Abstract
During early human pregnancy extravillous cytotrophoblasts invade the uterus and spiral arteries transforming them into large vessels of low resistance. Failure of trophoblast invasion and spiral artery transformation occurs in preeclampsia and fetal growth restriction (FGR); these processes are not well understood. Recent studies have suggested that cytotrophoblasts that invade spiral arteries mimic the endothelial cells they replace and express PECAM-1. It was also reported that in preeclampsia, cytotrophoblasts fail to express PECAM-1 and that failure to express endothelial cell adhesion molecules may account for failed trophoblast invasion. Despite the possible importance of adhesion molecules in trophoblast invasion, no study has systematically investigated the expression of PECAM-1 in the placental bed throughout the period of invasion, particularly in the myometrial segments where the key failure occurs. There are no studies on PECAM-1 expression in the placental bed in FGR. We have examined the expression of PECAM-1 in placental bed biopsies and placentas from 8 to 19 weeks of gestation and in the placenta and placental bed in the third trimester in cases of preeclampsia, FGR, and control pregnancies. PECAM-1 was expressed on endothelium of vessels in the placenta and placental bed but not by villous or extravillous trophoblasts in normal or pathological samples. These findings do not support a role for PECAM-1 in normal invasion or in the pathophysiology of preeclampsia or FGR.
During early human pregnancy, extravillous cytotrophoblasts (CTBs) from anchoring villi invade the decidualized endometrium and myometrium (interstitial trophoblasts) and also migrate in a retrograde direction along the spiral arteries (endovascular trophoblasts) transforming them into large diameter conduit vessels of low resistance. 1 Endovascular trophoblast invasion has been reported to occur in two waves; the first into the decidual segments of spiral arteries at 8 to 10 weeks of gestation and the second into myometrial segments at 16 to 18 weeks of gestation. 1 This physiological transformation is characterized by a gradual loss of the normal musculoelastic structure of the arterial wall and replacement by amorphous fibrinoid material in which trophoblast cells are embedded. 2-7 These physiological changes are required for a successful pregnancy.
Failure of trophoblast invasion and spiral artery transformation has been documented in preeclampsia (PE), one of the leading causes of maternal death. In this syndrome reduced uteroplacental perfusion is associated with widespread endothelial dysfunction and fetal growth restriction (FGR) leading to significant maternal and perinatal morbidity. 8 Similar spiral artery abnormalities have been reported in the placental bed of women with FGR and spontaneous abortion in the absence of maternal hypertension. 4,9-16 Thus failure of the spiral arteries to undergo physiological transformation may lead to a spectrum of pregnancy failures. Despite the importance of trophoblast invasion and vascular remodeling, these processes are still not well understood. However, they are thought to include changes in expression of cell adhesion molecules, matrix metalloproteinases and their tissue inhibitors, and growth factors and their receptors. 17,18
Platelet endothelial cell adhesion molecule (PECAM-1) is a member of the immunoglobulin family and is a transmembrane glycoprotein of ∼130 kd. 19 PECAM-1 is expressed by a wide variety of cells including endothelial cells, platelets, neutrophils, monocytes, and lymphocytes 19 and appears early in the development of the vascular system. 20 PECAM-1 is localized to cell-cell borders of adjacent endothelial cells suggesting a role in angiogenesis. 21 Several studies also support a role for PECAM-1 in leukocyte-endothelial interactions during leukocyte margination at times of inflammation. 22 Recent studies have suggested that PECAM-1 and other endothelial cell adhesion molecules (CAMs) may also play a role in spiral artery transformation. 23 It was suggested that CTBs that invade spiral arteries mimic the adhesion phenotype of the endothelial cells they replace and that extravillous CTBs in cell columns, interstitial and endovascular CTBs express PECAM-1. The authors also reported that in PE, the CTBs fail to express PECAM-1. 23,24 It was suggested that failure to express endothelial CAMs in PE may account for the failure of trophoblast invasion.
Despite the possible importance of cell adhesion molecules such as PECAM-1 in trophoblast invasion, no study has systematically investigated the expression of PECAM-1 in the placental bed throughout the period of trophoblast invasion and spiral artery transformation, particularly in the myometrial segments where the key failure in invasion in PE occurs. There are also no studies on PECAM-1 expression in the placental bed in FGR. Thus in this study we have used immunohistochemistry to examine the expression of PECAM-1 in placental bed biopsies and placentas from 7 to 19 weeks of gestation and in placenta and placental bed in the third trimester in cases of PE, FGR, and matched control pregnancies.
Materials and Methods
Study Participants and Sample Collection
Samples were obtained from pregnant women at the Royal Victoria Infirmary, Newcastle-on-Tyneside. The study was approved by the Joint Ethics Committee of Newcastle and North Ty Authority and the University of Newcastle. The procedure for collection of placentas and placental bed biopsies from first, second, and term pregnancies has been described previously. 25,26 First and second trimester samples were obtained from women undergoing termination of an apparently normal pregnancy. An initial ultrasound scan was performed to confirm fetal viability and to determine gestational age and placental position. After evacuation of the uterine contents, three placental bed biopsies were taken under ultrasound guidance using biopsy forceps (Wolf, Wimbleton, UK) introduced through the cervix. Forty-three placental bed biopsies spread evenly between 8 to 18 weeks of gestation were studied. Placental samples were collected from all cases.
For the third trimester study three groups of women were studied; control pregnancies with no hypertension or FGR (n = 18), women with pregnancies complicated by PE (n = 17), and women with pregnancies complicated by FGR in the absence of maternal hypertension (n = 8). Briefly, after delivery of the infant, the position of the placenta was determined by manual palpation. Six placental bed biopsies were then taken under direct vision using biopsy forceps. Placental samples were collected from all cases. PE was defined as pregnancy-induced hypertension (blood pressure, 140/90) and proteinuria (300 mg/24 hours) in women who were normotensive before pregnancy and had no other underlying clinical problems such as renal disease. FGR was defined ultrasonically as fetal abdominal circumference (AC) <10th centile with a decrease in AC SD score (SDS) of >1.5 SDS 27 and umbilical artery pulsatility index equaling the 95th centile. 28 We have previously shown that a fall in AC SDS of >1.5 SDS is the optimal cut-off to define a group of fetuses with evidence of wasting at birth and morbidity associated with FGR. 27 Birth weight centiles were obtained from charts of the Northern Region population of England. 29 Clinical details were compared using analysis of variance and post hoc testing was performed using the Fisher’s PLSD test.
All samples were frozen in liquid nitrogen-cooled isopentane and stored sealed at −70°C until required. Cryostat sections (7 μm) from each specimen were stained with hematoxylin and eosin for histological analysis. Placental bed biopsies were included in this study if they contained decidual and/or myometrial spiral arteries with interstitial trophoblasts.
Antibodies
Desmin (NCL-DES-DERII) and cytokeratin (NCL-LP34) monoclonal antibodies were obtained from Novocastra, Newcastle-upon-Tyne, UK. The PECAM-1 monoclonal antibody was obtained from R&D Systems, Abingdon, UK. The fluorescein isothiocyanate-conjugated anti-cytokeratin monoclonal antibody was obtained from Sigma Chemical Company (Poole, UK) and the Texas red anti-mouse IgG antibody was obtained from Vector Laboratories (Peterborough, UK). Aqueous mounting medium (Citifluor) was purchased from UKC Chemical Laboratory (Canterbury, UK) and diamidino-2-phenylindole from the Sigma Chemical Company. All other reagents were purchased from Sigma unless stated otherwise.
Western Blotting
Western blotting was used to determine that the PECAM-1 antibody detected the correct molecular weight species. Placental samples comprising full thickness blocks from chorionic plate through to basal plate were snap-frozen in liquid nitrogen. Tissue samples were ground to a fine powder in liquid nitrogen with a mortar and pestle and added to 4 volumes of cold lysis buffer (25 mmol/L Tris/0.25 mol/L sucrose/1 mmol/L ethylenediaminetetraacetic acid, pH 7.6 and 50 μl/g tissue protease inhibitor cocktail) (Sigma). Using a Polytron homogenizer at setting 10, the sample containers were surrounded by ice and homogenized for 3 × 10 second intervals. The homogenate was spun at 5000 × g for 10 minutes at 4°C to remove debris and the resultant supernatant was aliquoted and stored at −70°C. Protein concentrations were determined by the method of Bradford 30 using bovine serum albumin as a standard, and diluted to the required concentration.
Samples were mixed 1:1 with loading buffer (1.2 ml of 1 mol/L Tris, pH 6.8, 2 ml of glycerol, 4 ml of 10% sodium-dodecyl-sulfate, 2 ml of 1 mol/L dithiothreitol, and 0.8 ml of distilled water with bromophenol blue added to give a deep blue color) and boiled for 5 minutes before loading. Samples were separated on 10% sodium-dodecyl-sulfate polyacrylamide resolving gels with a 4% stacking gel using a minigel kit (BioRad, Hemelhempstead, UK) 31 at a constant current of 15 mA. Each well was loaded with 50 μg of protein. Molecular weight markers (SDS-7B prestained 33- to 205-kd range; Sigma) were loaded beside the samples.
Protein was transferred overnight in buffer containing 25 mmol/L Tris, 190 mmol/L glycine, 20% methanol at a constant 30 V to Hybond ECL nitrocellulose membranes (Amersham, UK). Filters were blocked for 1 hour at room temperature in PBST buffer (1 × PBS, 0.1% Tween 20) (containing 5% Marvel). The PECAM-1 antibody (1:1000) was prepared in PBST containing 1% Marvel and left to stand for 1 hour at room temperature before use to reduce nonspecific binding. The antibody was added for 1 hour at room temperature. The filters were rinsed twice for 5 minutes in PBST and were then incubated with horseradish peroxidase-conjugated sheep anti-mouse IgG (Diagnostics Scotland, Carluke, UK) diluted 1:1000 in PBST containing 1% Marvel for 1 hour at room temperature. Blots were then rinsed again and washed twice for 10 minutes in PBST followed by one 5-minute wash in distilled water. Proteins were detected using the Amersham ECL detection system and filters were exposed to Hyperfilm ECL (Amersham, Buckinghamshire, UK).
Immunohistochemistry
Sections were all stained on the same day (for each antibody) to eliminate day to day variations in immunostaining. Immunohistochemistry was performed using the Vectastain Universal kit (Vector Laboratories). Cryostat sections (7 μm) were mounted on 3-aminopropyl-triethoxysilane-coated glass slides. In addition, to assist in identification of spiral arteries and trophoblasts, sections from placental bed biopsies were immunostained for cytokeratin (1:200) to detect trophoblast and desmin (1:100) to detect muscle. Two separate immunohistochemical methods were used. In the first method, sections were fixed in acetone for 5 minutes, ethanol for 5 minutes, and then rehydrated in water for 5 minutes. Nonspecific binding sites were blocked with the universal kit horse serum at 37°C and after washing in phosphate-buffered saline (PBS) for 5 minutes, the sections were incubated with the PECAM-1 antibody (1:10,000) for 90 minutes in PBS at 37°C. After 2 × 5 minute PBS washes the biotinylated secondary antibody was added for 30 minutes at 37°C. Two more PBS washes were performed and then endogenous peroxidase activity was quenched by incubating the sections in 1% (v/v) hydrogen peroxide in methanol for 15 minutes. The remaining steps were performed according to the instructions supplied with the kit and were performed at room temperature. Immunoreactive proteins were detected with Fast diaminobenzidine tablets (Sigma). Sections were counterstained in Harris’s hematoxylin (BDH, Poole, UK) and mounted in synthetic resin. Omission of primary antibody or substitution of nonimmune serum for the primary antibody were both included as controls and resulted in no immunostaining. In the second method a double-immunofluorescence method was used. Samples were fixed as above and then the PECAM-1 antibody, diluted 1:10,000 in the blocking buffer supplied with the kit used in the first method, was added for 1 hour at 37°C. After 3 × 5-minute washes in PBS the second antibody (Texas Red anti-mouse IgG) was added at 1:100 in PBS for 60 minutes at 37°C. Next the cytokeratin-fluorescein isothiocyanate antibody (diluted 1:50 in blocking buffer) was added for 60 minutes at 37°C. After three further 5-minute washes in PBS the sections were mounted in aqueous mounting medium containing diamidino-2-phenylindole. Mounting medium was prepared by mixing 3 volumes of diamidino-2-phenylindole with 100 volumes of Citiflour. Coverslips were added and sealed with clear nail varnish. Sections were viewed using a Quips LS PathVysion Workstation,/Zeiss Axioplan epifluorescence microscope equipped with cooled charged coupled device camera equipped and filters that allow viewing of Texas red or fluorescein isothiocyanate labeling without cross-contamination (Applied Imaging, Newcastle, UK).
Results
Study Participants
The clinical details for patients used for the third trimester immunohistochemistry studies are shown in Table 1 ▶ . Umbilical artery PI was abnormally elevated in all of the FGR fetuses; five had absent and one had reversed end-diastolic frequencies. Birth weight was significantly reduced in the FGR and the PE group when compared with the control group. All infants in the FGR group had a birth weight less than the 10th centile with five less than the third centile. Two of the infants in the PE group had birth weights <10th centile.
Table 1.
Clinical Details for Placental Immunohistochemistry Studies
| Control (n = 18) | PE (n = 17) | FGR (n = 8) | |
|---|---|---|---|
| Age (years) | 30.77 ± 6.00 | 27.62 ± 7.38 | 30.14 ± 8.45 |
| Gestational age at delivery (weeks) | 37 ± 2.93 | 33.94 ± 4.11* | 34.12 ± 2.47 |
| Birth weight (kg) | 3.13 ± 0.84 | 2.18 ± 1.04* | 1.34 ± 0.38† |
| Systolic BP (mm Hg) | 118.07 ± 8.8 | 155.94 ± 13.93† | 120.63 ± 7.76 |
| Diastolic BP (mm Hg) | 70 ± 5.67 | 105.56 ± 7.46† | 70.63 ± 7.76 |
| Plasma urate (mmol/L) | — | 422 ± 68 | — |
Values are shown as mean ± SD.
*P < 0.05 compared with control pregnant group.
†P < 0.005 compared with control pregnant group.
Western Blots
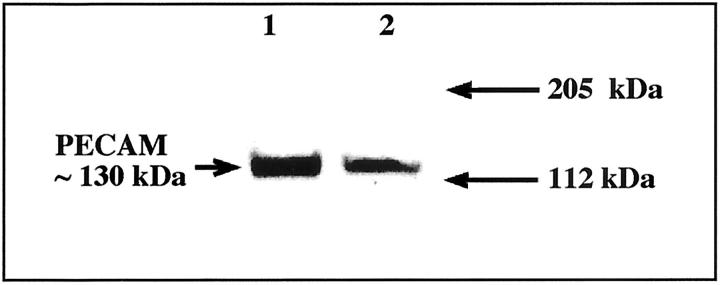
A representative Western blot of placental villous tissue is shown in Figure 1 ▶ . A band of ∼130 kd was identified in the samples that is consistent with the reported molecular weight for PECAM-1. 32 As shown in later immunohistochemistry experiments this band reflects PECAM-1 expressed on the villous endothelium.
Figure 1.
Western blot analysis of placental villous tissue to validate the PECAM-1 antibody. 1 and 2 represent two different term placentas.
Immunohistochemistry
All Placental Cases and First and Second Trimester Placental Bed Cases
The overall findings showed that CTB did not express PECAM-1 across gestation. The findings were consistent with both staining methods. Figure 2 ▶ summarizes the findings. Figures 2A ▶ is a positive control for the antibodies and shows a double-immunofluorescence result for a placenta at 38 weeks of gestation. The villous endothelial cells are positive for PECAM (red) but villous CTBs and syncytiotrophoblasts (green) did not express PECAM. These results were confirmed by the second single staining ABC method (Figure 2D) ▶ . Similar results were obtained for all placentas examined across gestation. Next we examined the expression of PECAM-1 in cell columns and superficial decidua. A representative case at 16 weeks of gestation stained using the double-immunofluorescence method is shown in Figure 2B ▶ . The start of the cell column is indicated by the arrow. None of the cells within the column expressed PECAM-1, however endothelial cells of blood vessels in the adjacent villi are clearly PECAM-1-positive. At the distal end of the columns and within the decidua CTBs were also PECAM-1-negative. Few occasional smaller cells that were cytokeratin-negative (presumably lymphocytes) were PECAM-1-positive and endothelium of blood vessels within the decidua were also PECAM-1-positive. These results were confirmed by the ABC method (Figure 2E) ▶ . A cell island adjacent to villous tissue is shown in Figure 2C ▶ . This case was at 8 weeks of gestation. Extravillous CTBs (EVT) in the cell island were also PECAM-1-negative and this was confirmed by the ABC method (Figure 2F) ▶ . Note the PECAM-1-positive blood vessels in the villous tissue in Figure 2C ▶ . Similar results were obtained for cell columns and cell islands at all gestations. CTB in the basal plate (38-week gestation sample shown) were cytokeratin-positive (Figure 2G ▶ , bottom) and PECAM-1-negative (Figure 2G ▶ , top).
Figure 2.

Double immunofluorescence for PECAM-1 (red) and cytokeratin (green) of a placenta at 38 weeks of gestation (A), a cell column at 16 weeks of gestation (B), and a cell island at 8 weeks of gestation (C). ABC method for PECAM-1 immunostaining in 38-week placenta (D), a cell column (E: left, cytokeratin; right, PECAM-1), cell island (F: left, cytokeratin; right, PECAM-1) and basal plate (G: lower panel, cytokeratin; upper panel, PECAM-1). H and I: Cytokeratin immunostaining (left) and PECAM-1 immunostaining (right) in myometrium and decidua respectively at 9 weeks of gestation. Double immunofluorescence for cytokeratin (J), PECAM-1 (K), and both (L) in a myometrial spiral artery at 18 weeks of gestation.
Within the placental bed CTBs were consistently PECAM-1-negative using both methods of immunostaining. We first used the ABC method to examine the expression of PECAM-1. Figure 2H ▶ (left) shows EVT surrounding a myometrial blood vessel. This example is at 9 weeks of gestation. The adjacent section shows that whereas the endothelium of the blood vessel is PECAM-1-positive, the CTBs are PECAM-1-negative. A section showing deep decidua at 9 weeks of gestation is shown in Figure 2I ▶ . A cytokeratin-positive gland and many EVT including giant cells can be seen (left panel) but all are PECAM-1-negative (right panel). Similar findings for all gestations were found using both staining methods. A representative double-immunofluorescence example of a myometrial spiral artery at 18 weeks of gestation that has undergone extensive invasion by CTBs is shown in Figure 2 ▶ ; J, K, and L. The endothelium is intact in places but in others has disappeared completely (Figure 2K) ▶ . Some of the CTBs have replaced the endothelium, some are lying on top and to the outside of the endothelium, and some are within the lumen itself (Figure 2L) ▶ . All of the CTBs shown are PECAM-1-negative. Because there was no double staining these findings show that invasive CTBs do not express PECAM-1.
Third Trimester Placental Bed Samples
By the third trimester the majority of spiral arteries from both normal and abnormal pregnancies had an intact endothelium that was PECAM-1-positive. In normal cases where CTBs were embedded in the vessel wall the majority were separated from the lumen by endothelium and few intraluminal CTBs were evident. None of the CK-positive CTB expressed PECAM-1. Figure 3 ▶ shows immunofluorescence results for a representative case from a placental bed from a placental bed biopsy obtained from a control pregnancy at 36 weeks of gestation (Figure 3; A, B, and C ▶ ), a case complicated by PE at 27 weeks of gestation (Figure 3; D, E, and F ▶ ) and a case complicated by FGR at 37 weeks of gestation (Figure 3; G, H, and I ▶ ). The normal case selected shows CTBs surrounding a myometrial vessel that has undergone complete physiological change; this vessel had almost no muscle remaining as assessed by desmin immunostaining (not shown). The inset shows another area of the same biopsy where CTBs are in contact with the endothelium. The biopsy shown from the case complicated by PE contains a myometrial vessel that has not undergone physiological change; most of the muscle surrounding this vessel was still intact. Despite the retention of the muscle, this and the other cases of PE still contained abundant interstitial CTBs. These CTBs were also PECAM-1-negative. Finally a case of FGR is shown. Note that the endothelium is complete and almost all of the CTBs are separated from the lumen by the endothelium. As for the control and PE groups, the CTBs in FGR placental bed biopsies did not express PECAM-1 as demonstrated by no double staining.
Figure 3.

Double immunofluorescence staining a placental bed biopsy obtained from a normal pregnancy (A–C), a pregnancy complicated by PE (D–F), and a pregnancy complicated by FGR (G–I). A, D, and G: Cytokeratin immunostaining. B, E, and H: PECAM-1 immunostaining. C, F, and I: Simultaneous detection of cytokeratin and PECAM-1 immunostaining.
Discussion
We believe that the present study is the most comprehensive investigation of PECAM-1 expression in the placenta and placental bed. Because no population of extravillous CTBs expressed PECAM-1 our findings do not support a role for PECAM-1 in the process of normal trophoblast invasion. We also found no differences in PECAM-1 expression on trophoblast from carefully selected cases of PE and FGR confirming our earlier observations in the placenta 33 and inferring no role for this adhesion molecule in the failed trophoblast invasion evident in these conditions.
As CTBs invade the uterus they up-regulate expression of MMP-9, 34 the 92-kd matrix metalloproteinase, HLA-G, 35 the trophoblast-specific HLA class 1 molecule that is thought to be important in avoidance of rejection of the fetus and hormones including human placental lactogen. They also down-regulate the oxygen sensing protein HIF-1α 36 and transforming growth factor-β3. 37 Expression of CAMs are thought to be pivotal to the process of invasion as they determine the adhesion of CTB to each other, to other cell types, and to the extracellular matrix. CTBs interact with components of the extracellular matrix through the integrin class of CAMs. 38 It has been suggested that it is a failure to acquire an invasive phenotype that underlies inadequate spiral artery transformation in PE. For example, villous CTBs express the laminin receptor; integrin α6β4 but as they invade the uterus they down-regulate α6β4 and up-regulate the fibronectin receptor α5β1 and the laminin/collagen receptor α1β1. 39,40 In PE all of the aforementioned are altered; CTBs fail to down-regulate α6β4 and to up-regulate α1β1 integrins, 41 fail to modulate MMP-9, 42 and show reduced expression of HLA-G. 42 Transforming growth factor-β3 expression is also reported to be overexpressed in the placenta in PE. 43 More recently the observations that integrins play a pivotal role in invasion have been extended to members of the cadherin, selectin, and immunoglobulin families. Zhou and colleagues 23 reported that invasive CTBs take on an endothelial phenotype and lose their epithelial phenotype. This group also reported that extravillous CTBs down-regulate E-cadherin during invasion but that expression persists in PE. In contrast VE-cadherin was not present on villous CTBs but was up-regulated during invasion and was detected on CTBs on columns and in the uterine wall of normal pregnancies and on the CTBs that had replaced endothelial cells in spiral arteries. In PE, VE-cadherin was not detected on any CTBs in the placental bed. The study of Zhou and colleagues 23 also investigated CAMs associated with leukocyte trafficking. Immunostaining for VCAM-1 was not present on villous CTBs but was detected on CTBs within the uterine wall. PECAM-1 was also expressed on CTBs in cell columns and on interstitial and endovascular CTBs from normal pregnancies. Neither VCAM-1 nor PECAM-1 were expressed on CTBs in PE cases. E-selectin expression was different from VCAM-1 and PECAM-1 in that expression was detected on villous CTBs as well as CTBs in columns and in decidua, however the expression of E-selectin in PE was not reported.
The conclusions drawn from the first and second trimester studies described above must be interpreted with caution because sample numbers were limited and based on placental samples collected with attached decidua rather than true placental bed. In particular, conclusions based on the time of the myometrial wave of invasion were based on one hysterectomy sample obtained at 22 weeks of gestation. No studies on FGR were performed. Some of the findings of Zhou and colleagues 23 are difficult to interpret when one tries to reconcile the findings with the published morphological data. For example spiral arteries are re-endothelialized in the third trimester 5,44 and the vessel lumen is not replaced by a lining of CTBs. In our third trimester specimens, consistent with the published data, we found that the majority of CTBs close to the lumen had endothelium over the top. Secondly, because PE is characterized by failure of CTBs to invade spiral arteries it is difficult to understand how these cells can fail to express PECAM-1 if they have not actually invaded the arteries. Finally Zhou and colleagues 23 imply that in PE there is a generalized failure of CTBs in the placental bed; interpretation of these data are not straightforward because interstitial migration of EVT into the decidua and myometrium proceeds normally in PE. 45
This study systematically examined the expression of PECAM-1 on CTBs. PECAM-1 was chosen as a representative endothelial CAM. We aimed to confirm and extend the previous study that suggested that PECAM-1 was expressed by invasive CTBs in normal pregnancy but not in PE by using larger numbers of cases, focusing on the myometrial phase of invasion, and by studying FGR. Instead we were unable to show PECAM-1 expression by any CTB population. There are few other studies of normal human pregnancy for comparison. Coukos and colleagues 46 examined placentas with attached decidua between 8 to 12 weeks of gestation for PECAM-1 expression. In parallel cultures CTBs were prepared for PECAM-1 analysis. This group reported that cultured CTBs express PECAM-1 and that PECAM-1 expression was strongly up-regulated on these cells after they have fused to form a syncytium. However, the majority of published studies suggest that villous CTBs and syncytiotrophoblasts do not express PECAM-1 23,33,47 and indeed Coukis and colleagues 46 were unable to identify PECAM-1-positive trophoblasts in placental villous tissue sections at any gestation stage or in the CTB shell. In agreement with the present study, and in contrast to Zhou and colleagues, 23 all CTBs within cell columns were PECAM-1-negative. Coukos and colleagues 46 reported that invasive CTBs within the decidua as a rule did not express PECAM-1 unless in contact with endothelium and the authors reported that sites of endothelial trophoblast contact were where PECAM-1 expression was prominent. The authors however, did not show conclusively that this PECAM-1 was associated with CTBs rather than with the actual endothelial cells and the illustrative examples of PECAM-1-positive CTBs that were cytokeratin-negative raise doubt as to whether these cells were endovascular CTBs. Our studies are consistent with the observations of Pijnenborg and colleagues 48 who reported that CTBs within the placental bed in the third trimester of normal pregnancies and pregnancies complicated by PE did not express PECAM-1. One other study, performed on the Macaque, suggests that trophoblasts express PECAM-1 during invasion. 49 However, the authors also reported strong staining of the apical surface of syncytiotrophoblast in this species that contrasts with reports in humans.
We studied a group of carefully selected women with PE and FGR. Consistent with our observations in normal pregnancy, CTBs from these groups was consistently PECAM-1-negative. This is consistent with our earlier studies of villous CTBs in PE and FGR. 33 We also examined a subset of selected cases with a different PECAM antibody and cytokeratin 7 antibody (Novocastra, UK) and on a subset of cases that had been paraffin embedded (data not shown). Exactly the same results were obtained. There are no other studies of FGR for comparison.
As discussed herein, a wide spectrum of obstetric disorders including PE and FGR have been reported to be associated with reduced modification of maternal spiral arteries by invasive trophoblasts with a consequent reduction in uteroplacental blood flow. 50 This can be demonstrated using Doppler ultrasound; 51,52 abnormal uterine artery Doppler waveforms are predictive of subsequent PE and FGR and the finding of absent physiological change in myometrial vessels is more often found in PE/FGR cases with abnormally uterine artery waveforms. 53,54 In PE, complete physiological change is present in <20% of myometrial spiral arteries. 17,55 This is consistent with the findings in the present study; 72% of the myometrial vessels in the PE cases studied had either completely intact or only partially disrupted muscle. Less is known about the placental bed in FGR. Absence of physiological change has been reported in 45 to 100% of cases of isolated FGR. 6,56,57 These studies have defined FGR according to body weight, typically less than the 10th centile. 17 The criteria used to select growth-restricted fetuses for inclusion in the present study were much stricter and the birth weight and extent of umbilical artery Doppler abnormalities attest to the severity of placental disease in this group. In the collection of FGR specimens used in the present study, 50% of myometrial vessels demonstrated completely intact or partially disrupted muscle. This is in keeping with the data of Gerretsen and colleagues 58 who showed that absence of physiological change in myometrial arteries was more likely in severely small infants (birth weight less than the 2.3 centile), which are more likely to be growth restricted, than in those with birth weights between the 2.3 to 10th centiles.
In summary we have demonstrated that CTB do not switch on expression of PECAM-1 as they become invasive. We have also found no evidence that CTBs that replace spiral artery endothelial cells in the first and second trimester express PECAM-1. We also found that CTBs in the third trimester placental bed in normal pregnancy and in PE and FGR remain PECAM-1-negative. Thus our data do not support a role for PECAM-1 in normal human trophoblast invasion or in the pathophysiology of failed invasion that is characteristic of PE and FGR.
Acknowledgments
We thank Mrs. Barbara Innes for technical assistance and Dr. Helen Simpson for assistance in sample collection.
Footnotes
Address reprint requests to Dr. Fiona Lyall, Maternal and Fetal Medicine Section, Institute of Medical Genetics, Yorkhill, Glasgow, G3 8SJ, United Kingdom. E-mail: f.lyall@udcf.gla.ac.uk.
Supported by Action Research British Heart Foundation and Tommy’s Campaign.
References
- 1.Pijnenborg R, Bland JM, Robertson WB, Brosens I: Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983, 4:397-414 [DOI] [PubMed] [Google Scholar]
- 2.Brosens I, Robertson WB, Dixon HG: The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 1967, 93:569-579 [DOI] [PubMed] [Google Scholar]
- 3.Pijnenborg R, Dixon G, Robertson WB, Brosens I: Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1:3-19 [DOI] [PubMed] [Google Scholar]
- 4.Sheppard BL, Bonnar J: The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. J Obstet Gynaecol Br Cwlth 1976, 83:948-959 [DOI] [PubMed] [Google Scholar]
- 5.De Wolf F, De Wolf-Peeters C, Brosens I: Ultrastructure of the spiral arteries in human placental bed at the end of normal pregnancy. Am J Obstet Gynecol 1973, 117:833-848 [DOI] [PubMed] [Google Scholar]
- 6.Khong TY, De Wolf F, Robertson WB, Brosens I: Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 1986, 93:1049-1059 [DOI] [PubMed] [Google Scholar]
- 7.Blankenship TN, Enders AC, King BF: Trophoblastic invasion and modification of uterine veins during placental development macaques. Cell Tissue Res 1993, 274:135-144 [DOI] [PubMed] [Google Scholar]
- 8.Roberts JM, Redman CW: Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 1993, 341:1447-1451 [DOI] [PubMed] [Google Scholar]
- 9.Michel MZ, Khong TY, Clark DA, Beard RW: A morphological and immunological study of human placental bed biopsies in miscarriage. Br J Obstet Gynaecol 1990, 97:984-988 [DOI] [PubMed] [Google Scholar]
- 10.Jauniaux E, Zaidi J, Jurkovic D, Campbell S, Hustin J: Comparison of colour Doppler features and pathological findings in complicated early pregnancy. Hum Reprod 1994, 12:2432-2437 [DOI] [PubMed] [Google Scholar]
- 11.Hustin J, Jauniaux E, Schaaps JP: Histological study of the materno-embryonic interface in spontaneous abortion. Placenta 1990, 11:477-486 [DOI] [PubMed] [Google Scholar]
- 12.Khong TY, Liddell HS, Robertson W: Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol 1987, 94:649-655 [DOI] [PubMed] [Google Scholar]
- 13.Khong TY: Placental changes in fetal growth retardation. Fetus and Neonate. Physiology and Clinical Applications, vol 3. Edited by MA Hanson, JAD Spencer, CH Rodeck. Cambridge, Cambridge University Press, 1995
- 14.McFadyen IR, Price AB, Geirsson RT: The relation of birth-weight to histological appearances in vessels of the placental bed. Br J Obstet Gynaecol 1986, 93:476-481 [PubMed] [Google Scholar]
- 15.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman AL, Van Assche FA: Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991, 98:648-655 [DOI] [PubMed] [Google Scholar]
- 16.Sheppard BL, Bonnar J: An ultrastructural study of utero placental arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol 1981, 88:695-705 [DOI] [PubMed] [Google Scholar]
- 17.Lyall F, Robson SC: Defective extravillous trophoblast function and pre-eclampsia. Kingdom JCP Jauniaux ERM O’Brien SPM eds. The Placenta: Basic Science and Clinical Practice. 2000, :pp 79-96 RCOG Press, London [Google Scholar]
- 18.Lyall F, Kaufmann P: The uteroplacental circulation: extravillous trophoblast. Baker PN Kingdom JCP eds. Intrauterine Growth Restriction. 2000, :pp 85-119 Springer-Verlag, London [Google Scholar]
- 19.De Lisser HM, Newman PJ, Albelda SM: Molecular and functional aspects of PECAM-1/CD31. Immunol Today 1994, 15:490-495 [DOI] [PubMed] [Google Scholar]
- 20.Baldwin HS, Shen HM, Yan H-C, DeLisser HM, Chung HM, Mickanin A, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, Buck CA: Platelet-endothelial adhesion molecule (PECAM-1/CD31) and its alternatively spliced isoforms are expressed during early mammalian cardiovascular development. Development 1994, 120:2539-2553 [DOI] [PubMed] [Google Scholar]
- 21.Muller WA, Ratti CM, McDonnell SL, Cohn ZA: A human endothelial cell-restricted externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med 1989, 170:399-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller WA, SAW, Deng X, Phillips DM: PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 1993, 178:439–447 [DOI] [PMC free article] [PubMed]
- 23.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH: Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997, 99:2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Damsky CH, Fisher SJ: Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. J Clin Invest 1997, 99:2152-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyall F, Robson SC, Bulmer JN, Kelly H, Duffie E: Human trophoblast invasion and spiral artery transformation: the role of nitric oxide. Am J Pathol 1999, 154:1105-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyall F, Barber A, Myatt L, Bulmer JN, Robson SC: Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J 2000, 14:208-219 [DOI] [PubMed] [Google Scholar]
- 27.Robson SC, Chang TC: Measurement of human fetal growth. Fetus and Neonate, vol 3. Edited by MA Hanson, JAD Spencer, CH Rodeck. Cambridge, Cambridge University Press, 1995
- 28.Arduini D, Rizzo G: Normal values of pulsatility index from fetal vessels; a cross-sectional study of 1556 healthy fetuses. J Perinat Med 1990, 18:165-172 [DOI] [PubMed] [Google Scholar]
- 29.Tin W, Wariyar UK, Hey EN: Selection biases invalidate current low birthweight-for-gestation standards. Br J Obstet Gynaecol 1997, 104:180-185 [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM: A refined and sensitive method for the quantitation of proteins utilizing the principle of protein-dye binding. Anal Biochem 1976, 72:248-254 [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227:680-685 [DOI] [PubMed] [Google Scholar]
- 32.Newman PJ: The biology of PECAM-1. J Clin Invest 1997, 99:3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyall F, Greer IA, Boswell F, Young A, Macara LM, Jeffers MD: Expression of cell-adhesion molecules in placentae from pregnancies complicated by preeclampsia and intrauterine growth retardation. Placenta 1995, 16:79-87 [DOI] [PubMed] [Google Scholar]
- 34.Librach CL, Werb Z, Fitzgerald ML, Chui K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ: 92-kD type IV collagenase mediates invasion of human trophoblasts. J Cell Biol 1991, 113:437-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovats SC, Librach P, Fisch EK, Main PM, Sondel SJ, Fisher SJ, DeMars R: Expression and possible function of the HLA-G chain in human cytotrophoblasts. Science 1990, 248:220-222 [DOI] [PubMed] [Google Scholar]
- 36.Caniggia I, Mostachfi H, Winter J, Grassmann M, Lye SJ, Kuliszewski M, Post M: Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGF-β3. J Clin Invest 2000, 105:577-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ: Inhibition of TGFBβ3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest 1999, 103:1641-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyall F: Cell adhesion molecules: their role in pregnancy. Fetal Maternal Med Rev 1998, 10:21-44 [Google Scholar]
- 39.Vicovac L, Jones CS, Aplin JD: Trophoblast differentiation during formation of anchoring in a model of the early human placenta in vitro. Placenta 1995, 16:41-56 [DOI] [PubMed] [Google Scholar]
- 40.Damsky CH, Fitzgerald ML, Fisher SJ: Distribution patterns of extracellular matrix components are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992, 89:210-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ: Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993, 91:950-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ: Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol 1997, 151:1809-1818 [PMC free article] [PubMed] [Google Scholar]
- 43.Caniggia I, Post M, Lye SJ: Restoration of trophoblast invasion capability in preeclamptic villous explants by antisense knock-down of TGFβ3. J Soc Gynecol Invest 1998, 5:66A [Google Scholar]
- 44.Khong TY, Sawyer IH, Heryet AR: An immunological study of endothelialization of uteroplacental vessels in human pregnancy—evidence that the endothelium is focally disrupted by trophoblast in pre-eclampsia. Am J Obstet Gynecol 1992, 167:751-756 [DOI] [PubMed] [Google Scholar]
- 45.Pijnenborg R: The placental bed. Hypertens Pregnancy 1996, 15:7-23 [Google Scholar]
- 46.Coukos G, Makrigiannakis A, Amin K, Albelda SM, Coutifaris C: Platelet endothelial cell adhesion molecule-1 is expressed by a subpopulation of human tropoblasts: a possible mechanism for trophoblast-endothelial interaction during haemochorial placentation. Mol Hum Reprod 1998, 4:357-367 [DOI] [PubMed] [Google Scholar]
- 47.Lakasing L, Campa JS, Parmar K, Poston R, Hunt BJ, Poston L: Normal expression of cell adhesion molecules in placentae from women with systemic lupus erythematosus. Placenta 2000, 21:142-149 [DOI] [PubMed] [Google Scholar]
- 48.Pijnenborg R, Vercruysse L, Verbist L, Van Assache FA: Interaction of interstitial trophoblast with placental bed capillaries and venules of normotensive and pre-eclamptic pregnancies. Placenta 1998, 19:569-575 [DOI] [PubMed] [Google Scholar]
- 49.Blankenship TN, Enders AC: Expression of platelet-endothelial cell adhesion molecule-1 (PECAM) by macaque trophoblast cells during invasion of the spiral arteries. Anat Rec 1997, 247:413-419 [DOI] [PubMed] [Google Scholar]
- 50.Lunell NO, Sarby B, Lewander R, Nylund L: Comparison of uteroplacental blood flow in normal and intrauterine growth-retarded pregnancy. Gynecol Obstet Invest 1979, 10:106-118 [DOI] [PubMed] [Google Scholar]
- 51.Matijevic P, Meekins JW, Walkinshaw SW, Neilson JP, McFadyen IR: Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet Gynecol 1995, 86:289-292 [DOI] [PubMed] [Google Scholar]
- 52.Bower S, Bewley S, Campbell S: Improved prediction of preeclampsia by two stage screening of uterine arteries using the early diastolic notch and color Doppler imaging. Obstet Gynecol 1993, 82:78-83 [PubMed] [Google Scholar]
- 53.Olofsson P, Laurini RN, Marsal K: A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation. Eur J Obstet Gynecol Reprod Biol 1993, 49:161-168 [DOI] [PubMed] [Google Scholar]
- 54.Voigt HJ, Becker V: Doppler flow measurements and histomorphology of the placental bed in uteroplacental insufficiency. J Perinat Med 1992, 20:139-147 [DOI] [PubMed] [Google Scholar]
- 55.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Assche A: A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994, 101:669-674 [DOI] [PubMed] [Google Scholar]
- 56.Brosens IA: Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol 1977, 4:573-593 [PubMed] [Google Scholar]
- 57.De wolf F, Brosens I, Renaer M: Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol 1980, 87:177-200 [DOI] [PubMed] [Google Scholar]
- 58.Gerretsen G, Huisjes HJ, Elema JD: Morphological changes of spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol 1981, 88:876-881 [DOI] [PubMed] [Google Scholar]