Abstract
Caroli’s disease (congenital intrahepatic biliary dilatation) associated with congenital hepatic fibrosis is an autosomal recessive polycystic kidney disease. Recently, the polycystic kidney (PCK) rat, a spontaneous mutant derived from a colony of Crj:CD rats with polycystic lesions in the liver and an autosomal recessive mode of inheritance, was reported. In the present study, the pathology of the hepatobiliary system and the biliary cell-kinetics were evaluated in fetuses (day 18 to 21 of gestation) and neonates and adults (1 day to 4 months after delivery) of PCK rats. Crj:CD rats were used as a control. Multiple segmental and saccular dilatations of intrahepatic bile ducts were first observed in fetuses at 19 days of gestation. The dilatation spread throughout the liver and the degree of dilatation increased with aging. Gross and histological features characterizing ductal plate malformation were common in the intrahepatic bile ducts. Overgrowth of portal connective tissue was evident and progressive after delivery. These features were very similar to those of Caroli’s disease with congenital hepatic fibrosis. Proliferative activity in the biliary epithelial cells was greater in PCK rats than controls during the development. In contrast, the biliary epithelial apoptosis was less extensive in PCK rats than the controls until 1 week after delivery, but greater after 3 weeks, suggesting that the remodeling defect in immature bile ducts associated with the imbalance of cell kinetics plays a role in the occurrence of intrahepatic biliary anomalies in PCK rats. The PCK rat could be a useful and promising animal model of Caroli’s disease with congenital hepatic fibrosis.
Hepatic fibropolycystic disease consists of autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), choledochal cyst, Meckel syndrome, solitary or simple hepatic cysts, and Von Meyenburg complex. 1-3 Although ADPKD shows an autosomal dominant inheritance, ARPKD is known to show an autosomal recessive heritance and variable clinical manifestations. Congenital hepatic fibrosis (CHF) and Caroli’s disease are regarded as a clinicopathological form of ARPKD, and these two diseases are frequently associated in an individual liver. 4
Programed cell death, or apoptosis, is a key mechanism in developing organisms, playing an important role in their differentiation and maturation. In the ontogenesis of the intrahepatic biliary tree of humans, apoptosis plays a role in remodeling. 5 It has been reported that impaired remodeling or failure of the ductal plate, the protostructure of the intrahepatic biliary system, to disappear during the fetal and neonatal developmental stages results in so-called “ductal plate malformation”. Disordered cell kinetics including apoptosis are pathogenetically related to such ductal plate malformation. Interestingly, the above-mentioned hepatic fibropolycystic diseases belong to “ductal plate malformation.” 6 In these diseases, there is also a deposition of fibrous connective tissue in portal tracts.
There are many spontaneously occurring animal models for human polycystic kidney disease such as the cpk mouse, and these animals are used for the genetic and phenotypic study of cyst formation. 7-9 However, no animal models suitable for the investigation of ARPKD with constant liver involvement such as CHF and Caroli’s disease are available. Caroli’s disease is characterized by multiple cystic and segmental saccular dilatations of the intrahepatic bile ducts, and is frequently associated with CHF, which is characterized by overgrowth of portal connective tissue and tortuous and dilated bile ducts and ductules at microscopic levels. The latter ductal abnormality reflects ductal plate malformations. Both diseases are included in ARPKD. So far, there are no suitable animal models for Caroli’s disease with CHF, and the genetic mechanism and pathogenesis of these diseases remain to be fully clarified.
Recently, a novel polycystic kidney (PCK) rat was reported by Katsuyama and colleagues. 10 This rat was a spontaneous mutant animal model derived from a colony of Crj:CD rats (Crj:CD is the registered name for Sprague-Dawley rats at Charles River Japan, Inc.), and was found to show constant renal and hepatic cysts with gross enlargement of kidney as well as liver. 10 Development of the PCK rat was initiated by sibling mating of the female offspring, and continuous sibling mating since 1996 has led to the establishment of this rat model, which is now in its twelfth generation. In a preliminary study with mating experiments, Katsuyama and colleagues 10 found that hepatic and renal phenotypes of the PCK rat were controlled by an autosomal recessive gene.
In this study, we tried to characterize the hepatobiliary lesions in the PCK rat and to test whether this rat could be used as an animal model for ARPKD including Caroli’s disease and CHF. The cystic changes of the liver of the PCK rats were found not to be true cysts but multiple segmental and saccular (cystic) dilatations of the intrahepatic biliary tree.
Materials and Methods
Animals and Tissues
Male and female PCK rats were obtained from Charles River Japan, Inc. (Sagamihara, Japan) in the ninth generation, and a colony of PCK rats was developed and maintained at the Laboratory Animal Institute of Kanazawa University School of Medicine. As controls, Crj:CD rats were also obtained from Charles River Japan, Inc., and were developed and maintained similarly at the same institution. The PCK and control rats were handled according to the National Institutes of Health guidelines for the care and use of laboratory animals. Pregnant female PCK as well as control rats were sacrificed at 18, 19, and 21 days of gestation, and the fetuses were removed. In addition, neonates and adults were sacrificed in sequence at 1 day, 4 days, 1 week, 3 weeks, 6 weeks, 10 weeks, and 4 months, respectively.
Livers with the extrahepatic biliary tree and kidneys were removed from fetal, neonatal, and adult rats. Then, they were fixed in 10% neutral-buffered formalin and embedded in paraffin. More than 10 serial sections, 4-μm thick, were cut from each paraffin block. Several of these sections were stained with hematoxylin and eosin (H&E) and Azan-Mallory, routinely, the remainder were subjected to the immunohistochemical staining for cytokeratin 20 (CK 20) and Ki-67 protein as well as for the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) for detection of apoptosis. It has been reported that CK 20 is the biliary type cytokeratin in rat liver 11 and antibody to Ki-67 protein is a marker of cell proliferation. 12,13
Classification and Development of the Biliary Tree in Rats
Classification
The biliary tree of rats was divided into two parts (extrahepatic and intrahepatic bile duct) in this study as follows: the extrahepatic bile duct refers to the biliary tree that derives from the duodenum and goes to the hepatic lobules. The intrahepatic bile ducts that are located within the hepatic lobules, are branches of the extrahepatic bile ducts. Smaller bile ducts including bile ductules are included in the intrahepatic bile duct in this study.
Development
The intrahepatic bile duct development of the rats could be divided into four stages based on histological observations and cytokeratin immunostainings. 14 In the first stage (fetuses before 15 days of gestation), biliary elements are not identifiable. At the second stage (fetuses from 17 to 21 days of gestation), immature biliary cells are identifiable at the junction between the mesenchyma and liver parenchyma around the large portal vein in the hepatic hilum. At 17 days of gestation, these immature biliary cells around the portal vein close to the hepatic hilum assumed one of the following forms: slit-like lumen, incomplete, or complete bile ductule-like structures, appearing as pearl-like structures. 14,15 In the third stage (neonates from 0 to 4 days after birth), the immature biliary elements identifiable at the junction between portal tracts and liver parenchyma began to bud into the mesenchyme of the portal areas. A few mature interlobular bile ducts are recognizable in the portal tract. At the fourth stage (neonates from 7 to 42 days after birth), pearl-like structures generally disappear and the mature bile ducts and bile ductules are present in the portal tracts.
Characteristics of Congenital Malformation of the Biliary Tree in PCK Rats
The exact ductal plate, which is an important anatomical structure in the development of intrahepatic bile ducts in humans, 16-20 is not seen in mice and rats. Instead, pearl-like structures equivalent to the ductal plate were seen in rats. 14,15 They undergo similar remodeling processes in the development of the intrahepatic biliary tree. Therefore, the changes of pearl-like structures and intrahepatic bile ducts resembling ductal plate malformation in the PCK rats were called “ductal plate malformation” in this study, a well-known and universally accepted pathological term for such features.
Immunohistochemistry for Cytokeratin 20 (CK 20) and Ki-67
Deparaffinized and rehydrated sections of fetal livers at 21 days of gestation and neonatal and adult livers at 1 day, 4 days, 1 week, 3 weeks, and 10 weeks of age (from three or four livers of individual fetal, neonatal, and adult rats for each age) were used for the immunostaining. The deparaffinized sections were treated with proteinase K (diluted 1:50 at room temperature for 7 minutes; DAKO, Carpinteria, CA) for CK 20 and heated in an 800-W microwave oven in citrate buffer (pH 6.0) for 20 minutes (4 cycles, 5 minutes) for Ki-67 protein, respectively. Then, after the blocking of endogenous peroxidase in methanolic-H2O2 and incubation in normal goat serum, the sections were incubated with primary antibodies against CK 20 (mouse monoclonal, diluted 1:100; DAKO, Glostrup, Denmark) and Ki-67 protein (mouse monoclonal, MIB-5, diluted 1:50; Immunotech, Marseille, France) overnight at 4°C, respectively. Next, the sections were incubated with goat anti-mouse immunoglobulins conjugated to peroxidase labeled-dextran polymer (DAKO Envision+, neat; DAKO) for 60 minutes. Finally, reaction products were visualized by the diaminobenzidine reaction, and the sections were weakly counterstained with hematoxylin. CK 20 was strongly and diffusely detected in the cytoplasm of all biliary epithelial cells whereas hepatocytes showed cell membranous staining, weakly and inconsistently. Ki-67 was detected in the nuclei. Negative control stainings were performed by substituting nonimmune mouse serum for either of the primary antibodies, which always resulted in no staining.
TUNEL Method for Detection of Apoptosis
DNA fragmentation because of apoptosis was detected by a modified TUNEL method. That is, the deparaffinized and rehydrated sections were obtained from the same blocks used for Ki-67 protein and CK 20 immunostaining. After proteinase K digestion and endogenous peroxidase blocking, the sections were stained by using a commercial kit (In Situ cell death detection kit, POD; Roche Diagnostics GmbH, Mannheim, Germany). After the benzidine reaction, sections were weakly counterstained with hematoxylin and mounted. For a negative control, TdT containing fluorescein-labeled dUTP and dATP was substituted with phosphate-buffered saline, which resulted in no staining in the nuclei.
Evaluation of Cell Proliferation and Apoptosis
Ki-67 protein- and TUNEL-positive signals were recognized in the nuclei, and only nuclei with a clear brown color were regarded as positive. The Ki-67-labeling index (LI) and TUNEL-LI were defined as follows. In the liver sections at each developmental stage of fetus, neonate, and adult PCK and control rats, more than 500 biliary epithelial cells of the intrahepatic bile ducts were surveyed and the Ki-67 protein-positive and TUNEL-positive biliary epithelial cells were counted. The percentages of biliary epithelial cells positive for Ki-67 protein and TUNEL were expressed as the Ki-67-LI and TUNEL-LI, respectively.
Three-Dimensional Observation of Intrahepatic Biliary Tree (Silicon Rubber Cast Study)
The livers from one control rat and also from one PCK rat at 4-months old, respectively, were used for a silicon rubber biliary cast (Microfil compound, MV-112; Flow Tech. Inc., Carver, MA) injection study. That is, a polyethylene cannula was inserted into the extrahepatic bile duct. Microfil compound was injected by manual pressure using a syringe and cannula into the biliary tree. When a white reticular pattern was observed on the surface of the liver, the infusion was stopped. Microfil compound-injected liver specimens were placed in a refrigerator at 4°C overnight to allow polymerization. Then, they were immersed in 25% ethyl alcohol solution for 1 day. At 24-hour intervals, the ethyl alcohol concentration was raised to 50, 75, 95, and 100%. Finally, the specimens were immersed in methyl salicylate for clearing of the tissue. The intrahepatic and extrahepatic biliary casts in the control and PCK rats were then observed under a stereoscopic microscope.
Results
Macroscopic Observations
Main findings of the liver and kidney of the PCK rats are shown in Table 1 ▶ .
Table 1.
Macroscopic Findings of Liver and Kidney in Control and PCK Rats
| Organ | Findings | Fetus | Neonate and adult | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 days | 19 days | 21 days | 1 day | 4 days | 1 week | 3 weeks | 6 weeks | 10 weeks | 4 months | ||
| Liver | Enlargement | − | − | − | − | − | − | + | + | ++ | ++ |
| Cyst | − | − | − | − | − | − | − | + | + | + | |
| Extrahepatic bile duct | Dilatation | − | − | − | − | − | − | +* | +* | +* | +* |
| Kidney | Enlargement | − | − | − | − | − | − | + | ++ | ++ | ++ |
| Cyst | − | − | − | − | − | − | + | + | + | +, ++ | |
−, No abnormality; +, slight enlargement and dilatation, and a few cysts; ++, moderate enlargement and numerous cysts.
*The dilatation of extrahepatic bile duct is cylindrical, and the degree parallels the enlargement of the liver.
Kidneys
Enlargement and cyst formations of the kidney of the PCK rats were first observed grossly at 3 weeks of age. These lesions in the kidneys became gradually more severe with aging up to 4 months.
Livers
Enlargement and multiple cyst formations of the livers of the PCK rats were first observed grossly at 3 weeks and 6 weeks of age, respectively. Enlargement of livers became gradually more severe with aging up to 4 months. The cysts in the livers were concentrated or localized around the intrahepatic portal tracts and increased in number and distribution slightly up to 4 months of age (Figure 1A) ▶ . There were frequently bridges or bulbar protrusions in the cystically dilated bile duct as seen in Caroli’s disease associated with CHF in humans. 4 Some of these cysts were also recognizable from the surface. The extrahepatic bile duct showed cylindrical dilatation at 3 weeks of age but the degree was slight even at 4 months. This cylindrical dilatation was evident when compared to the control rats. In PCK rats, the degree of dilatation of the extrahepatic bile duct seemed to parallel the enlargement of the liver.
Figure 1.
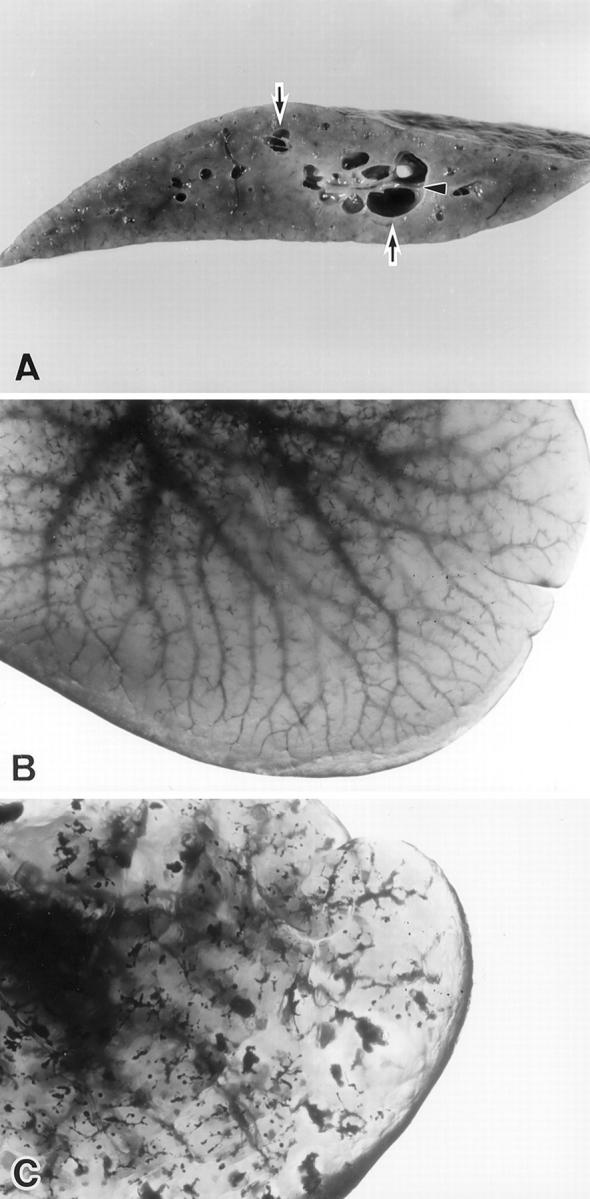
A: The liver of the PCK rat at 4 months of age. Dilated intrahepatic bile ducts (arrows) and bridge formation (arrowheads) in the dilated lumen are seen. B: The liver of the Crj: CD rat at 4 months. A silicon rubber cast of the intrahepatic biliary tree shows fine branching. C: The liver of the PCK rat at 4-months old. A silicon rubber cast of the intrahepatic biliary tree. Multiple segmental and saccular dilatations in the intrahepatic biliary tree are seen, and its fine branches become unclear, compared to the Crj: CD rat (B).
Three-Dimensional Observation of Intrahepatic Biliary Tree (Biliary Casts)
In the control rat liver, Microfil compound filled in the extrahepatic and intrahepatic bile ducts peripheral to small bile ducts and a fine tree-like or reticular structure of intrahepatic biliary tree was clearly observed (Figure 1B) ▶ . On the other hand, in the PCK rats, multiple segmental and saccular dilatations of the intrahepatic bile ducts (manifested as pools of silicon rubber) were observed here and there, although the fine branching of the intrahepatic bile ducts was obscure and discontinuous (Figure 1C) ▶ , when compared to the control.
Histopathological Observations
Main findings of the liver and kidney of the PCK rats are shown in Table 2 ▶ .
Table 2.
Histopathological Findings of Liver and Kidney in Control and PCK Rats
| Organ | Findings | Fetus | Neonate and adult | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 days | 19 days | 21 days | 1 day | 4 days | 1 week | 3 weeks | 6 weeks | 10 weeks | 4 months | ||
| Liver | Dilatation of intrahepatic bile duct | − | + | + | + | + | + | ++ | ++ | ++ | ++, +++ |
| Bulbar protrusion and bridge formation in the inside of intrahepatic bile duct | − | − | − | − | + | + | ++ | ++ | ++ | ++ | |
| Expansion of portal tract (dilated bile duct and proliferation of immature connective tissue or mature fibrous tissue) | − | − | − | + | ++ | ++ | ++ | ++ | ++ | ++,+++ | |
| Lymph follicle formation in portal tract and cluster or mass of polymorphonuclear leukocytes in intrahepatic biliary lumen | − | − | − | − | − | − | − | − | + | + | |
| Extrahepatic bile duct | Dilatation | − | − | − | − | − | − | +* | +* | +* | +* |
| Kidney | Cystic dilatation of renal tubule | − | − | − | − | − | − | ++ | ++ | ++ | ++, +++ |
−, No abnormality; +, slight change; ++, moderate change; +++, severe change.
*The dilatation of extrahepatic bile duct is cylindrical, and the degree parallels the enlargement of the liver.
Kidneys
The right and left kidneys of the PCK rats showed identical changes. That is, small cysts were histologically recognizable at 3 weeks, and there were cystic dilatations of renal tubules at the corticomedullary junction and outer layer of medulla. Renal papillae were spared. These cystic dilatations seemed to spread to the cortex in older animals, although there were no glomerulocystic lesions.
Livers
By a combination of H&E staining and CK20 immunostaining, the biliary epithelial cells and bile ducts were easily recognizable. That is, irregular dilatations with pearl-like structures were observed around the portal veins in the fetal livers of PCK rats at 19 days of gestation (Figure 2, A and B) ▶ . Frequently, these dilated biliary structures surrounded the portal connective tissue. These features resembled ductal plate malformations in humans. After birth, enlargement of portal areas by dilated bile ducts and fibrous connective tissue was increasingly observed (Figure 2C) ▶ . Overgrowth of portal connective tissue was also evident around birth and increased thereafter (edematous and immature at first and then more collagenous). At around 3 weeks, portal areas came to be occupied by the dilated and connecting dilated bile ducts and fibrous tissue. Dilated bile ducts spread throughout the liver and the degree of dilatation increased with aging (Figure 2, D and E) ▶ . In addition, the hepatic parenchyma was subdivided by fibrously enlarged portal tracts and their septa containing many dilated small bile ducts, particularly at 10 weeks and 4 months of age (Figure 2F) ▶ . The subdivided parenchyma lacked regenerative features such as multicell thickness and the hepatic lobular pattern itself was preserved. Moreover, bulbar protrusion and bridge formation of portal connective tissue in the dilated lumen of bile ducts suggesting dilated ducts of pearl-like structure were noted in the livers of 4-day-old and older neonates and adults (Figure 2D) ▶ .
Figure 2.
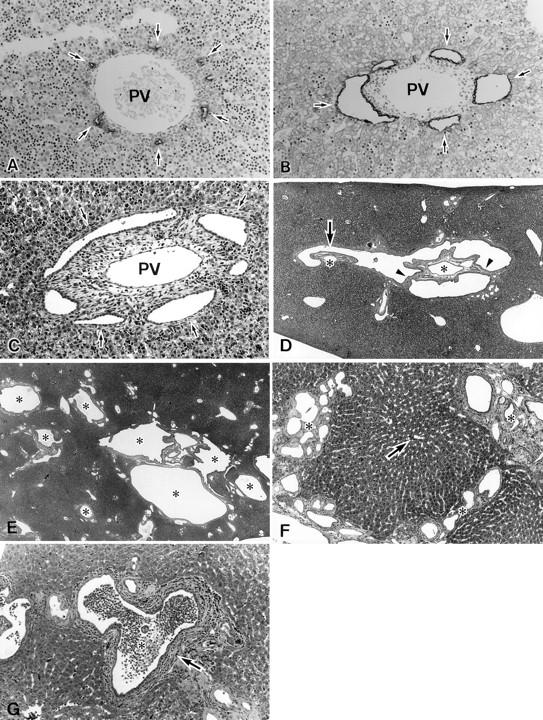
A: The liver of Crj:CD rat fetus at 21 days of gestation. Pearl-like structures (arrows) are positive for CK 20. Immunostaining for CK20. PV, portal vein. Original magnification, ×260. B: The liver of PCK rat fetus at 21 days of gestation. Dilatations in the pearl-like structures (immature biliary elements) are seen (arrows). Immunostaining for CK20. PV, portal vein. Original magnification, ×260. C: The liver of PCK rat neonate at 1 day of age. Dilatations of the small intrahepatic bile ducts (arrows) corresponding to the pearl-like structures and increased connective tissue in the portal area are seen. PV, portal vein. H&E staining. Original magnification, ×200. D: The liver of PCK rat at 3 weeks of age. The bulbar protrusion (arrow) and bridge formations (arrowheads) are seen in the dilated intrahepatic bile ducts. Portal veins (asterisk) and other portal connective tissue surrounded by dilated bile duct. H&E staining. Original magnification, ×40. E: The liver of PCK rat at 10 weeks of age. Dilated intrahepatic bile ducts (asterisk) are seen in almost all portal areas. H&E staining. Original magnification, ×40. F: The liver of PCK rat at 4 months of age. The hepatic parenchyma is divided by increased fibrous tissue (asterisk) with many small dilated bile ducts. The subdivided parenchyma lacks regenerative activities such as multicell thickness and a central vein is located in the central part (arrow). H&E staining. Original magnification, ×100. G: The liver of PCK rat at 10 weeks of age. Clusters of polymorphonuclear leukocytes (luminal abscess) (asterisk) are seen in the lumen of intrahepatic bile duct (arrow) showing chronic cholangitis. H&E staining. Original magnification, ×200.
In addition, portal tracts showed variable lymphocytic infiltration and occasionally lymph follicle formation around the bile ducts at around 10 weeks after birth. Some bile ducts contained clusters or masses of polymorphonuclear leukocytes in their lumen (Figure 2G) ▶ .
Ki-67-LI and TUNEL-LI in Biliary Tree
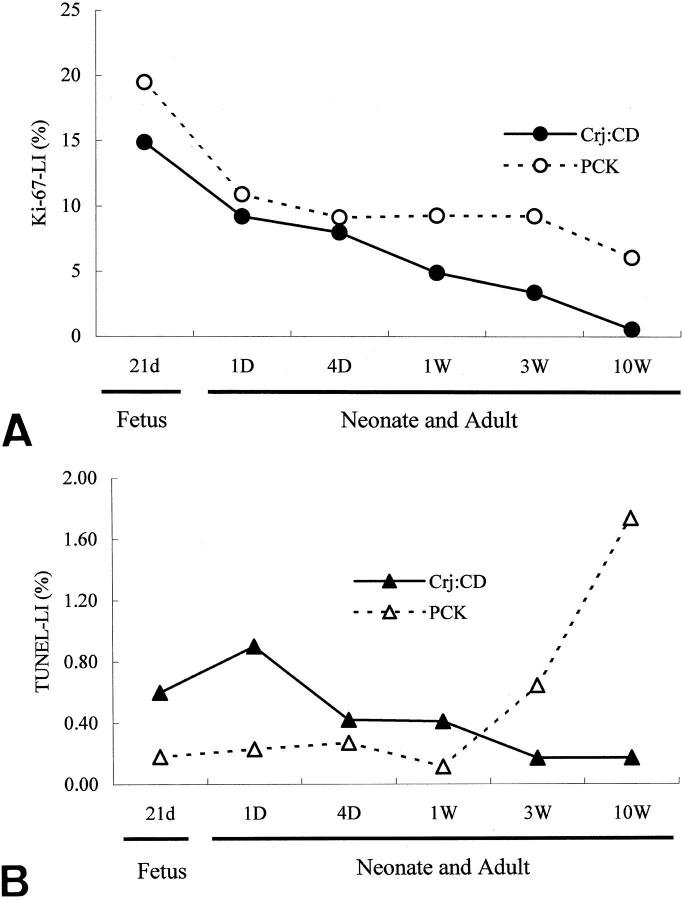
The Ki-67-LI of biliary epithelial cells in the intrahepatic bile duct decreased gradually up to 10 weeks in the PCK rats as well as in the control rats. The Ki-67-LI in the PCK rats was higher than that of the control rats at all ages examined (Figure 3A) ▶ .
Figure 3.
A: Changes of the Ki-67-LI in intrahepatic biliary epithelial cells in PCK rat and control rat (Crj:CD) at fetal, neonatal, and adult stages. B: Changes of TUNEL-LI in intrahepatic biliary epithelial cells in fetus, neonate, and adult PCK and control (Crj:CD) rats.
The TUNEL-LI of biliary epithelial cells in the intrahepatic bile ducts decreased gradually up to 10 weeks of age in the control rats. On the other hand, the TUNEL-LI was lower in the PCK than control rats until 1 week, but was higher at 3 weeks and thereafter (Figure 3B) ▶ .
Discussion
To better understand the pathogenesis of congenital biliary anomalies, a knowledge of the normal development and maturation of intrahepatic bile ducts as well as comparative studies of congenital biliary abnormalities between human diseases and animal models, are important and mandatory. There are many animal models for polycystic diseases involving the liver and biliary tree. 7-9,21-31 For example, the kat2J/kat2J mouse, 28 the Han:SPRD (cy/+) rat, 31 and gene targeting 27,29 of Pkd1 and Pkd2 that cause ADPKD in humans, have been recently reported. These models also develop polycysts in liver as well as kidney, and have characteristics of ADPKD. Aged C57BL/6J-cpk/+ mice are reported to develop cystic dilatations of biliary ducts 7 and BALB/c-bpk/bpk mice develop biliary epithelial hyperplasia, chaotic portal areas and dilatation of the extrahepatic bile duct. 21 However, the hepatic lesions of C57BL/6J-cpk/+ mice are focal, and intrahepatic cysts or shape abnormalities of the biliary lumen were not detected by using biliary casts and morphometry in BALB/c-bpk/bpk mice. At present, no animal models suitable for the investigation of ARPKD with constant liver involvement such as CHF and Caroli’s disease are available.
The PCK rat is a novel model of polycystic disease in which visible multiple hepatic polycysts in addition to renal cysts are constantly detectable during development. The outline and profile of the PCK rat such as survival rate and major anatomical findings, have been briefly reported by Katuyama and colleagues. 10 In this study, we tried to clarify the hepatobiliary lesions of this animal and to test whether the PCK rat could be an animal model of ARPKD such as Caroli’s disease and CHF.
Gross and microscopic examination as well as the three-dimensional observation of intrahepatic biliary casts revealed that the hepatic polycysts in PCK rats were multiple segmental and saccular dilatations of the intrahepatic bile ducts. Furthermore, findings suggestive of ductal plate malformation such as bulbar protrusions of the duct wall to the lumen and bridge formation of the duct wall across the lumen were observed in the intrahepatic bile ducts. Overgrowth of portal connective tissue associated with biliary lesions also become evident after birth and increased thereafter. This pattern of hepatic fibrosis with many dilated bile ducts and a preserved hepatic lobular pattern is known to be a feature of CHF. 4,6 All of these changes are almost identical to those of human Caroli’s disease associated with CHF. 4,32 In addition, these changes were noted at 19 days of gestation and became evident more clearly thereafter. Taken together, it is suggested that the PCK rat is a useful and promising animal model for Caroli’s disease associated with CHF. The inheritance of the polycystic changes of the PCK rat was autosomal recessive and the kidneys showed cystic dilatation of renal tubules, 10 also supporting this suggestion.
The lumen of immature bile ducts around portal veins (pearl-like structures) in fetal livers in the PCK rat were dilated variably at 19 days of gestation, suggesting that the intrahepatic bile duct abnormalities start along with the development of pearl-like structures. In humans, hepatic fibropolycystic diseases caused by a failure of remodeling of the ductal plate and disappearance of excessive immature biliary elements were also observed in the fetal period. 6 That is, excessive immature biliary elements are temporarily formed during the fetal period in both humans and rats. Then, these excessive structures disappear and remodeling gradually occurs along with development and maturation. 14-20,33-35 Therefore, it is suggested that the impaired remodeling of immature biliary elements might have caused the development of saccular and segmental dilatations of the intrahepatic biliary tree appearing as hepatic polycysts in the PCK rats. The normal development and maturation of the intrahepatic biliary tree requires a balance of proliferation and apoptosis of biliary epithelial cells. 5 However, the index of proliferation and of apoptosis for intrahepatic biliary epithelial cells differed between the PCK rats and the control rats, suggesting that this imbalance of cell proliferation and cell death is involved in the dilatation and tortuosity of the intrahepatic bile ducts presenting with pearl-like structures and features of Caroli’s disease and CHF. As for the overgrowth of portal connective tissue, fibrogenetic factors may be involved, and further studies are mandatory.
It has been suggested that the saccular dilatations of the intrahepatic bile ducts in human Caroli’s disease are a predisposing factor in stagnation of bile and bacterial infection associated with cholangitis, and this condition may give rise to biliary sludge, intraductal lithiasis and even cholangiocarcinoma. 6 Intrahepatic cholangitis was found in older PCK rats, and calculi were occasionally encountered in dilated intrahepatic bile ducts (unpublished data). Further long-term studies of the PCK rats are needed. Furthermore, the identification and characterization of the gene(s) involved in the PCK rat and in ARPKD should be performed by molecular methods.
As for the renal lesions of the PCK rats, they seem to belong to a spectrum of ARPKD associated with CHF 36 rather than ADPKD, because the renal tubules at the corticomedullary junction were mainly affected, the glomeruli as well as renal papillae were spared, and the cystic lesions of the liver and kidneys exhibited autosomal recessive inheritance. 10 However, the appearance of renal lesions at 3 weeks and the increase in these cystic lesions in size and extent thereafter, as well as the rather multifocal cystic dilatations of renal tubules contrasts with the typical renal lesions of ARPKD characterized by diffuse cylindrical dilatations of collecting tubules. The renal lesions of ARPKD, particularly those associated with CHF are variable and even controversial, 36 so the renal lesions of PCK rats presented here could be a manifestation of CHF with Caroli’s disease.
In conclusion, the present study revealed that the hepatic polycysts in the PCK rats were actually multiple segmental and saccular dilatations of the intrahepatic bile ducts. In addition, the gross and histological features of the hepatobiliary system were very similar to those of Caroli’s disease and CHF. Moreover, it is suggested in this study that the imbalance between biliary epithelial proliferation and apoptosis as well as impaired remodeling of immature intrahepatic bile ducts play an important role in the development of dilatation of intrahepatic bile ducts in the PCK rat. The PCK rat might be a useful and promising animal model of human hepatic fibropolycystic diseases, particularly Caroli’s disease associated with CHF.
Footnotes
Address reprint requests to Yasuni Nakanuma M.D., Second Department of Pathology, Kanazawa University School of Medicine, Kanazawa 920-8640, Japan. E-mail: pbcpsc@kenroku.kanazawa-u.ac.jp.
References
- 1.Sherlock S, Dooley J (Eds): Cysts and congenital biliary abnormalities. Diseases of the Liver and Biliary System, ed 10. Oxford, Blackwell Science Ltd., 1997, pp 579–591
- 2.Sergi C, Kahl P, Otto HF: Contribution of apoptosis and apoptosis-related proteins to the malformation of the primitive intrahepatic biliary system in Meckel syndrome. Am J Pathol 2000, 156:1589-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishak KG, Sharp HL: Developmental abnormalities and liver disease in childhood. ed 3 MacSween RNM Anthony PP Scheuer PJ Burt AD Portmann BC eds. Pathology of the Liver, 1994, :pp 83-122 Churchill Livingstone Ltd., Edinburgh [Google Scholar]
- 4.Nakanuma Y, Terada T, Ohta G, Kurachi M, Matsubara F: Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver 1982, 2:346-354 [DOI] [PubMed] [Google Scholar]
- 5.Terada T, Nakanuma Y: Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol 1995, 146:67-74 [PMC free article] [PubMed] [Google Scholar]
- 6.Desmet VJ: Congenital diseases of intrahepatic bile ducts. Variations on the theme “ductal plate malformation.” Hepatology 1992, 16:1069-1083 [DOI] [PubMed] [Google Scholar]
- 7.Crocker JFS, Blecher SR, Givner ML, McCarthy SC: Polycystic kidney and liver disease and corticosterone changes in the cpk mouse. Kidney Int 1987, 31:1088-1091 [DOI] [PubMed] [Google Scholar]
- 8.Grimm PC, Crocker JFS, Malatjalian DA, Ogborn MR: The microanatomy of the intrahepatic bile duct in polycystic disease: comparison of the cpk mouse and human. J Exp Pathol Oxford 1990, 71:119-131 [PMC free article] [PubMed] [Google Scholar]
- 9.Guay-Woodford LM, Green WJ, Lindsey JR, Beier DR: Germline and somatic loss of function of the mouse cpk gene causes biliary ductal pathology that is genetically modulated. Hum Mol Genet 2000, 9:769-778 [DOI] [PubMed] [Google Scholar]
- 10.Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H: Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim 2000, 49:51-55 [DOI] [PubMed] [Google Scholar]
- 11.Faa G, Van Eyken P, Roskams T, Miyazaki H, Serreli S, Ambu R, Desmet VJ: Expression of cytokeratin 20 in developing rat liver and in experimental models of ductular and oval cell proliferation. J Hepatol 1998, 29:628-633 [DOI] [PubMed] [Google Scholar]
- 12.Schlüter C, Duchrow M, Wohlenberg C, Becker MHG, Key G, Flad HD, Gerdes J: The cell proliferation-associated antigen of antibody Ki-67. A very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol 1993, 123:513-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerlach C, Sakkab DY, Scholzen T, Dassler R, Alison MR, Gerdes J: Ki-67 expression during rat liver regeneration after partial hepatoectomy. Hepatology 1997, 26:573-578 [DOI] [PubMed] [Google Scholar]
- 14.Sanzen T, Yoshida K, Sasaki M, Terada T, Nakanuma Y: Expression of glycoconjugates during intrahepatic bile duct development in the rat. An immunohistochemical and lectin-histochemical study. Hepatology 1995, 22:944-951 [PubMed] [Google Scholar]
- 15.Van Eyken P, Sciot R, Desmet V: Intrahepatic bile duct development in the rat. A cytokeratin-immunohistochemical study. Lab Invest 1988, 59:52-59 [PubMed] [Google Scholar]
- 16.Bloom W: The embryogenesis of human bile capillaries and ducts. Am J Anat 1926, 36:451-465 [Google Scholar]
- 17.Enzan H, Ohkita T, Fujita H, Iijima S: Light and electron microscopic studies on the development of periportal bile ducts of human embryo. Acta Pathol Jpn 1974, 24:427-447 [DOI] [PubMed] [Google Scholar]
- 18.Van Eyken P, Sciot R, Callea F, Van Der Steen K, Moerman P, Desmet VJ: The development of the intrahepatic bile ducts in man. A keratin-immunohistochemical study. Hepatology 1988, 8:1586-1595 [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Nakanuma Y: Development of human intrahepatic peribiliary glands. Histological, keratin immunohistochemical, and mucus histochemical analyses. Lab Invest 1993, 68:261-269 [PubMed] [Google Scholar]
- 20.Terada T, Nakanuma Y: Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation. A lectin-histochemical and immunohistochemical study. Hepatology 1994, 20:388-397 [PubMed] [Google Scholar]
- 21.Nauta J, Ozawa Y, Sweeney WE, Rutledge JC, Avener ED: Renal and biliary abnormalities in a new murine model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 1993, 7:163-172 [DOI] [PubMed] [Google Scholar]
- 22.Werder AA, Amos MA, Nielsen AH, Wolfe GH: Comparative effects of germfree and ambient environments on the development of cystic kidney disease in CFWwd mice. J Lab Clin Med 1984, 103:399-407 [PubMed] [Google Scholar]
- 23.Fry JL, Koch WE, Jennette JC, Mcfarland E, Fried FA, Mandell J: A genetically determined murine model of infantile polycystic kidney disease. J Urol 1985, 134:828-833 [DOI] [PubMed] [Google Scholar]
- 24.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NLA, Wilkinson JE, Woychik RP: Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 1994, 264:1329-1333 [DOI] [PubMed] [Google Scholar]
- 25.Flaherty L, Bryda EC, Collins D, Rudofsky U, Montgomery JC: New mouse model for polycystic kidney disease with both recessive and dominant gene effects. Kidney Int 1995, 47:552-558 [DOI] [PubMed] [Google Scholar]
- 26.Gattone VH, MacNaughton KA, Kraybill AL: Murine autosomal recessive polycystic kidney disease with multiorgan involvement induced by the cpk gene. Anat Rec 1996, 245:488-499 [DOI] [PubMed] [Google Scholar]
- 27.Wu G, D’Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Kucherlapati R, Edelmann W, Somlo S: Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 1998, 93:177-188 [DOI] [PubMed] [Google Scholar]
- 28.Vogler C, Homan S, Pung A, Thorpe C, Barker J, Birkenmeier EH, Upadhya P: Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J Am Soc Nephrol 1999, 10:2534-2539 [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Fan X, Basora N, Babakhanlou H, Law T, Rifai N, Harris PC, Perez-Atayde AR, Rennke HG, Zhou J: Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat Genet 1999, 21:160-161 [DOI] [PubMed] [Google Scholar]
- 30.McGeoch JEM, Darmady EM: Polycystic disease of kidney, liver and pancreas. A possible pathogenesis. J Pathol 1976, 119:221-228 [DOI] [PubMed] [Google Scholar]
- 31.Kränzlin B, Schieren G, Gretz N: Azotemia and extrarenal manifestations in old female Han: SPRD(cy/+) rats. Kidney Int 1997, 51:1160-1169 [DOI] [PubMed] [Google Scholar]
- 32.Marchal GJ, Desmet VJ, Proesmans WC, Moerman PL, Van Roost WW, Van Holsbeeck MT, Baert AL: Caroli disease. High frequency US and pathologic findings. Radiology 1986, 158:507-511 [DOI] [PubMed] [Google Scholar]
- 33.Wilson JW, Groat CS, Leduc EH: Histogenesis of the liver. Ann NY Acad Sci 1963, 111:8-24 [DOI] [PubMed] [Google Scholar]
- 34.Wood RL: An electron microscope study of developing bile canaliculi in the rat. Anat Rec 1965, 151:507-530 [DOI] [PubMed] [Google Scholar]
- 35.Shiojiri N, Lemire JM, Fausto N: Cell lineages and oval cell progenitors in rat liver development. Cancer Res 1991, 51:2611-2620 [PubMed] [Google Scholar]
- 36.Eggi KD, Hartman DS: Autosomal recessive polycystic kidney disease. Renal cystic disease. AFIP Atlas of Radiologic-Pathologic Correlation. 1989:pp 73-107 WB Saunders Co., Edited by AJ Davidson. Philadelphia