ap) and control (Co) fetal cells. 9 The phenotypic characteristics induced by the mutation in these cells have been recently described. 9-11 Briefly, mutant Ap cells display increased expression of osteoblast marker genes and increased in vitro osteogenesis compared to normal Co cells, a phenotype that is similar to the pathological feature observed in the mutant suture in vivo. 10 Ap and Co cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with glutamine (292 mg/L), 10% heat inactivated fetal calf serum, and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin).
Cell Viability
To evaluate cell viability in vitro, trypan blue staining was used for determination of dead cells by dye exclusion. After addition of trypan blue [0.4% in phosphate-buffered saline (PBS)], the percentage of Ap cells and Co cells exhibiting both nuclear and cytoplasmic trypan blue staining (nonviable cells) was determined. A total of 1500 cells per well were counted for each cell type and the results were expressed as percentage of total cells.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP-Biotin Nick End Labeling (TUNEL) Analysis in Vivo and in Vitro
To determine the effect of the FGFR-2 Apert mutation on apoptosis in vivo, DNA cleavage was assessed on normal and Apert coronal sutures by the TUNEL assay, as described by the manufacturer (Boehringer Mannheim, Mannheim, Germany). Briefly, paraffin-embedded tissues were deparaffinized in xylene and rehydrated through a graded series of ethanol. Sections were digested with 1 μg/ml proteinase K for 15 minutes. Endogenous peroxidase was quenched with 3% H2O2, permeabilized with 0.1% Triton X-100 at 4°C for 2 minutes, and incubated for 1 hour at 37°C with the TUNEL reaction mixture containing the terminal deoxynucleotidyl transferase. TUNEL signal was revealed with diaminobenzidine and mounted. To assess the effect of the mutation in vitro, apoptotic cells were detected on Ap and Co cells cultured on Labtek chambers in basal conditions and under the different treatments described below, using the TUNEL assay. Cells were washed with PBS, fixed with paraformaldehyde (4% in PBS), and endogenous peroxidase was quenched with 0.3% H2O2. Then TUNEL assay was performed as described above. TUNEL-positive cells were revealed by brown nuclei and apoptotic morphology, reflecting the specific dNTP transfer to 3′-hydroxy ends of DNA. The number of total and TUNEL-positive Ap and Co cells was then counted, and the results were expressed as percent of total cells. Positive controls consisted of sections and cells treated with DNase I for 10 minutes. Negative controls were obtained by omitting the transferase from the reaction.
Quantitation of DNA Fragmentation
Quantitative analysis of DNA fragmentation was performed as described. 36 Briefly, Ap and Co cells were prelabeled with [3H]-thymidine (1 μCi/ml) in 1% bovine serum albumin (BSA) for 24 hours. Cells were washed, trypsinized, and then lysed in TTE [10 mmol/L Tris, pH 7.4, 10 mmol/L EDTA, and 0.2% (v/v) Triton X-100]. Fragmented DNA was separated from intact chromatin by centrifugation, and the pellet was suspended in TTE. [3H]-Thymidine incorporated into both soluble and unfragmented DNA was determined by liquid scintillation counting. Percentage of fragmented DNA was calculated as the ratio of fragmented/fragmented plus intact chromatin.
Determination of Caspase Activity
To determine the implication of caspases involved in apoptosis in mutant cells, Ap and Co cells were plated in 6-well plates with 1% BSA in serum-free medium. After 24 hours, the cells were lysed in 400 μl of lysis buffer (10 mmol/L Tris, pH 7.4, 200 mmol/L NaCl, 5 mmol/L EDTA, 10% glycerol, 1% Nonidet P-40) for 30 minutes on ice and stored at −20°C. The activity of effector caspases (caspase-3, -6, -7) and initiator caspases (caspase-2, -8, -9) was determined by the cleavage of synthetic fluorogenic substrates containing the amino acid sequence recognized by specific caspases. The substrates were as follows: WEHD (Thr-Glu-His-Asp) for caspase 1, DEVD (Asp-Glu-Val-Asp) for caspase-3-like, IETD (Ile-Glu-Thr-Asp) for caspase-8, and were combined to a fluorophore (7-amino-4-methylcoumarin, AMC). On cleavage of the substrate by caspases, free AMC fluorescence emission was detected using a spectrofluorometer (F-2000, Hitachi, Japan). For the assay, aliquots of 100 μl were incubated for 2 hours at 37°C with 200 μl reaction buffer (0.1 mmol/L phenylmethyl sulfonyl fluoride, 10 mmol/L dithiothreitol, 10 mmol/L Hepes/NaOH, pH 7.4) containing 5-μl specific substrate (20 μmol/L final concentration). The fluorescence released in samples was measured by excitation at 367 nm and reading was made at 440 nm. The negative control was buffer mix and the positive control was free AMC (10 μmol/L in PBS). The free AMC fluorescence emission by caspases was related to protein level and was expressed as arbitrary units.
Immunocytochemistry of IL-1
To determine the expression of IL-1 that might be involved in apoptosis, Ap and Co cells were cultured to confluence, fixed in 4% paraformaldehyde at 4°C for 30 minutes, washed in PBS/0.01% Triton X-100, incubated with 0.1% BSA/3% goat serum to block unspecific binding, then exposed for 1 hour at room temperature to rabbit polyclonal anti-human IL-1α or IL-1β antibodies (Genzyme) diluted 1:100. Control cells were incubated with the appropriate solution (rabbit IgG). After 1 hour exposure at room temperature, cells were washed three times for 10 minutes in PBS and exposed to second anti-rabbit antibody (1:50) linked to colloidal gold particles (IntenSETMM; Amersham, Arlington Heights, IL) for 1 hour at room temperature. The gold particle staining was enhanced by precipitation of metallic silver (ImmunoGold Silver Staining), then washed before visualization.
Western Blot Analysis
IL-1, CD95/Fas, Bax, and Bcl2 protein levels were determined by Western blot analysis in Ap and Co cells. Co and Ap cells were washed twice with cold PBS and scrapped into 300 μl of ice-cold lysis buffer (10 mmol/L Tris-HCl, 5 mmol/L EDTA, 150 mmol/L NaCl, 30 mmol/L sodium pyrophosphate, 50 mmol/L NaF, and 1 mmol/L Na3VO4) containing 10% glycerol and protease inhibitors (Boehringer Mannheim). Protein samples were solubilized in 2× Laemmli sodium dodecyl sulfate loading buffer and boiled at 95°C for 5 minutes. Fifty micrograms of proteins, determined using the DC protein assay (Bio-Rad Laboratories, Hercules, CA), were resolved on 12% acrylamide gel, then transferred onto polyvinylidene difluoride-Hybond-P membranes (Amersham). Blots were saturated overnight with 1% blocking solution (Boehringer Mannheim) in Tris-buffered saline buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl) containing 0.1% Tween-20. Membranes were then incubated with rabbit polyclonal anti-human IL-1α or IL-1β (1 μg/ml, Chemicon), mouse monoclonal anti-human CD95/Fas (1 μg/ml, Immunotech), mouse monoclonal anti-human Bax (0.5 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-human Bcl2 (0.5 μg/ml, Santa Cruz), or rabbit polyclonal anti-human β-actin (1.5 μg/ml, Sigma) in 0.5% blocking buffer. After 1 hour at room temperature, the membranes were washed twice with Tris-buffered saline/0.1% Tween 20 and 0.5% blocking buffer, and incubated for 1 hour with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. After incubation with the appropriate secondary antibodies and washes, the signals were visualized with Boehringer Mannheim chemiluminescence blotting substrate. The specific bands on the autoradiograms were quantitated by densitometry.
Inhibition of PKC Signaling and IL-1 Expression
To determine the signal transduction pathways involved in apoptosis in mutant cells, Ap and Co cells were cultured in the presence of the selective PKC inhibitor calphostin C (2 μmol/L). After 24 hours, IL-1α and IL-1β protein levels were determined by Western blot, as described above. In parallel, apoptosis in Ap cells was determined by the TUNEL assay, and the activity of caspases-1, -3, -8, and -9 was determined as described above. To determine the implication of IL-1 on apoptosis, Ap cells were treated with neutralizing IL-1α or IL-1β antibody (0 to 15 μg/ml), the combined treatment (30 μg/ml) or IgG at equivalent amount, and cell viability was determined by trypan blue exclusion. Apoptosis was also determined by TUNEL analysis as described above. In parallel experiments, the activity of caspase-3 and -8, and CD95/Fas protein levels were determined as described above.
Data Analysis
The results are expressed as the mean ± SEM and were analyzed using the statistical package super-ANOVA (Macintosh, Abacus Concepts, Inc., Berkeley, CA). Differences between the mean values were evaluated with a minimal significance of P < 0.05.
Results
Premature Osteoblast Apoptosis in the Human Apert Suture
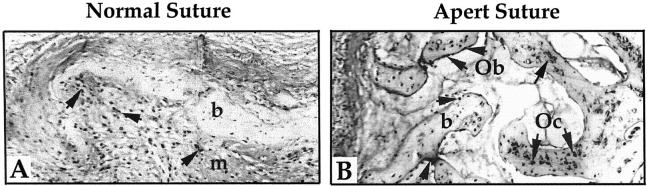
To analyze the effect of the Apert mutation on apoptosis in situ, coronal sutures from Apert fetus with the S252W FGFR-2 mutation and normal fetus were subjected to TUNEL analysis. As shown in Figure 1 ▶ , the fused Apert suture differed from the normal age-matched suture. The fused suture is composed of multiple and large bone trabeculae (∼1/10 of the suture area is shown here), whereas the normal suture is composed of a bone plate surrounded by multiple mesenchymal cells (only 1/2 of the suture is shown here). The pattern of TUNEL-positive apoptotic cells differed markedly n the Apert sample compared to the age-matched normal suture. Specifically, most differentiated osteoblasts and osteocytes were apoptotic in Apert suture (Figure 1B) ▶ . In contrast, in the normal suture, apoptosis was found in mesenchymal cells and pre-osteoblasts whereas very few osteoblasts and osteocytes were TUNEL-positive (Figure 1A) ▶ . The TUNEL labeling of cells was specific, as shown by the absence of TUNEL label in control sections and the positive staining of all cells treated with DNase I (not shown). These results revealed that the S252W FGFR-2 mutation induces premature apoptosis of differentiated osteoblasts and osteocytes in vivo in the fused Apert suture.
Figure 1.
The Apert S252W FGFR-2 mutation induces premature osteoblast apoptosis in the human suture. Normal (A) and Apert (B) coronal sutures were prepared for TUNEL analysis. The Apert suture shows numerous TUNEL-positive mature osteoblasts (Ob) along the bone trabeculae and TUNEL-positive osteocytes (Oc) in the bone (b) matrix (arrows) whereas only mesenchymal (m) cells were found to be TUNEL-positive in the normal suture. Original magnification, ×125.
Increased in Vitro Apoptosis in Mutant Apert Osteoblasts
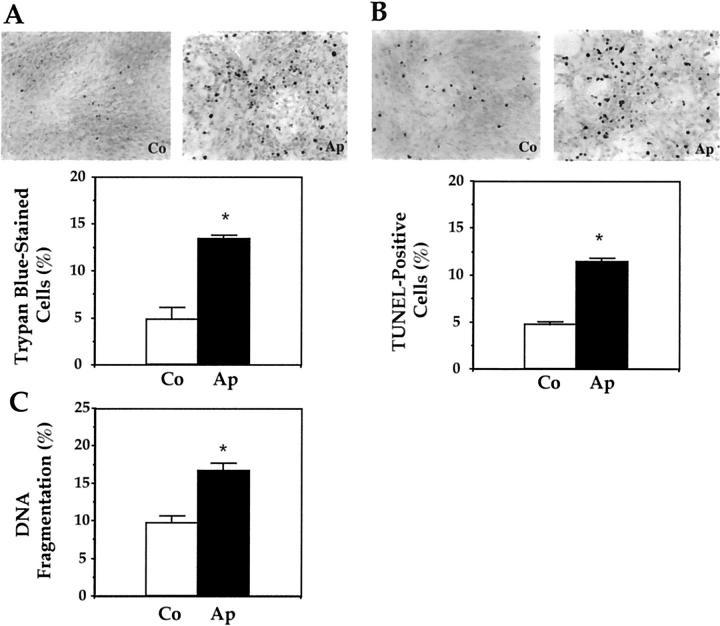
To further document the effect of the mutation on apoptosis, mutant Ap cells and normal Co cell viability was studied by the trypan blue exclusion assay. Figure 2A ▶ shows that most Co cells were viable in culture, with only a small percentage of trypan blue-stained cells. In contrast, numerous trypan-blue stained cells were found in Ap cultures, showing decreased cell viability induced by the mutation (Figure 2A) ▶ . To further determine the effect of the mutation on osteoblast apoptosis, mutant Ap cells and normal Co-cultured cells were subjected to TUNEL analysis to assess DNA fragmentation by specific labeling of double-strand DNA. Figure 2B ▶ shows that few Co cells were TUNEL-positive when cultured in 1% BSA serum-free conditions. In contrast, numerous TUNEL-positive Ap cells were found. Quantification of trypan blue-stained cells and TUNEL-positive Ap and Co cells revealed that the FGFR-2 mutation induced a twofold to threefold increase in the number of apoptotic cells in vitro (Figure 2, A and B) ▶ . Notably, the percentage of Ap TUNEL-positive apoptotic cells did not significantly differ from the percentage of trypan blue-positive Ap cells (representing apoptotic and necrotic cells) (11.4 ± 0.43% versus 13.3 ± 0.40%, ns), indicating that the mutation affected apoptosis rather than necrosis in mutant osteoblasts.
Figure 2.
Increased in vitro apoptosis induced by the S252W FGFR-2 mutation in Apert mutant osteoblasts. Apert (Ap) and control (Co) cells were stained with trypan blue (A) or TUNEL (B) and the number of trypan blue-stained or TUNEL-positive cells was counted. C: DNA fragmentation was determined as indicated in Materials and Methods. The data are the mean ± SEM of four values. *, Significant difference with Co cells (P < 0.05).
The final resulting effect of apoptosis is DNA fragmentation, leading to cell death. To firmly establish the effect of the FGFR-2 Apert mutation on cell death, DNA fragmentation was analyzed in Ap and Co cells cultured in 1% BSA conditions. As shown in Figure 2C ▶ , the percentage of DNA fragmentation, measured by the ratio of fragmented/fragmented plus intact chromatin, was increased in Ap cells compared to Co cells. Overall, these data show that the S252W FGFR-2 mutation constitutively increases apoptosis in mutant osteoblasts.
Increased Caspase-8 and -3 Activity in Mutant Apert Osteoblasts
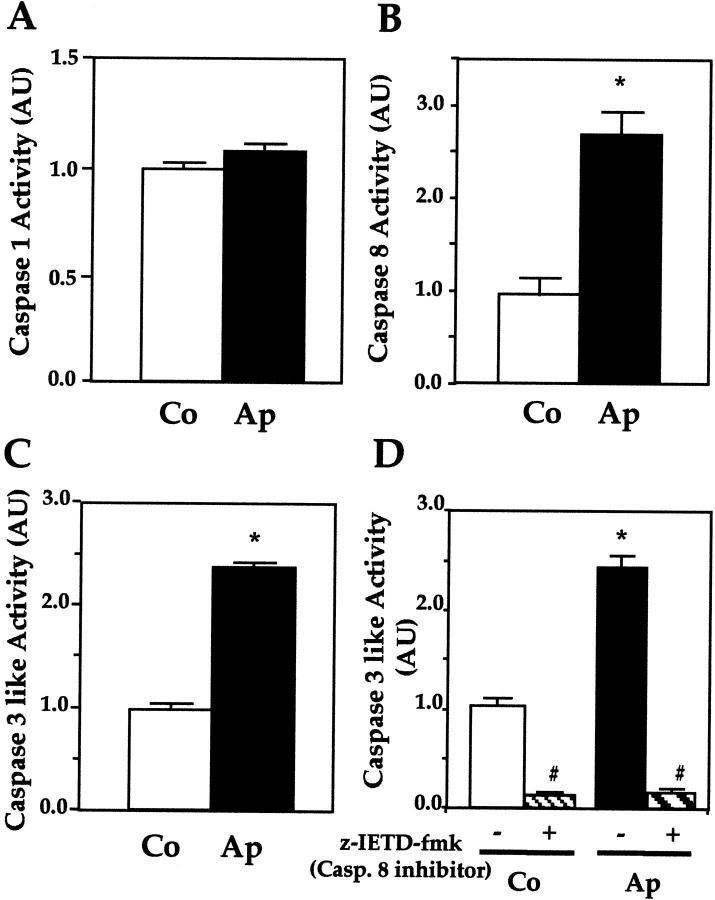
To begin investigating mechanisms involved in the constitutive increase in apoptosis in mutant cells, Ap and Co cells were cultured in basal conditions, and the activity of caspase-1, -3, and -8 was determined using specific substrates. Figure 3 ▶ shows that, in basal conditions, caspase-1 activity was similar in normal and mutant osteoblasts (Figure 3A) ▶ . In contrast, the activity of the initiator caspase-8 was increased threefold in Ap cells compared to Co cells (Figure 3B) ▶ . The activity of the effector caspases (caspase-3-like) was also dramatically increased in mutant cells (Figure 3C) ▶ . Moreover, suppression of caspase-8 activity, using the specific inhibitor z-IETD-fmk, abolished caspase-3-like activity in normal Co cells and mutant Ap cells (Figure 3D) ▶ . This reveals that apoptosis is associated with constitutive activation of caspase-8, leading to activation of downstream effector caspases in human mutant osteoblasts.
Figure 3.
Increased caspase-8- and -3-like activity in mutant osteoblasts. Caspase-1 (A), caspase-8 (B), and caspase-3, -6, and -7 (caspase-like) (C) were determined in Co and Ap cells. Caspase-3, -6, and -7 activity was also determined in the absence or presence of caspase-8 inhibitor (z-IETD-fmk) (D). Ap cells showed increased caspase-8 and caspase-3 activities, and the latter was inhibited by the specific caspase-8 inhibitor. The data are the mean ± SEM of four values. *, P < 0.05 versus Co cells; #, P < 0.05 versus Co or Ap cells untreated with the caspase-8 inhibitor.
Role of PKC in Caspase Activity and Apoptosis in Mutant Osteoblasts
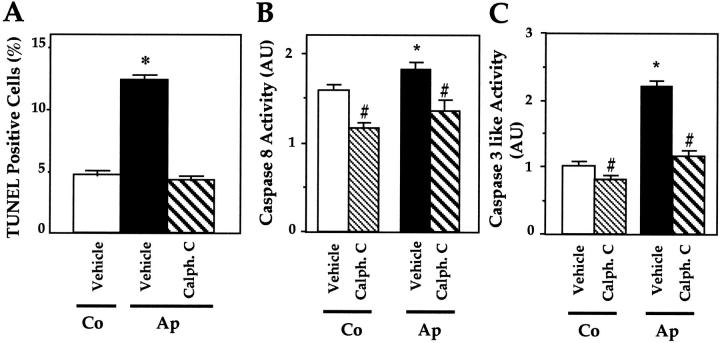
We recently reported that the S252W FGFR-2 mutation constitutively activates PKC signaling whereas erk1,2 and p38 MAP kinases are not affected in mutant Ap cells. 11 To assess whether PKC activity may be involved in the increased apoptosis in mutant cells, Ap cells were treated with calphostin C, a selective inhibitor of PKC, and apoptosis was determined by TUNEL analysis. As shown in Figure 4A ▶ , the number of TUNEL-positive Ap cells was greater than in Co cells, and treatment of Ap cells with 2 μmol/L calphostin C restored the number of TUNEL-positive Ap cells to normal levels (Figure 4A) ▶ . Moreover, suppression of PKC activity by calphostin C reduced both caspase-8 and caspase-3 activity to the basal level in Co cells (Figure 4, B and C) ▶ . Thus, inhibition of PKC activity abolished the constitutive increase in caspase-8 and -3 activity as well as the increased apoptosis induced by the mutation in Ap mutant osteoblasts.
Figure 4.
Role of PKC in caspase-8- and -3-like activity and apoptosis in mutant osteoblasts. Mutant Apert (Ap) cells were treated with 2 μmol/L calphostin C, a specific PKC inhibitor, and TUNEL-positive apoptotic cells (A), caspase-8 (B), and caspase-3, -6, and -7 (caspase-like) activity (C) were determined. The data are the mean ± SEM of four values. *, P < 0.05 versus Co cells; #, P < 0.05 versus vehicle-treated Co or Ap cells.
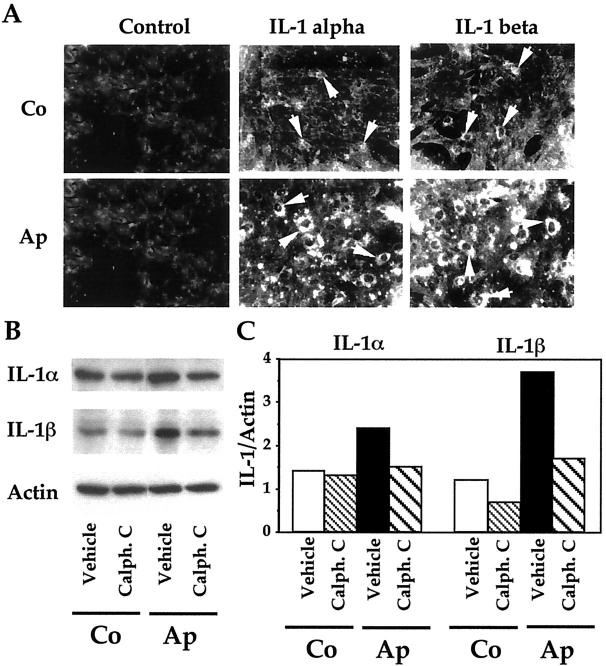
Role of PKC in the Increased IL-1α and IL-1β in Mutant Osteoblasts
Until now, the results showed that the increased apoptosis induced by the S252W FGFR-2 Apert mutation in osteoblasts involved increased PKC activity and, subsequently, increased effector caspase activity. Another possible mechanism may be CD95/Fas-mediated apoptosis, which is promoted by IL-1 in osteoblasts. We addressed this hypothesis by first investigating the in vitro expression of IL-1α and IL-1β in cultured Ap and Co cells. As shown in Figure 5A ▶ , Co cells showed weak IL-1α and IL-1β immunostaining. By contrast, Ap cells showed high IL-1α and IL-1β immunoreactivity compared to Co cells (Figure 5A) ▶ . The expression of IL-1α and IL-1β protein levels was further analyzed by Western blot. Figure 5B ▶ shows that IL-1α and IL-1β protein levels were increased in Ap cells compared to Co cells, confirming the immunocytochemical analysis (Figure 5A) ▶ . In parallel experiments, we found increased IL-1α and IL-1β immunolabeling in osteoblasts in the Apert suture compared to normal coronal suture (not shown). These results show that the FGFR-2 mutation induces a constitutive increase in IL-1α and IL-1β expression in mutant osteoblasts. To assess whether the increased IL-1α and IL-1β expression may result from PKC activation in mutant cells, Ap cells were treated with the PKC inhibitor calphostin C and IL-1α and IL-1β levels were analyzed by Western blot. As shown in Figure 5, B and C ▶ , treatment of Ap cells with calphostin C restored IL-1α and IL-1β protein levels to control levels in Co cells. Thus, PKC activation induced by the mutation is responsible for the increased IL-1α and IL-1β expression in mutant osteoblasts.
Figure 5.
Role of PKC in the increased IL-1α and IL-1β expression in mutant osteoblasts. A: Apert (Ap) and control (Co) cells were immunostained for IL-1α and IL-1β using specific antibodies or IgG (control). Strong IL-1α and IL-1β immunostaining was found in Ap cells compared to Co cells (arrows). Western blot analysis (B) and densitometric analysis (C) show that the IL-1α and IL-1β overexpression in Ap cells was suppressed by the PKC inhibitor calphostin C (2 μmol/L).
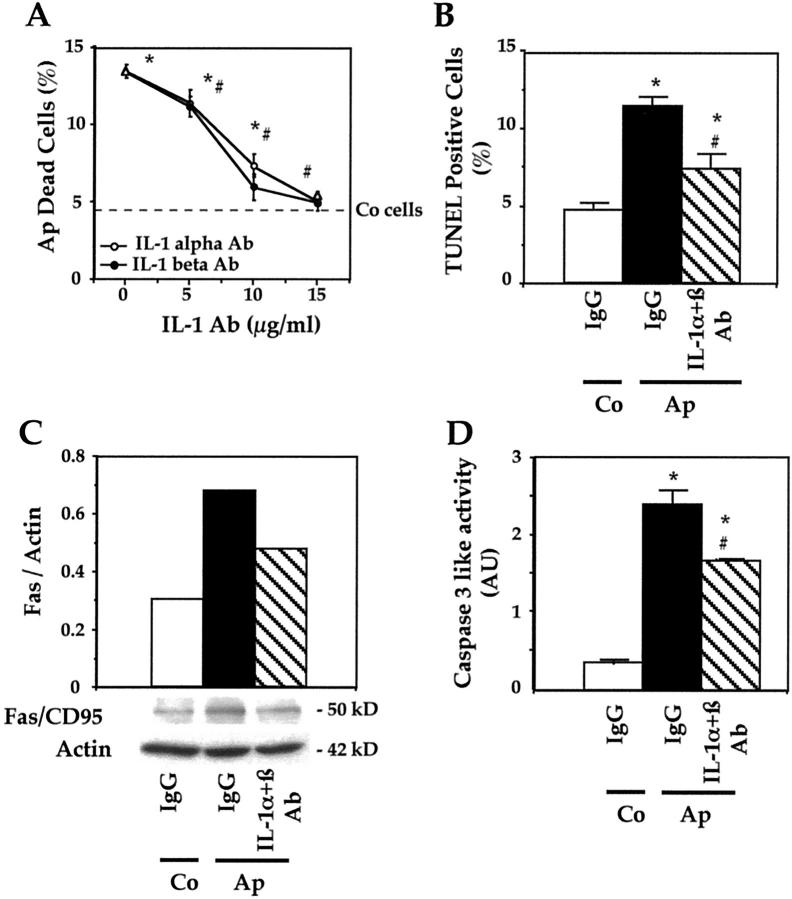
Role of IL-1α and IL-1β in Apoptosis in Mutant Osteoblasts
To assess whether the increased IL-1α and IL-1β expression is involved in apoptosis induced by the mutation, we tested the effects of anti-IL-1α and anti-IL-1β neutralizing antibodies or IgG on apoptosis and caspase-3 activity. Apoptosis was determined by trypan blue exclusion because necrosis is low in these conditions. As shown in Figure 6A ▶ , incubation of Ap cells with IL-1α antibody dose-dependently decreased the number of trypan blue-stained Ap cells. A similar effect of anti-IL-1β was found (Figure 6A) ▶ . The number of trypan blue-stained Ap cells did not significantly differ from normal levels in Co cells in the presence of either IL-1α antibody or IL-1β antibody (15 μg/ml) (Figure 6A) ▶ . Furthermore, quantification of TUNEL-positive (apoptotic) Ap cells showed that IL-1α and IL-1β antibodies at optimal dosage (total dose, 30 μg/ml) decreased apoptosis in Ap mutant cells. However, the number of Ap apoptotic cells remained higher than in Co cells (Figure 6B) ▶ , suggesting that suppression of IL-1 did not completely abolish apoptosis in mutant cells.
Figure 6.
Role of IL-1 and Fas in the increased apoptosis in mutant osteoblasts. A: Apert (Ap) cells were treated with anti-IL-Iα or anti-IL-1β antibodies at different doses and the number of trypan blue-stained cells was recorded. The dotted bar indicates the normal level in control (Co) cells. B: Ap cells were treated with neutralizing IL-1α and IL-1β antibodies (15 μg/ml each) or IgG, and the number of TUNEL-positive cells was counted. In parallel experiments, Fas protein levels were determined by Western blot analysis and the data recorded by densitometric analysis were corrected for β-actin (C). Caspase-3, -6, and -7 (caspase-like) activity was determined in the same culture conditions (D). The data are the mean ± SEM of four values. *, P < 0.05 versus Co IgG-treated cells; #, P < 0.05 versus Ap IgG-treated cells.
To determine whether IL-1 overexpression was associated with increased Fas levels, Fas was determined in the presence of IL-1 antibodies in mutant cells. As shown in Figure 6C ▶ , CD95/Fas protein levels were increased in Ap cells compared to Co cells. The addition of neutralizing IL-1α and IL-1β antibodies (total dose, 30 μg/ml) decreased CD95/Fas expression in mutant osteoblasts compared to a nonspecific IgG (Figure 6C) ▶ . This suggests a role of IL-1 in Fas overexpression in Ap cells. However, Fas levels in Ap cells were not restored to normal levels in Co cells. To further determine the role of IL-1α and IL-1β in the apoptotic process in mutant cells, Ap cells were treated with IL-1α and IL-1β antibodies (total dose, 30 μg/ml) or IgG and caspase-3 activity was determined. As shown in Figure 6D ▶ , treatment of Ap cells with neutralizing IL-1α and IL-1β significantly inhibited caspase-3 activity in Ap cells (Figure 6D) ▶ . Again, caspase-3-like activity was not completely restored to normal. Similar results were obtained with caspase-8 activity (data not shown). These results indicate that suppression of IL-1α and IL-1β expression leads to reduced CD95/Fas overexpression, caspase-3 activity and apoptosis in mutant osteoblasts. However, caspase-3 activity and apoptosis were not completely abolished by neutralizing IL-1 antibodies, suggesting that another pathway may be involved.
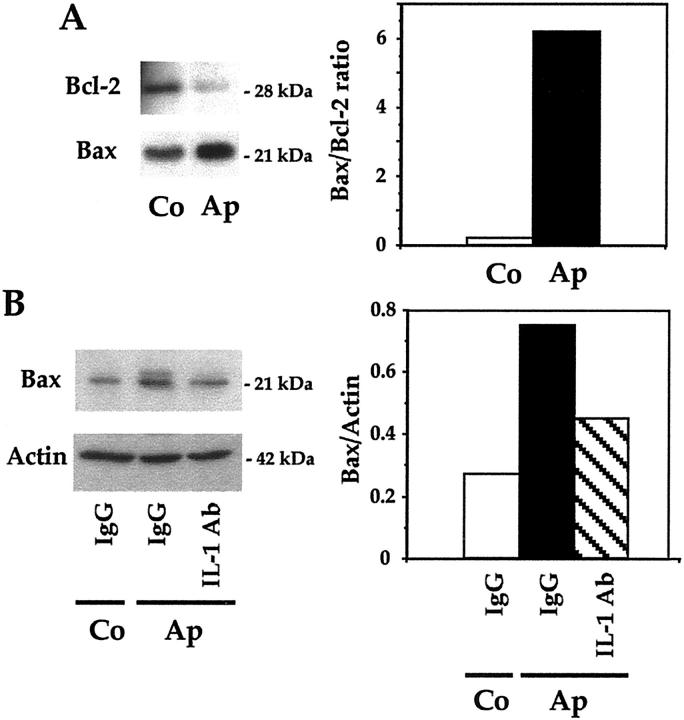
Bax and Bcl2 Expression in Mutant Apert Osteoblasts
Apoptosis is governed in part by Bax and Bcl-2 that are pro-apoptotic and prosurvival proteins, respectively. To determine the implication of these proteins in apoptosis induced by the Apert FGFR-2 mutation, we examined their expression in Ap mutant cells. Western blot analysis showed that Bax protein levels were increased in Ap cells compared to normal Co cells cultured in basal conditions (Figure 7A) ▶ . In contrast, Bcl2 levels were lower in Ap cells than in Co cells, which resulted in a huge increase in the Bax/Bcl2 ratio in mutant cells (Figure 7A) ▶ . Furthermore, we found that IL-1α and IL-1β neutralizing antibodies (total dose, 30 μg/ml) reduced by 50% Bax expression in Ap cells (Figure 7B) ▶ . These results suggest that the increase in Bax and decreased Bcl-2 may contribute to apoptosis in Ap cells, and that IL-1 overexpression mediates in part the increased Bax level in Ap mutant cells.
Figure 7.
Role of Bax and Bcl-2 in apoptosis in mutant osteoblasts. The levels of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 were determined by Western blot analysis in Apert (Ap) and control (Co) cells in basal culture conditions, the bands were scanned and the ratio of Bax/Bcl-2 was determined (A). Bax protein levels were determined in Ap cells in the presence of specific neutralizing IL-1α and IL-1β antibodies (15 μg/ml each) or IgG, and the levels were corrected for β-actin (B).
Discussion
In this study, we show that the S252W FGFR-2 mutation in Apert craniosynostosis induces premature programmed cell death in osteoblasts by a PKC-dependent pathway involving IL-1α and IL-1β, CD95/Fas, caspase-8 activity, and Bax/Bcl2 levels. We first showed that premature apoptosis is a hallmark of human osteoblast abnormalities induced by the Apert S252W FGFR-2 mutation in vivo. Increased apoptosis was restricted to more mature osteoblasts and osteocytes in the Apert suture, showing that apoptosis induced by the mutation affects more differentiated cells. This is supported by the observation that apoptosis increases with osteoblast maturation in vitro and is a prominent feature during the late development of the mature osteoblast phenotype. 16 This is also consistent with the recent finding that transfection with the S252W FGFR-2 mutation induces apoptosis in mouse differentiating calvaria cells. 12 The present data in human Apert osteoblasts supports the previous hypothesis that FGFR activating mutations affect apoptosis in human skeletal cells. Indeed, recurrent mutations in FGFR-3 in thanatophoric dysplasia were found to induce apoptosis in chondrocytes. 37 Conversely, the overexpression of a mouse dominant-negative FGFR-1 mutation was found to suppress apoptosis. 38
We sought mechanistic insight into the pro-apoptotic effect of the S252W FGFR-2 mutation in human calvaria osteoblasts by examining the signal transduction pathways and the intracellular mechanisms activated by the mutation. Several serine/threonine protein kinases have been proposed to control apoptotic mechanisms, including Erk1,2 mitogen-activated protein kinase (MAPK), p38 Map kinase, c-Jun N terminal kinase (JNK), and protein kinases A, B, and C. 39,40 Recent reports indicate that FGFR-2 mutations increase PKC expression and activity in human mutant osteoblasts. 8,11,41 More specifically, we found that the S252W FGFR-2 mutation activates PLCγ and PKC whereas Erk 1,2 or p38 kinases are not activated in Apert human osteoblasts. 11 Our finding that the PKC inhibitor calphostin C decreased apoptosis suggests an important role for PKC in apoptosis induced by the mutation in Ap cells. Although activation of PKC has been previously observed to contribute to apoptotic signaling, 39 novel PKC isoforms seem to be pro-apoptotic whereas classical and atypical PKC isoenzymes are associated with cell survival. 40 Human osteoblasts with FGFR-2 mutations express numerous PKC isoforms. 8,11 However, the precise role of each isoenzyme in the apoptotic effect of FGFR-2 mutations in Apert syndrome remains to be clarified. In this regard, Apert osteoblasts display increased PKCα activity 8,11 and expression, 41 suggesting a role for the PKCα isoform in apoptosis in mutant cells.
Having shown that the increased apoptosis induced by the FGFR-2 mutation is PKC-dependent, we assessed the downstream events involved in apoptosis in mutant cells. Evidence that apoptosis in Apert osteoblasts involves IL-1 and Fas pathways is supported by several findings. Mutant osteoblasts constitutively overexpress IL-1α and IL-1β protein levels that were corrected by the PKC inhibitor calphostin C, indicating a role for PKC in IL-1 overexpression in mutant cells. Our finding that neutralizing anti-IL-1 antibodies reduced Fas overexpression and apoptosis suggests that Fas-mediated apoptosis is mediated by IL-1 overexpression in mutant cells. Thus, apoptosis in Apert osteoblasts seems to be primarily mediated by a PKC-dependent overexpression of IL-1 and subsequent activation of Fas-mediated apoptosis. Because Fas expression may be directly activated by PKC, 42 it is also possible that PKC activation may directly increase Fas expression in mutant cells. A role for Fas and IL-1 in apoptosis induced by the Apert S252W FGFR-2 mutation is consistent with the finding that IL-1 and Fas-mediated apoptosis controls cell death in osteoblasts. 29,30 Fas-mediated apoptosis is known to activate a series of complex mechanisms, leading to procaspase cleavage and formation of caspases. 43,44 Procaspase-8 is cleaved after ligation of specific transmembrane death receptors such as Fas, 25 and activation of caspase-8 is one of the signaling pathways leading from Fas to apoptosis. 45,46 Our finding that neutralizing IL-1 antibodies reduced the constitutive increase in caspase-8 and caspase-3 activities indicates that the IL-1 and Fas-mediated pathway activates caspase-8 and ultimately, effector caspases and DNA degradation in Apert osteoblasts. Although numerous diseases have been previously associated with increased Fas levels, 47 this study is the first to present evidence for increased Fas-mediated apoptosis in abnormal (premature) membranous ossification induced by a genetic FGFR mutation in humans.
Because Bax and Bcl-2 are known to play critical roles in programmed cell death in several cell types and are influenced by FGFs 33,34 we hypothesized that apoptosis in Apert osteoblasts may involve alteration in the balance between Bax and Bcl-2. The increased Bax levels and decreased Bcl-2 levels in mutant cells indicates that the alteration of Bax/Bcl-2 may contribute to apoptosis induced by the FGFR-2 mutation. This is consistent with the previous finding that apoptosis induced by FGF-1 is associated with increased Bax and delayed Bcl-2 in differentiating murine calvaria cells. 12 The increased Bax in Ap cells seems to result from IL-1 overexpression, which indicates that IL-1 overexpression may induce premature cell death in mutant cells in part by altering the Bax/Bcl-2 ratio. Because apoptosis in Ap mutant cells was not completely corrected by anti-IL-1 antibodies, apoptosis may also arise from IL-1-independent mechanisms. The present data are compatible with a model in which the increased apoptosis induced by the S252W FGFR-2 mutation is triggered by a PKC-dependent pathway involving IL-1, Fas, caspase-8, and also Bax/Bcl-2 that ultimately converge to increase effector caspases in mutant osteoblasts.
The mechanisms by which FGF signaling controls cranial suture ossification are still unclear. Although FGFs are important factors controlling osteoblast proliferation and differentiation, 48 recent data indicate that the effects of FGF depend on the maturation stage of osteoblasts. Indeed, FGFs induce opposite effects on calvaria cell proliferation, differentiation, and apoptosis depending on the differentiation stage, and distinct responses to FGF were found in immature and mature osteoblasts. 12,49 Thus, the apparently different alterations of cell proliferation and differentiation reported in FGFR-2 mutant human and murine osteoblasts 8,9,12 may arise from variable FGF signaling mechanisms leading to distinct effects in mature and immature calvaria osteoblasts. One possible mechanism for the variable effects of FGF signaling may be a distinct expression of FGFRs in immature and mature calvaria cells. In this regard, we found that FGFR-2 is down-regulated in differentiated mutant Apert osteoblasts in vitro and in vivo. 10,48 FGFR-2 down-regulation in more differentiated mutant osteoblasts may limit the proliferative activity of the cells and contribute to the premature osteoblast differentiation. 50 Thus, a combination of increased differentiation and apoptosis in mature osteoblasts may accelerate the osteogenic process and contribute to premature cranial ossification in Apert syndrome. Further analysis of FGF/FGFR signaling pathways in relation to cell differentiation and apoptosis, now in progress in our laboratory, may shed more light on the mechanisms leading to the premature suture ossification in Apert syndrome.
Acknowledgments
The authors thank Dr. A-L. Delezoide, Hopital R. Debré, AP-HP, Paris, France, for supplying the bone samples; and Dr. J. Bonaventure, INSERM U 393, Hôpital Necker, Paris, France, for the genetic mutation analyses.
Footnotes
Address reprint requests to P. J. Marie, Ph.D., INSERM U 349, Lariboisière Hospital, 2 rue Ambroise Paré, 75475 Paris Cedex 10, France. E-mail: pierre.marie@inserm.lrb.ap-hop-paris.fr.
References
- 1.Muenke M, Schell U: Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet 1995, 11:308-313 [DOI] [PubMed] [Google Scholar]
- 2.Naski MC, Ornitz DM: FGF signaling in skeletal development. Front Biosci 1998, 3:D781-D794 [DOI] [PubMed] [Google Scholar]
- 3.Burke D, Wilkes D, Blundell TL, Malcolm S: Fibroblast growth factor receptors: lessons from the genes. Trends Biochem Sci 1998, 23:59-62 [DOI] [PubMed] [Google Scholar]
- 4.Hehr U, Muenke M: Craniosynostosis syndromes: from genes to premature fusion of skull bones. Mol Genet Metab 1999, 68:139-151 [DOI] [PubMed] [Google Scholar]
- 5.Mangasarian K, Li Y, Mansukhani A, Basilico C: Mutation associated with Crouzon syndrome causes ligand-independent dimerization and activation of FGF receptor-2. J Cell Physiol 1997, 172:117-125 [DOI] [PubMed] [Google Scholar]
- 6.Robertson SC, Meyer AN, Hart KC, Galvin BD, Webster MK, Gonoghue DJ: Activating mutations in the extracellular domain of the fibroblast growth factor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci USA 1998, 95:4567-4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotnikov AN, Hubbard SR, Schlessinger J, Mohammadi M: Crystal structures of two FGF-FGFR complexes reveal the determinants of ligand-receptor specificity. Cell 2000, 101:413-424 [DOI] [PubMed] [Google Scholar]
- 8.Lomri A, Lemonnier J, Hott M, de Perseval N, Lajeunie E, Munnich A, Renier D, Marie PJ: Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J Clin Invest 1998, 101:1310-1317 [PMC free article] [PubMed] [Google Scholar]
- 9.Lemonnier J, Hott M, Delannoy P, Lomri A, Modrowski D, Marie PJ: The Ser252Trp fibroblast growth factor receptor-2 (FGFR-2) mutation induces PKC-independent downregulation of FGFR-2 associated with premature calvaria osteoblast differentiation. Exp Cell Res 2000, 256:158-167 [DOI] [PubMed] [Google Scholar]
- 10.Fragale A, Tartaglia M, Bernardini S, Michela Di Stasi AM, Di Rocco C, Velardi F, Teti A, Battaglia PA, Migliacco S: Decreased proliferation and altered differentiation in osteoblasts from genetically and clinically distinct craniosynostotic disorders. Am J Pathol 1999, 154:1465-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemonnier J, Hay E, Delannoy P, Lomri A, Modrowski D, Caverzasio J, Marie PJ: Role of N-cadherin and protein kinase C in osteoblast gene activation induced by the S252W fibroblast growth factor receptor 2 mutation in Apert craniosynostosis. J Bone Miner Res (in press) [DOI] [PubMed]
- 12.Mansukhani A, Bellosta P, Sahni M, Basilico C: Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol 2000, 149:1297-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furtwangler JA, Hall SH, Koskinen-Moffett LK: Sutural morphogenesis in the mouse calvaria: the role of apoptosis. Acta Anat (Basel) 1985, 124:74-80 [DOI] [PubMed] [Google Scholar]
- 14.Bourez RL, Mathijssen IM, Vaandrager JM, Vermeij-Keers C: Apoptotic cell death during normal embryogenesis of the coronal suture: early detection of apoptosis in mice using annexin V. J Craniofac Surg 1997, 8:441-445 [DOI] [PubMed] [Google Scholar]
- 15.Lynch MP, Capparelli C, Stein JL, Stein GS, Lian JB: Apoptosis during bone-like tissue development in vitro. J Cell Biochem 1998, 68:31-49 [PubMed] [Google Scholar]
- 16.Rice D, Kim H, Thesleff I: Apoptosis in murine calvarial bone and suture development. Eur J Oral Sci 1999, 107:265-275 [DOI] [PubMed] [Google Scholar]
- 17.Manolagas SC: Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000, 21:115-137 [DOI] [PubMed] [Google Scholar]
- 18.Schwartzman RA, Cidlowski JA: Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 1993, 14:133-151 [DOI] [PubMed] [Google Scholar]
- 19.Israels LG, Israels ED: Apoptosis. Oncologist 1999, 4:332-339 [PubMed] [Google Scholar]
- 20.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM: Serine/threonine protein kinases and apoptosis. Exp Cell Res 2000, 256:34-41 [DOI] [PubMed] [Google Scholar]
- 21.Loeffler M, Kroemer G: The mitochondrion in cell death control: certainties and incognita. Exp Cell Res 2000, 256:19-26 [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi A, Dixit VM: Death receptors: signaling and modulation. Science 1998, 281:1305-1308 [DOI] [PubMed] [Google Scholar]
- 23.Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75:1169-1178 [DOI] [PubMed] [Google Scholar]
- 24.Friesen C, Herr I, Krammer PH, Debatin KM: Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nat Med 1996, 2:574-577 [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Harvey NL: Role of multiple cellular proteases in the execution of programmed cell death. FEBS Lett 1995, 375:169-173 [DOI] [PubMed] [Google Scholar]
- 26.Thornberry NA, Lazebnik Y: Caspases: enemies within. Science 1998, 281:1312-1316 [DOI] [PubMed] [Google Scholar]
- 27.Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994, 124:1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonsson B, Martinou JC: The Bcl-2 protein family. Exp Cell Res 2000, 256:50-57 [DOI] [PubMed] [Google Scholar]
- 29.Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, Fujiyama K, Kiriyama T, Nakashima T, Nakane PK, Nagataki S: Fas and Fas ligand interaction is necessary, for human osteoblast apoptosis. J Bone Miner Res 1997, 12:1637-1646 [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi M, Kawakami A, Nakashima T, Matsuoka N, Urayama S, Kawabe Y, Fujiyama K, Kiriyama T, Aoyagi T, Maeda K, Eguchi K: Tumor necrosis factor-alpha and interleukin-1 beta increase the Fas-mediated apoptosis of human osteoblasts. J Lab Clin Med 1999, 134:222-231 [DOI] [PubMed] [Google Scholar]
- 31.Weinstein RS, Bellido T, Parfitt AM, Manolagas SC: Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res 1998, 13:793-802 [DOI] [PubMed] [Google Scholar]
- 32.Gohel A, McCarthy MB, Gronowicz G: Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology 1999, 140:5339-5347 [DOI] [PubMed] [Google Scholar]
- 33.Funato N, Moriyama K, Shimokawa H, Kuroda T: Basic fibroblast growth factor induces apoptosis in myofibroblastic cells isolated from rat palatal mucosa. Biochem Biophys Res Commun 1997, 240:21-26 [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Maloof P, Wang H, Fenig E, Stein D, Nichols G, Denny TN, Yahalom J, Wieder R: Basic fibroblast growth factor downregulates Bcl-2 and promotes apoptosis in MCF-7 human breast cancer cells. Exp Cell Res 1998, 238:177-187 [DOI] [PubMed] [Google Scholar]
- 35.De Pollak C, Renier D, Hott M, Marie PJ: Increased bone formation and osteoblastic cell phenotype in premature cranial suture ossification (craniosynostosis). J Bone Miner Res 1996, 11:401-407 [DOI] [PubMed] [Google Scholar]
- 36.Kleuser B, Cuvillier O, Spiegel S: 1α,25-dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res 1998, 58:1817-1824 [PubMed] [Google Scholar]
- 37.Legeai-Mallet L, Benoist-Lasselin C, Delezoide AL, Munnich A, Bonaventure J: Fibroblast growth factor receptor 3 mutations promote apoptosis but do not alter chondrocyte proliferation in thanatophoric dysplasia. J Biol Chem 1998, 273:13007-13014 [DOI] [PubMed] [Google Scholar]
- 38.Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA: FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development 1995, 121:4383-4393 [DOI] [PubMed] [Google Scholar]
- 39.Lucas M, Sanchez-Margalet V: Protein kinase C involvement in apoptosis. Gen Pharmacol 1995, 26:881-887 [DOI] [PubMed] [Google Scholar]
- 40.Zeidman R, Pettersson L, Sailaja PR, Truedsson E, Fagerstrom S, Pahlman S, Larsson C: Novel and classical protein kinase C isoforms have different functions in proliferation, survival and differentiation of neuroblastoma cells. Int J Cancer 1999, 81:494-501 [DOI] [PubMed] [Google Scholar]
- 41.Lomri A, Lemonnier J, Delannoy P, Marie PJ: Identification of genes induced by the Ser252Trp FGFR-2 Apert mutation in osteoblasts using atlas human expression arrays: evidence for increased expression of PKCα, IL-1α and RhoA GTPase. J Bone Miner Res (in press) [DOI] [PubMed]
- 42.Wang R, Zhang L, Yin D, Mufson RA, Shi Y: Protein kinase C regulates Fas (CD95/APO-1) expression. J Immunol 1998, 161:2201-2207 [PubMed] [Google Scholar]
- 43.Nagata S: Fas-induced apoptosis, and diseases caused by its abnormality. Genes Cells 1996, 1:873-879 [DOI] [PubMed] [Google Scholar]
- 44.Depraetere V, Golstein P: Fas and other cell death signaling pathways. Semin Immunol 1997, 9:93-107 [DOI] [PubMed] [Google Scholar]
- 45.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM: An induced proximity, model for caspase-8 activation. J Biol Chem 1998, 273:2926-2930 [DOI] [PubMed] [Google Scholar]
- 46.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES: Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA 1996, 93:14486-14491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waring P, Mullbacher A: Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol 1999, 77:312-317 [DOI] [PubMed] [Google Scholar]
- 48.Marie PJ, Debiais F, Lomri A, Lemonnier J: Fibroblast growth factors and osteoblasts. Canalis E eds. Skeletal Growth Factors. 2000, :pp 179-196 Lippincott, Williams and Wilkins, Baltimore [Google Scholar]
- 49.Debiais F, Hott M, Graulet AM, Marie PJ: The effects of fibroblast growth factor-2 on human neonatal calvaria osteoblastic cells are differentiation stage specific. J Bone Miner Res 1998, 13:645-654 [DOI] [PubMed] [Google Scholar]
- 50.Iseki S, Wilkie AO, Morriss-Kay GM: Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development 1999, 126:5611-5620 [DOI] [PubMed] [Google Scholar]