Abstract
A minimally invasive laser-induced injury model is described to study thrombus development in mice in vivo. The protocol involves focusing the beam of an argon-ion laser through a compound microscope on the vasculature of a mouse ear that is sufficiently thin such that blood flow can be visualized by intravital microscopy. Two distinct injury models have been established. The first involves direct laser illumination with a short, high-intensity pulse. In this case, thrombus formation is inhibited by the GPIIb/IIIa antagonist, G4120. However, the anticoagulants, hirulog, PPACK, and NapC2 have minimal effect. This indicates that thrombus development induced by this model mainly involves platelet interactions. The second model involves low-intensity laser illumination of mice injected with Rose Bengal dye to induce photochemical injury in the region of laser illumination. Thrombi generated by this latter procedure have a slower development and are inhibited by both anticoagulant and anti-platelet compounds.
The development of transgenic and gene-targeting technologies has allowed the mouse to become a valuable genetic model to study the role of particular genes in normal and pathophysiological processes. In particular, mice have been generated with mutations and deficiencies in most components of the coagulation 1-17 and fibrinolytic 18-20 systems. Such animals provide the resources to study the role of particular factors in establishing and maintaining hemostasis in vivo.
Although mice provide an appropriate genetic model, their diminutive size presents a challenge in the study of hemostasis. Vascular injury models developed in larger mammals are sometimes difficult to miniaturize to apply to the mouse. Despite these difficulties, several murine vascular injury models have been developed. These include use of electrical 21 and mechanical injuries, 22-25 FeCl3, 26,27 laser-induced injuries, 28,29 and light-induced injuries using photoactivatable dyes. 30-34 Most of these models involve surgical intervention that can lead to artifactual activation of the hemostatic system. The use of intravital microscopy to analyze photochemical injury to vessels of the ear of the hairless mouse addresses this concern. 32,33 This latter model has been modified and used herein to induce injury with irradiation from an argon-ion laser. Specifically, laser radiation is introduced through an optical port of the microscope and focused through the objective on the target tissue. Because the laser energy is focused at the focal plane of the microscope, the intensity of the laser beam is adjusted so that injury is only induced at a particular depth in the tissue. It is thus possible to induce injury on the luminal surface of the vessel and monitor thrombogenesis of individual thrombi by intravital microscopy. This report describes the major characteristics of this injury model.
Materials and Methods
Mice
Mice for the studies included C57BL6 and Swiss Webster wild-type mice. Housing and procedures involving experimental animals were approved by Institutional Animal Cure and Use Committee of the University of Notre Dame.
Laser-Induced Injury
The experimental arrangement of components to induce and record vascular injury in real time in vivo is illustrated in Figure 1 ▶ . The beam of an argon-ion laser (Innova 90-5, Coherent, CA) was introduced into an optical port of a TwinEpi attachment (Prairie Technologies, Madison, WI) to a Nikon Eclipse 600 (Nikon, Tokyo, Japan) compound microscope and directed through the objective to be focused on a sample. The laser was focused on the target with a low intensity light (using power input to the laser that does not induce lasing). An injury was induced with a short high-intensity laser burst. After laser exposure, the image of the injury generated by backlighting of the injured vessel was recorded with a charge-coupled device camera (Optronics, Goleta, CA). The recorded video image was digitized and then analyzed with Bioquant True Color Windows software (Biometrics, Nashville, TN). The design of the TwinEpi attachment also permitted illumination with a mercury lamp to generate epifluorescent images of the thrombus.
Figure 1.
Diagram of the experimental arrangement for laser-induced vascular injury. The beam of the laser is introduced through the optical port of the microscope and focused through the objective at the target vessel. The charge-coupled device camera records images of the developing thrombus generated by back-illumination of the target vessel with the microscope illuminator. The images are recorded for analysis.
Image Analysis
Video recordings of the injury were analyzed by capturing and digitizing the recorded image at prescribed intervals (1 or 2 seconds). The captured frames were analyzed using the BioQuant True Color Windows software package (Biometrics, Nashville TN). A region of interest (ROI) was defined for the target vessel that included a portion of the target vessel that is larger than the maximum injured area. Using software tools, thresholds were set to define the thrombus in the ROI. The software was programmed to review each frame, identify the thrombus in the ROI, and to measure several different parameters including area and intensity.
The increase in the integrated optical density (IOD) of the thrombus was determined by first using the BioQuant software to adjust the brightness of the video image such that the mean brightness of the pixels in the ROI was at midrange (a brightness level of 128 in a scale from 0 to 255). The background intensity was defined as the average intensity in the ROI from a captured frame corresponding to the vessel before injury. The IOD is defined as the Σ − (log[Ip/Ib] for each of the pixels in the ROI where Ip is the light intensity at the pth pixel and Ib is the background intensity. The increase of the IOD is determined by subtracting the average IOD of the first two captured frames corresponding to the vessel before injury from the IOD of subsequent frames.
Histological and Immunohistochemical Analyses
After the induction of injury to the vessels of the ear, the anesthetized animals were sacrificed and the animals perfused by pumping saline followed by buffered formalin into the left ventricle of the heart. Histological analyses were performed on paraffin-embedded serial sections of the injured ears. Sections at 80-μm intervals were stained with hematoxylin and eosin (H&E) for microscopic evaluation.
Electronmicroscopic Analysis
After laser illumination, mice were perfused with saline Karnovsky’s solution (1% paraformaldahyde/2.5% glutaraldehyde, in 0.1 mol/L Na-cacodylate buffer, in which the osmolarity was adjusted with NaCl and sucrose). The injured section of ear was excised, and incubated in Karnovsky’s solution for 16 hours. The samples were rinsed twice in 0.1 mol/L Na-cacodylate buffer for 30 minutes each, and postfixed in 4% osmium tetroxide for 1 hour, and rinsed again in the same buffer. After dehydration through progressive alcohol incubations, the samples were embedded in epoxy resin. Semithin sections (0.5 μm) were stained in toluidine blue and examined with a compound microscope to identify regions of interest. Once the thrombus was identified, thin sections (90 nm) were placed onto copper grids and stained with Reynold’s lead citrate and (2%) uranyl acetate for transmission electron microscopy analysis. 35
Results
Description of Model
The experimental set-up illustrated in Figure 1 ▶ permits photo-induced injury at specific sites within the vasculature of the ear of a mouse. The hair on the ear of an anesthetized mouse is removed and the animal positioned in a plastic restraint on a microscope stage. The restraint is designed so one ear can be taped to a glass platform so that blood flow through the vasculature of the ear can be visualized by transillumination. Although circulation in both arteries and veins is easily visualized, this report presents a characterization of the induction of venous injuries. Although the architecture of the vasculature of the ear varies among mice, generally there are either one or two primary veins proximal to the head with a diameter of ∼200 to 350 μm. This primary vein(s) is fed by secondary veins of ∼150 to 250 μm, which in turn are fed by tertiary vessels of 100 to 200 μm. The primary, secondary, and tertiary veins are targeted in these studies because the increased pigmentation in more distal segments of the ear introduces experimental complications reducing the reproducibility of the model.
Using the microscope, laser, and imaging system described in Figure 1 ▶ , injury can be induced in the vessel by two distinct methods. The first involves direct laser injury of the wall of the vessel by focusing a high-energy laser pulse at the luminal surface of the vessel wall. The second involves intravenous injection of the photoactivatable dye, Rose Bengal. In this latter case, subsequent laser illumination of the target vessel results in photochemical injury at the site of illumination. Both models are minimally invasive, generate reproducible injuries, and can produce multiple injuries in a single animal. Although the two methods for inducing injuries are similar, thrombus development by the two procedures exhibits different sensitivities to anticoagulants and platelet antagonists.
Direct Laser Injury
After positioning the anesthetized animal in a restraint on the microscope platform, the laser was focused on the target vessel. Because the laser beam focused through the microscope objective is confocal with the image generated by transmitted light, the laser energy is concentrated to a small volume around the focal plane. Initial studies established parameters to generate subocclusive thrombi. Laser illumination of larger venules (primary to tertiary venules in the ear) with the 517-nm line at 100 mW for 1 second consistently induced a subocclusive thrombus that grew in size and intensity during the course of several minutes (Figure 2A) ▶ . After reaching its maximum size (∼25 to 75% the diameter of the vessel), the thrombus began to fade and then usually to embolize. Several minutes after the laser pulse, the image of the vessel stabilizes and a detectable mural thrombus remains.
Figure 2.
Effects of GPIIb/IIIa antagonist, G4120, on thrombus development. Top: Thrombus development after direct-laser induced injury (1 second, 75 mW) in an untreated mouse. Bottom: Injury in a mouse given G4120 (10 μg/g i.v.) before laser illumination.
Laser illumination for longer times or at a higher laser intensity induces more severe injuries. For example, illumination at 100 mW for 5 seconds or longer consistently induced occlusive thrombi (data not shown). On the other hand, illumination at 20 mW for >3 minutes failed to generate detectable injury.
Effects of Antithrombotics
To determine the nature of the thrombi generated in this model, the effects of coagulation inhibitors on thrombus development were examined. Anesthetized animals were administered the anti-GPIIb/IIIa inhibitor, G4120, 36 in normal saline at a dose of 1 μg/g body weight by intravenous tail vein injection. The panels in Figure 2 ▶ illustrate the progression of two injuries induced in a C57BL6 mouse in the absence (Figure 2 ▶ , top) or presence (Figure 2 ▶ , bottom) of the inhibitor. In Figure 3 ▶ , the areas of thrombi generated after multiple injuries as determined with the BioQuant imaging system in the presence or absence of G4120 are displayed graphically. These images (Figure 2) ▶ and analyses (Figure 3) ▶ show that thrombus development is inhibited in the presence of the antiplatelet compound.
Figure 3.
Thrombus development after laser-induced injury in the vasculature of the ear. The area of the thrombi was measured at 2-second intervals after laser-induced injury. The solid squares represent thrombus growth after laser-induced injury in three animals before administration of G4120. The open circles represent four injuries made in two mice after an intravenous injection of the GPllb/IIIa antagonist G4120.
The results with anticoagulant compounds were ambiguous. Although some inhibition of thrombus development was seen with the FVII inhibitor, NapC2, 37 and the thrombin inhibitor, PPACK, 38 the results were variable. Tail vein injection of 100 μl of 10 μmol/L PPACK delayed thrombus development after direct laser injury, but thrombi did form. The results were not as consistent as with the anti-GPIIb//IIIa inhibitor, G4120.
Because measuring the area of the thrombus (as plotted in Figure 3 ▶ ) does not take into consideration the intensity or brightness of the clot, the increase of the IOD of the developing thrombus was determined. The IOD calculates the log of the ratio of the intensity at each pixel to the background intensity before injury and sums that value for each pixel in the ROI. The ROI is drawn to include a region of the target vessel that extends beyond the maximum extent of the clot. The increase in the IOD of the clot can be calculated by subtracting the value of the IOD from images of the vessel before injury from the value of the IOD after injury. Pixels in the ROI that do not change in intensity during the course of the injury (corresponding to uninjured sections of the vessel in the ROI) thus do not contribute to the increase in IOD.
The increase in IOD for multiple laser-induced thrombi generated in three animals is shown in Figure 4 ▶ . The general shape of the tracings for each thrombus is very similar. The IOD increases rapidly within the first 30 seconds after injury and then is maintained or increases slightly throughout the next several minutes of observation. In addition, the maximal increase in the IOD (IODmax) for each thrombus was determined. The average values of the (IODmax) for thrombi generated in each of three animals are displayed in Table 1 ▶ . Interestingly, the average increase in the IOD for injuries made in each animal is very reproducible; the average (IODmax) calculated for different animals in this series of injuries was 184 ± 20%.
Figure 4.
Thrombus development after laser-induced injury in the vasculature of the ear. The increase in the IOD was measured at 5-second intervals after laser-induced injury of three wild-type Swiss Webster mice. Each line traces the development of an individual injury made in four mice (three to four injuries per mouse).
Table 1.
Maximum Increase in Integrated Optical Density for Injuries Made in Multiple Animals
| Mouse (no. of injuries) | Average (IODmax) |
|---|---|
| 1 (3) | 184 ± 43 |
| 2 (4) | 148 ± 59 |
| 3 (3) | 222 ± 97 |
| Total (10) | 184 ± 37 |
The maximum increase in IOD (IODmax) for each thrombus was determined and the mean for all injuries in each animal was calculated.
Histological and Immunohistochemical Analysis
To analyze the nature of the laser-induced injury histologically after injury, animals were perfused by ventricular puncture first with saline, then with buffered formalin. The region of the ear surrounding the injury was excised and embedded in paraffin. The target vein was followed through serial sections enabling identification of sections of the target vessel with the induced thrombus. The panels in Figure 4 ▶ represent histological characterization of sections through a single clot induced by direct laser injury. As expected, sections preceding the injury were free of detectable clot, consistent with the local nature of the injury recorded by intravital microscopy (Figure 5A) ▶ . The periphery of the thrombus includes less condensed fibrous material (Figure 5B) ▶ whereas the main body of the thrombus contains condensed material attached to the vessel wall. Histochemical analysis of other laser-induced thrombi indicates the less condensed fibrous material stains with antibodies recognizing fibrin(ogen) (data not shown). Unfortunately, the antibody recognizes both fibrinogen and fibrin, so it is not possible to tell whether fibrin generation and polymerization are required for thrombus generation. The thrombus also contains P-selectin antigen (data not shown) suggesting the presence of activated platelets within the clot. This result is consistent with the sensitivity of thrombus development to the anti-GPIIb/IIIa inhibitor G4120 (Figures 2 and 3) ▶ ▶ . Sections stained for von Willebrand factor (data not shown), which serves as a marker for the vascular endothelium, indicate that the lumen of the vein is ringed with a layer of endothelial cells. At the resolution of the micrographs for immunohistochemical analysis, there does not seem to be major disruption of the endothelial lining of the vein. In addition, it was noted in these figures that there was no apparent damage to the tissue surrounding the target vessel. This observation supports the contention that the focusing of the laser induces injury in a small volume within the target rather than burning a vertical column through the ear.
Figure 5.
Histological characterization of thrombus generated by direct laser injury. The micrographs represent regions of the ear surrounding the target vein. The sections show the adjacent artery and nearby nerve bundle. Serial sections (8 μm) adjacent (A), at the periphery (B), and within the body of a thrombus (C) were stained with H&E. The micrographs demonstrate the presence of a localized, condensed thrombus attached to wall of the target vein. The laser does not seem to induce detectable damage to tissue surrounding the target vessel.
Ultrastructural Analysis
Ultrastructural analysis has been initiated to further characterize the thrombus generated by laser-induced injury. Figure 6a ▶ presents a transmission electron micrograph of a thrombus induced by direct laser injury. The figure shows polymorphonuclear cells (PMN) attached to damaged, highly vacuolated endothelium. There are numerous platelets at various stages of activation. Although the endothelium is injured, it is still attached to the substratum of the vessel wall. However, there is one site at which the subendothelial matrix is exposed and a projection from a neutrophil is shown to make contact with the subendothelial matrix (Figure 6a ▶ , insert). From this, it seems that thrombus formation does not require major denudation of the endothelium and exposure of platelets or coagulation factors to the subendothelial matrix. The micrographs also confirm the local nature of the injury. Sections ∼100-μm proximal or distal to the thrombus are free of injury. In addition, composite micrographs that allow tracing along the luminal surface indicate that while the endothelium at the site of injury is damaged, the lining on the opposite surface of the vessel appears normal (data not shown).
Figure 6.
Electron micrographs of laser-induced thrombi. a: Direct laser injury was induced by a 1-second pulse of an argon laser (517 nm line at 75 mW) directed through the objective of a compound. After injury, the animal was perfused and thin sections prepared for electron microscopic examination. The section shows a polymorphonuclear cell (N) attached to damaged endothelium (En) and a collection of platelets (P) at various stages of activation. The insert is an expanded view of the region identified with an arrow where an appendage of the PMN contacts the subendothelial matrix. b: Electron micrograph of an injury generated by laser-induced photoactivation of Rose Bengal. The mouse was injected with 100 μl of 10 mg/ml Rose Bengal in saline and the vein of the ear then illuminated with a 30-second pulse of 20 mW from the argon laser.
Laser-Induced Photochemical Injury
In addition to the induction of direct laser injury with a relatively high-energy pulse for a short duration, the experimental apparatus shown in Figure 1 ▶ can also be used to induce photochemical injury. In the photochemical injury mode, mice were administered 40 μg/g body weight by tail vein injection of a 10 mg/ml solution of Rose Bengal dye dissolved in normal saline. Subsequent laser illumination induced mural clots in the target vessels near the site of illumination. Presumably, photoactivation of the Rose Bengal dye results in the generation of singlet oxygen, which induces chemical damage to the endothelium.
Parameters to Generate Subocclusive Thrombi
Initial experiments were designed to determine parameters necessary to generate subocclusive thrombi in the target vessels in 8- to 12-week-old Swiss-Webster mice. Irradiation at 10 mW failed to generate thrombi in the presence or absence of Rose Bengal, even after 4 minutes of continuous illumination. Irradiation at 20 mW for 30 seconds in the presence of Rose Bengal at 40 μg/g body weight occasionally induced a faint thrombus. However, irradiation at 30 mW for 20 to 30 seconds consistently generated subocclusive thrombi in target veins. In contrast, more intense irradiation (75 mW) for 20 seconds at the same Rose Bengal concentration consistently generated occlusive clots near the site of illumination. These results indicate that the extent of thrombus development in the primary, secondary, or tertiary veins of the ear is a function of the intensity of irradiation. Similarly, the extent of injury is also affected by the duration of irradiation and concentration of Rose Bengal. Furthermore, it is possible to identify irradiation parameters to consistently generate subocclusive thrombi in the particular target vessel. In particular, subocclusive clots in secondary veins are consistently generated by 30 seconds of irradiation at 30 mW in Swiss Webster mice injected with Rose Bengal with a dose of 40 μg/g body weight. As might be expected, the parameters required to induce an injury of a particular intensity vary with the genetic background of the mouse. For instance, irradiation at 20 mW for 30 seconds induces extensive subocclusive thrombi in 129/C57BL6 mice, parameters that only induce a faint thrombus in Swiss-Webster mice.
Effects of Coagulation Inhibitors
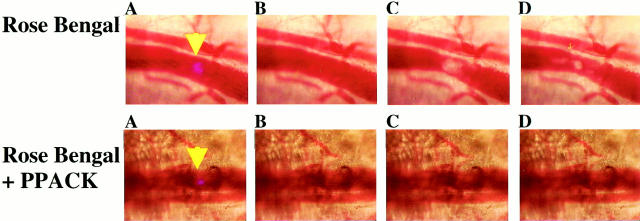
To explore the role of the coagulation system on thrombus development after photochemical injury, injuries were made in the presence or absence of the thrombin inhibitor PPACK. Anesthetized mice were administered Rose Bengal either with or without PPACK by intravenous injection into the tail vein. Figure 7 ▶ illustrates the effect of the thrombin inhibitor PPACK on photochemical injury. Figure 7 ▶ , top, shows photochemical injury in the absence of PPACK. The blue light in panel A indicates the target site for the laser, whereas panel B shows the vessel before laser illumination. Panels C and D show the vessel 30 and 180 seconds after laser injury and indicate the growth and development of the thrombus. Panels E through H represent similar images after irradiation in an animal injected with PPACK and Rose Bengal. In this latter case, no detectable thrombus was detected near the site of illumination. These results indicate that thrombin activity is required for thrombus development after laser-induced photochemical injury.
Figure 7.
Effects of PPACK on laser-induced photochemical injury. Mice were injected via tail vein with 100 μl of a 10 mg/ml solution of Rose Bengal (top) or 10 mg/ml Rose Bengal and 10 mmol/L PPACK (bottom). The laser beam was targeted to the vessel with a low intensity light (yellow arrowhead) from the laser (using an energy input to the laser that does not cause “lasing”) (A). B: The vessel before laser illumination. The vessels are illuminated with a 30-second pulse of 20 mW. C and D: The vessel at 50 and 100 seconds after the start of laser illumination, respectively.
Ultrastructural Analysis
Transmission electron micrographs of a thrombus generated by laser-induced photoactivation of Rose Bengal (Figure 6b) ▶ show the aggregation of activated platelets at the site of injury. Although the endothelium is intact and there is no detectable exposure of the subendothelial matrix. The micrographs show the endothelial cells are damaged and highly vacuolated. Examination of composite micrographs that trace around the entire vessel indicate that the damage to the endothelium extends to a larger area, and is not limited to the region of illumination as was the case with the direct laser injury (data not shown). This is consistent with phototoxins being generated at the site of illumination, but damaging cells in a somewhat wider area.
Discussion
Developments in transgenic and gene targeting technology have allowed the mouse to become a valuable animal to study the role of particular genetic factors in physiological processes. These genetic approaches have been applied to the study of the hemostatic system in which mice with deficiencies of most known coagulation genes have been developed. However, to investigate the effects of particular coagulation genes on hemostasis, it is necessary to test these genetically modified animals in appropriate vascular injury models. In this regard, a number of vascular injury models have been developed including mechanical-, electrical-, chemical-, photochemical-, and laser-induced lesions.
The goal of this study was to develop a vascular injury model incorporating some of the advantages described in earlier models. In particular, a noninvasive photochemical injury model for the vasculature of the ear has been described that involved illuminating the ear with a mercury lamp after injection with fluorescein isothiocyanate-labeled dextran. 32 In addition, there have been several reports of laser-induced photo-injuries to the rodent microvasculature. It is possible to focus the laser beam through the compound objective of a microscope to concentrate the laser radiation at the focal plane of the objective. 27,28 If the laser is aimed at the inside surface of the vessel wall and the focal plane is adjusted so blood flowing in the vein is in sharp focus, then it is possible to induce injury consistently to the luminal surface of the vessel. Furthermore, the use of intravital microscopy to monitor thrombus development by transillumination in real time has been described. 22 Relevant features of these models have been incorporated to noninvasively generate and monitor in real time laser-induced injury to the vasculature of the ear.
The models described in the present study offer a number of advantages over those previously described. They are noninvasive and require minimal animal handling and preparation. Because the injuries that are generated are small, there is no major perturbation of the coagulation system and no significant consumption of coagulation factors. Therefore, multiple injuries can be induced and recorded in a single animal. In addition, the model permits monitoring of thrombogenesis after individual injuries. Furthermore, parameters have been established that allow subocclusive injuries to be generated reproducibly. Therefore, the effects of genetic or pharmacological manipulations that shift the hemostatic balance in either thrombotic or hemorrhagic directions can be detected.
Using the experimental set-up described in Figure 1 ▶ , two types of laser-induced injuries can be induced. In the first model of direct laser injury, illumination in the range of 75 to 200 mW by the argon laser for ∼1 to 2 seconds induces reproducible injuries in veins of the ear. The focusing of the laser beam by the objective of the microscope results in concentration of the energy of the laser beam at the focal point. If the time and power during irradiation are adjusted appropriately, tissue damage is limited to the focal point of the targeted laser beam. By focusing the laser to the luminal surface of the vein, it is possible to make reliable injuries inside the vessel. Because the energy density of the beam is too low both above and below the focal plane, no injury is induced above and below the vessel. Therefore, injuries inside the vein can be generated in a noninvasive manner.
Although it is possible to define parameters for making reproducible subocclusive injuries in the vein of the ear, the elaboration of conditions to provoke reproducible injuries in the artery has been problematic. Conceivably, the thicker wall of the artery and smaller diameter of the vessel disrupts the optical path of the laser beam and prevents reliable focusing of the beam to the luminal surface of the artery.
Because photo-injury models have been described to induce vascular damage in a number of different animal systems, the possibility of using the experimental system described in Figure 1 ▶ was explored for inducing photochemical damage. If animals are administered Rose Bengal dye, subsequent illumination of vessels with light of a wavelength of 540 nm leads to singlet oxygen production, chemical damage to endothelial cells, and thrombus development near the site of illumination. Although initial reports describe the use of xenon lamps to provide the illumination, the 514-nm band of the argon laser has been used to induce photochemical injury in rat spinal cord and middle cerebral arteries. 39,40
Therefore, the possibility of focusing the laser through the microscope to induce photochemical injury in vessels of the ear was explored. Using mice injected with a Rose Bengal solution, parameters were identified to generate reproducible subocclusive thrombi in secondary veins of the ear. Similarly, conditions have been developed to induce reproducible thrombi in ear arteries.
Both direct laser-induced injury and photochemical injury lead to the development of platelet-rich thrombi. Platelets were detected histochemically by staining with anti-P-selectin antibodies and by transmission electron microscopy. Although anti-murine fibrin antibodies detect antigen in the thrombi, distinction cannot be made as to whether the antigen is fibrin or fibrinogen. Initial ultrastructural analyses using transmission of thrombi generated by these models fail to detect significant cross-linked fibrin in the clot. Thus, both models appear to generate platelet-rich thrombi that are more characteristic of arterial injuries even though they are induced in a vein.
The sensitivity of the thrombi to various anti-thrombotic compounds has been explored. The anti-GPIIb/IIIa compound, G4120, inhibits thrombus formation in the direct laser injury model, consistent with the platelet-rich nature of the developing thrombi. However, tests with anticoagulants were less consistent. The anti-thrombin compound, PPACK, and coagulation inhibitor, NapC2 (which targets the FXa/FVIIa/TF complex), did not consistently inhibit thrombogenesis. Because thrombin is thought to be a physiologically important trigger of platelet activation in vivo, it was somewhat surprising that coagulation inhibitors failed to block thrombogenesis. However, both coagulation inhibitors act after the appropriate zymogens have been activated. Therefore, small but sufficient concentrations of thrombin (in the case of PPACK) or FVIIa and FXa (in the case of NapC2) could be generated locally at the site of injury and result in significant platelet activation.
In contrast to the direct laser injury, the development of Rose Bengal-induced thrombi are inhibited by PPACK. Conceivably, the slower rate at which these clots develop might prevent the accumulation of local concentrations of thrombin at the site of injury to promote platelet aggregation. Determining the components required for thrombogenesis in these models is under further investigation.
Ultrastructural analyses indicate that both injuries do not lead to denudation of the endothelium lining the lumen of the vessel. The endothelial cells at the site of injury are more highly vacuolated than undamaged endothelial cells, but the cell membrane is intact and the cells are attached to subendothelial matrix. Thus, thrombogenesis does not require exposure of blood components to subendothelial matrix.
In summary, minimally invasive photo-injury models of vessels in the ear have been described. Using the same experimental system in which a laser beam is focused through the objective of a compound microscope, injuries can be induced by direct laser illumination or by photochemical damage after the administration of photoactivatable dyes. These systems offer significant advantages including that the procedures are minimally invasive; no surgical procedures that could activate or otherwise modulate the hemostatic system are involved other than anesthetization of the animal. Additionally, multiple injuries can be made on a single animal. Furthermore, variation in the development of multiple thrombi in a single animal is similar to the variation in development of injuries between animals. Finally, the system enables monitoring and recording of individual thrombus development in real time. This system will be valuable for the investigation of the role of specific components of the hemostatic system in thrombogenesis in vivo.
Footnotes
Address reprint requests to Dr. Elliot D. Rosen or Dr. Francis J. Castellino, Department of Chemistry and Biochemistry, Nieuwland Science Hall, University of Notre Dame, Notre Dame, Indiana 46556. E-mail: rosen.1@nd.edu or castellino.1@nd.edu.
Supported by National Institutes of Health grants HL-13423 and HL-19982 (to F. J. C.), a grant from the W. M. Keck Foundation, by the Kleiderer/Pezold Family Endowed Professorship (to F. J. C.)., and a grant from the American Heart Association Midwest Affiliate (to E. D. R.).
References
- 1.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MS, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL: Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA 1996, 93:6258-6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlanderen I, Demunck H, Kasper M, Breier G, Evrard P, Muller M, Risau W, Edgington T, Collen D: Role of tissue factor in embryonic blood vessel development. Nature 1996, 383:73-75 [DOI] [PubMed] [Google Scholar]
- 3.Toomey JR, Kratzer KE, Laskey NM, Stanton JT, Broze GJ: Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood 1996, 88:1583-1587 [PubMed] [Google Scholar]
- 4.Suh TS, Holmback K, Jensens NJ, Daugherty CC, Kersten S, Simon DI, Potter SS, Degen JL: Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 1995, 9:2020-2033 [DOI] [PubMed] [Google Scholar]
- 5.Sun WY, Witte DP, Degen JL, Colbert MC, Burkart MC, Holmback K, Xiao Q, Bugge TH, Degen SJ: Prothrombin deficiency results in embryonic and neonatal lethality in mice. Proc Natl Acad Sci USA 1998, 95:7597-7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue J, Wu O, Westfield LA, Tuley EA, Lu D, Zhang O, Sadler JE: Incomplete embryonic lethality and fatal neonatal hemorrhage caused by prothrombin deficiency in mice. Proc Natl Acad Sci USA 1998, 95:7603-7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, O’Shea KS, Purkayastha A, Saunders TL, Ginsburg D: Fatal haemorrhage and incomplete block to embryogenesis in mice lacking coagulation factor V. Nature 1996, 384:66-68 [DOI] [PubMed] [Google Scholar]
- 8.Rosen ED, Chan JC, Idusogie E, Clotman F, Vlasuk G, Luther T, Jalbert LR, Albrecht S, Zhong L, Lissens A, Schoonjans L, Moons L, Collen D, Castellino FJ, Carmeliet P: Mice lacking factor VII develop normally but suffer fatal perinatal bleeding. Nature 1997, 390:290-294 [DOI] [PubMed] [Google Scholar]
- 9.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazaian HH: Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet 1995, 10:119-121 [DOI] [PubMed] [Google Scholar]
- 10.Lin HF, Maeda N, Smithies O, Straight DL, Stafford DW: A coagulation factor IX-deficient mouse model for human hemophilia B. Blood 1997, 90:3962-3966 [PubMed] [Google Scholar]
- 11.Kundu RK, Sangiorgi F, Wu LY, Kurachi K, Anderson WF, Maxson R, Anderson WF: Targeted inactivation of the coagulation factor IX gene causes hemophilia B in mice. Blood 1998, 92:168-174 [PubMed] [Google Scholar]
- 12.Dewerchin M, Liang Z, Moons L, Carmeliet P, Castellino FJ, Collen D, Rosen ED: Blood coagulation Factor X deficiency causes partial embryonic lethality and fatal neonatal bleeding in mice. Thromb Haemost 2000, 83:185-190 [PubMed] [Google Scholar]
- 13.Gailani D, Laskey N, Broze GJ: A murine model of factor XI deficiency. Blood Coagul Fibrinolysis 1997, 8:134-144 [DOI] [PubMed] [Google Scholar]
- 14.Jalbert LR, Rosen ED, Moons L, Chan JCY, Carmeliet P, Collen D, Castellino FJ: Inactivation of the gene for anticoagulant protein C causes lethal perinatal consumptive coagulopathy in mice. J Clin Invest 1998, 102:1481-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy AM, Rayburn HB, Rosenberg RD, Weiler H: Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci USA 1995, 92:850-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang ZF, Higuchi D, Lasky N, Broze GJ: Tissue factor pathway inhibitor gene disruption produces intrauterine lethality in mice. Blood 1997, 90:944-951 [PubMed] [Google Scholar]
- 17.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD: A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci USA 1998, 95:9524-9529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P, Kieckens L, Schoonjans L, Ream B, Van Nuffelen A, Prendergast G, Cole M, Bronson R, Collen D, Mulligan RC: Plasminogen activator inhibitor-1 gene-deficient mice. I. Generation by homologous recombination and characterization. J Clin Invest 1993, 92:2746-2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D: Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation 1995, 92:2585-2593 [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC: Physiological consequences of loss of plasminogen activator gene function in mice. Nature 1994, 368:419-424 [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet P, Moons L, Stassen J-M, DeMol M, Bouche A, van den Oord JJ, Kockx M, Collen D: Vascular wound healing and neointima formation induced by perivascular electric injury in mice. Am J Pathol 1997, 150:761-776 [PMC free article] [PubMed] [Google Scholar]
- 22.Stockmans F, Deckmyn H, Gruwez J, Vermylen J, Acland R: Continuous quantitative monitoring of mural, platelet-dependent, thrombus kinetics in the crushed rat femoral vein. Thromb Haemost 1991, 65:425-431 [PubMed] [Google Scholar]
- 23.Stockmans F, Stassen JM, Vermylen J, Hoylaerts MF, Nystrom A: A technique to investigate mural thrombus formation in small arteries and veins: I. Comparative morphometric and histological analysis. Ann Plast Surg 1997, 38:56-62 [DOI] [PubMed] [Google Scholar]
- 24.Busuttil SJ, Drumm C, Ploplis VA, Plow EF: Endoluminal arterial injury in plasminogen-deficient mice. J Surg Res 2000, 91:159-164 [DOI] [PubMed] [Google Scholar]
- 25.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED: Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol 2000, 20:335-342 [DOI] [PubMed] [Google Scholar]
- 26.Kurz KD, Main BW, Sandusky GE: Rat model of arterial thrombosis induced by ferric chloride. Thromb Res 1990, 60:269-280 [DOI] [PubMed] [Google Scholar]
- 27.Farrehi PM, Ozaki CK, Carmeliet P, Fay WP: Regulation of arterial thrombolysis by plasminogen activatorinhibitor-1 in mice. Circulation 1998, 97:1002-1008 [DOI] [PubMed] [Google Scholar]
- 28.Doutremepuich F, Aguejouf O, Imbault P, Azougagh F, Doutremepuich C: Effect of the low molecular weight heparin/non steroidal anti-inflammatory drugs association on an experimental thrombosis induced by laser. Thromb Res 1995, 77:311-319 [DOI] [PubMed] [Google Scholar]
- 29.Rosenblum WI, Nelson GH, Pvlishock JT: Laser-induced endothelial damage inhibits endothelium-dependent relaxation in the cerebral microcirculation of the mouse. Circ Res 1987, 60:169-176 [DOI] [PubMed] [Google Scholar]
- 30.Matsuno H, Kozawa O, Niwa M, Ueshima S, Matsuo O, Collen D, Uematsu T: Differential role of components of the fibrinolytic system in the formation and removal of thrombus induced by endothelial injury. Thromb Haemost 1999, 81:601-604 [PubMed] [Google Scholar]
- 31.Kawasaki T, Kaida T, Arnout J, Vermylen J, Hoylaerts MF: A new animal model of thrombophilia confirms that high plasma factor VIII levels are thrombogenic. Thromb Haemost 1999, 81:306-311 [PubMed] [Google Scholar]
- 32.Roesken F, Ruecker M, Vollmar B, Boeckel N, Morgenstern E, Menger MD: A new model for quantitative in vivo microscopic analysis of thrombus formation and vascular recanalisation: the ear of the hairless (hr/hr) mouse. Thromb Haemost 1997, 78:1408-1414 [PubMed] [Google Scholar]
- 33.Roesken F, Vollmar B, Rucker M, Seiffge D, Menger MD: In vivo analysis of antithrombotic effectiveness of recombinant hirudin on microvascular thrombus formation and recanalization. J Vasc Surg 1998, 28:498-505 [DOI] [PubMed] [Google Scholar]
- 34.Mori M, Sakata I, Hirano T, Obana A, Nakajima S, Hikida M, Kumagai T: Photodynamic therapy for experimental tumors using ATX-S10(Na), a hydrophilic chlorin photosensitizer, and diode laser. Jpn J Cancer Res 2000, 91:753-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds ES: The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 1963, 1:208-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu HR, Gold HK, Wu Z, Yasuda T, Pauwels P, Rapold HJ, Napier M, Bunting S, Collen D: G4120, an Arg-Gly-Asp containing pentapeptide, enhances arterial eversion graft recanalization with recombinant tissue-type plasminogen activator in dogs. Thromb Haemost 1992, 67:686-691 [PubMed] [Google Scholar]
- 37.Stassens P, Bergum PW, Gansemans Y, Jespers L, Laroche Y, Huang S, Maki S, Messens J, Lauwereys M, Cappello M, Hotez PJ, Lasters I, Vlasuk GP: Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc Natl Acad Sci USA 1996, 93:2149-2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghebrehiwet B, Randazzo BP, Dunn JT, Silverberg M, Kaplan AP: Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest 1983, 71:1450-1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markgraf CG, Kraydieh S, Prado R, Watson BD, Dietrich WD, Ginsberg MD: Comparative histopathologic consequences of photothrombotic occlusion of the distal middle cerebral artery in Sprague-Dawley and Wistar rats. Stroke 1993, 24:286-292 [DOI] [PubMed] [Google Scholar]
- 40.Bunge MB, Holets VR, Bates ML, Clarke TS, Watson BD: Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp Neurol 1994, 127:76-93 [DOI] [PubMed] [Google Scholar]