Abstract
The changing process of protease expression phenotype was studied after transplantation of peritoneal mast cells (PMCs). To pursue the fate of the transplanted PMCs, we obtained PMCs from WBB6F1-c-kit+/c-kit+ mice with a transgene encoding green fluorescent protein (GFP). A large (n = 104) or small (n = 500) number of PMCs was injected into the stomach wall of genetically mast cell-deficient WBB6F1-c-kitW/c-kitWv mice without the GFP transgene. The original PMCs expressed messenger (m) RNAs of both mast cell carboxypeptidase A (MC-CPA) and mouse mast cell protease (mMCP)-2. The MC-CPA+/mMCP-2+ phenotype did not change in both the muscularis propria and mucosa when 104 PMCs were injected. In contrast, when 500 PMCs were injected, the mast cells that developed in the muscularis propria showed MC-CPA+/mMCP-2− phenotype and those that appeared in the mucosa showed MC-CPA−/mMCP-2+ phenotype. On day 1 after the injection of 500 PMCs, only ∼20 GFP+ cells were detected in the muscularis propria and no GFP+ cells in the mucosa. The proportion of Alcian blue+ cells decreased until day 7 and increased thereafter. The GFP+ but Alcian blue− cells were considered as degranulated PMCs. The remarkable decrease or degranulation seemed to be necessary for the alteration of protease expression phenotype.
Phenotype of mast cells is influenced by the tissue environment in which they develop. 1,2 The phenotype may be changed by transplantation of differentiated mast cells to a different tissue environment. 2-6 Peritoneal mast cells (PMCs) contain heparin glycosaminoglycan but mast cells in the mucosa of gastrointestinal tract do not contain it. 3-7 Mucosal mast cells contain chondroitin sulfate. 8 We transplanted PMCs of (WB × C57BL/6)F1-c-kit+/c-kit+ (hereafter WBB6F1-c-kit+/c-kit+) mice into the muscularis propria of the stomach of WBB6F1-c-kitW/c-kitWv mice that genetically lack mast cells. 4,9 Mast cells that developed in the stomach mucosa of WBB6F1-c-kitW/c-kitWv mice were not stained with berberine sulfate, indicating that they did not contain heparin glycosaminoglycan. 3,4
Mast cells of mice contain various proteases. The complementary (c) DNAs and genes that encode mast cell carboxypeptidase A (MC-CPA) and 9 of 10 mouse mast cell proteases (mMCPs) have been cloned and sequenced. 10-20 The type of expressed proteases seems more suitable for characterization of mast cells than the type of glycosaminoglycans. The type of glycosaminoglycans is a result of actions of numerous enzymatic steps, whereas the type of expressed proteases represents only an action of each protease gene. We have demonstrated that in situ hybridization histochemistry of proteases is useful for identification of the protease expression phenotypes of mast cells in tissues of mice. 21,22
When mast cells are identified by staining their specific granules, it is difficult to investigate the fate of relatively small numbers of mast cells. In the previous experiment, we transplanted 20 PMCs of WBB6F1-c-kit+/c-kit+ mice into the muscularis propria of WBB6F1-c-kitW/c-kitWvmice. 4,22 Ten days after the transplantation, we could not detect any mast cells by staining with Alcian blue (AB). 4 We did not know whether this represented the actual loss of the injected PMCs or the degranulation of the PMCs. In the present experiment, we used PMCs that were obtained from the transgenic mice expressing green fluorescent protein (GFP) of a jellyfish. 23 The transgenic mice were generated by Okabe and colleagues, 24 and showed the enhanced expression of GFP under the control of chicken β-actin promoter and cytomegalovirus enhancer. Cells from the transgenic mice can be identified easily under the fluorescent microscope. We produced WBB6F1-c-kit+/c-kit+ mice with a GFP transgene (hereafter WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice), and confirmed the alteration of protease expression phenotypes of GFP-labeled PMCs when a relatively small number of PMCs of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice were injected into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice.
Materials and Methods
Mice
WBB6F1-c-kit+/c-kit+ and WBB6F1-c-kitW/c-kitWv mice were purchased from Japan SLC (Hamamatsu, Japan) and used at 2 to 3 months of age. The mouse with the GFP transgene was generated as previously reported using the eggs from B6 strain. 24 Briefly, EGFP cDNA (a subtype of GFP originated from a jellyfish Aequorea victoria), driven by chicken β-actin promoter and cytomegalovirus enhancer 25 was injected to pronucleus of fertilized eggs. Thus established transgenic mouse line B6 TgN (act-EGFP15) Osb1 was maintained by the serial backcrosses to our own B6 colony. One-day-old B6 pups expressing GFP (hereafter B6-c-kit+/c-kit+; GFP+/GFP−) were identified by placing them under a hand-held fluorescent illuminator (model UVL-56; UVP Upland, CA). WB-c-kit+/c-kit+; GFP−/GFP− females were crossed to B6-c-kit+/c-kit+; GFP+/GFP− males and the resulting WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice were identified and used for the experiment. Because WBB6F1-c-kitW/c-kitWv mice had no GFP transgene, they are described as WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice hereafter.
Purification of PMCs
Purification of PMCs was performed according to the method described by Yurt and colleagues. 7 In brief, peritoneal cells (6 to 10 × 107) of 6-month-old WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice were suspended in 1 ml of Tyrode’s buffer, layered on 2 ml of 22.5% (w/v) metrizamide (density, 1.120 g/ml; Sigma Chemical Co., St. Louis, MO) and centrifuged at room temperature for 15 minutes at 400 × g. The cells remaining at the buffer-metrizamide interface were aspirated and discarded; the cells in the pellet were washed and resuspended in 1 ml of Tyrode’s buffer. Mast cells represented 70 to 80% of the nucleated cells in this preparation. To obtain PMC suspensions of ≧99% purity, the procedure just described was repeated using the 70 to 80% pure mast cell suspensions. Cells were counted with a standard hemocytometer.
Transplantation into the Stomach Wall
Recipient WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice were anesthetized with Nembutal (Pitman-Moore, Inc., Washington, NJ), the peritoneal cavity was opened, and the stomach was exposed. PMCs from WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice were injected into the wall of the glandular stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. 4 PMCs (n = 500 or 104) suspended in 0.1 ml of α-minimal essential medium (ICN Biomedicals, Cosa Mesa, CA) were injected with a tuberculin syringe. Each mouse received two injections marked by tattooing with India ink (Pelikan, Hannover, Germany). WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice were killed 1, 3, 7, 14, and 35 days after the injection. Injection sites identified by the presence of India ink were removed.
Detection of Cells
The stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice were harvested on various days after the injection of PMCs, opened, and flattened onto a rubber plate. To count the GFP-positive (GFP+) cells, the tissues were fixed with freshly prepared 4% paraformaldehyde in 0.1 mol/L of phosphate buffer (pH 7.4) for 6 hours at 4°C. Sites of PMC injection were embedded in Tissue-Tek OCT compound (Miles Inc., Elkhart, IN) and quickly frozen in liquid nitrogen. Serial frozen sections (8-μm thick) were cut with the cryostat (Leica, Heerbrugg, Switzerland). The number of GFP+ cells was counted with a fluorescence microscope (BX50; Olympus, Tokyo, Japan). The numbers of GFP+cells were counted in one of every five serial sections; total numbers of cells per each injection site were calculated by multiplying the sum of counted cells by five. After the observation with the fluorescent microscope, the same sections were used for either AB staining or in situ hybridization. Sections stained with AB were counterstained with nuclear fast red. AB+ cells were counted as described above in the case of GFP+ cells. Proportions of AB+ cells to GFP+ cells were calculated.
Preparation of Probes for in Situ Hybridization
Total RNA was extracted from cultured mast cells of WBB6F1-c-kit+/c-kit+; GFP−/GFP− mice. Single-strand cDNA was synthesized with an antisense primer by reverse transcriptase (Takara, Kyoto, Japan). The cDNAs for mMCP-2 and MC-CPA were amplified by a Perkin-Elmer Cetus (Norwalk, CT) DNA thermal cycler using Taq DNA polymerase (Takara). 26 PCR products were subcloned into the EcoRV site of the Bluescript KS (−) plasmid (Stratagene, La Jolla, CA), which contains T3 and T7 promoters to generate probes. The sequence was confirmed with a model 373A DNA sequencer (Applied Biosystems, Foster, CA).
In Situ Hybridization
The same sections for counting GFP+ cells was also used for in situ hybridization. Hybridization was performed as described previously with minor modifications. 27 Digoxigenin-labeled single-strand RNA probes were prepared using a DIG RNA labeling kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Controls included 1) hybridization with the sense probe, 2) RNase A treatment (20 μg/ml) before hybridization, and 3) withholding of the antisense RNA probe and the anti-digoxigenin antibody. 27 None of the three controls showed any positive signals. The numbers of GFP+ cells and those of mMCP-2 or MC-CPA mRNA-expressing cells were counted in the same sections as described in the case of GFP+ cells, and the proportions of protease mRNA+ cells to GFP+ cells were calculated.
Staining with Berberine Sulfate
Stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice were harvested on day 35 after the injection of 10 4 PMCs of WBB6F1-c-kitW/c-kitWv; GFP+/GFP− mice, opened, and flattened onto a rubber plate. The tissues were fixed with Carnoy’s fluid. Tissues including the injection site were embedded in paraffin. These procedures abolished the fluorescence of GFP. Serial sections (8-μm thick) were made. One section was stained with AB. The adjacent section was stained with 0.025% berberine sulfate (Sigma) at pH 4.0 as described by Enerback. 28 Tissues stained with berberine sulfate were examined with the Olympus fluorescence microscope.
Results
PMCs were purified from the peritoneal fluid of WBB6F1-c-kit+/c-kit+ mice with the GFP transgene (ie, WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice), and 500 or 10 4 GFP+ PMCs were injected into the muscularis propria of the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. The recipient WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice were killed 1, 3, 7, 14, and 35 days after the injection. Frozen sections of the stomachs were cut, and the appearance of GFP+ cells were examined under the fluorescent microscope.
When 10 4 PMCs were injected, GFP+ cells were detected in both the muscularis propria and mucosa of stomachs of most WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice throughout the observation period (Table 1) ▶ . On the other hand, when 500 PMCs were injected, the proportion of appearance of GFP+ cells was influenced by both the observation site (muscularis propria or mucosa) and the duration between the injection and observation. GFP+ cells were detected in the muscularis propria of ∼60% of the injection sites from day 1 after the injection, but GFP+ cells were not detected in the mucosa of these WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice on days 1, 3, and 7 after the injection (Table 1) ▶ . GFP+ cells appeared in the mucosa from day 14 after the injection. The proportion of the appearance in the mucosa increased thereafter (Table 1) ▶ .
Table 1.
Proportion of Appearance of GFP+ Cells in the Stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− Mice at Various Days after the Injection of PMCs of WBB6F1-c-kit+/c-kit+; GFP+/GFP− Mice
| Days after injection | Proportion of appearance (injection sites with GFP+ cells/total injection sites) | |||
|---|---|---|---|---|
| Muscularis propria | Mucosa | |||
| 500 PMC injection | 104 PMC injection | 500 PMC injection | 104 PMC injection | |
| 1 | 9 /14 | 6 /6 | 0 /14 | 5 /6 |
| 3 | 5 /8 | 4 /4 | 0 /8 | 3 /4 |
| 7 | 14 /22 | 7 /8 | 0 /22 | 6 /8 |
| 14 | 7 /12 | 6 /6 | 4 /12 | 5 /6 |
| 35 | 19 /28 | 4 /4 | 10 /28 | 3 /4 |
When 10 4 PMCs were injected, the number of GFP+ cells that appeared in both the muscularis propria and mucosa of stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice did not change throughout the observation period (Figure 1A) ▶ . On the other hand, when 500 PMCs were injected, the number of GFP+ cells increased from day 1 to 35 in the muscularis propria and from day 14 to 35 in the mucosa of stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice (Figure 2A) ▶ .
Figure 1.

Changes of various parameters after injection of 10 4 PMCs from WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. A: Number of GFP+ cells. B: Proportion of AB+ cells to CFP+ cells. C: Proportion of MC-CPA mRNA+ or mMCP-2 mRNA+ cells to GFP+ cells in the muscularis propria. D: Proportion of MC-CPA mRNA+ or mMCP-2 mRNA+ cells to GFP+ cells in the mucosa. Each point represents the mean of four to eight injection points. Bar shows the SE. Column represents the value observed in the injected PMCs (mean ± SE of four experiments) or the value observed in each tissue of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice (mean ± SE of four mice).
Figure 2.
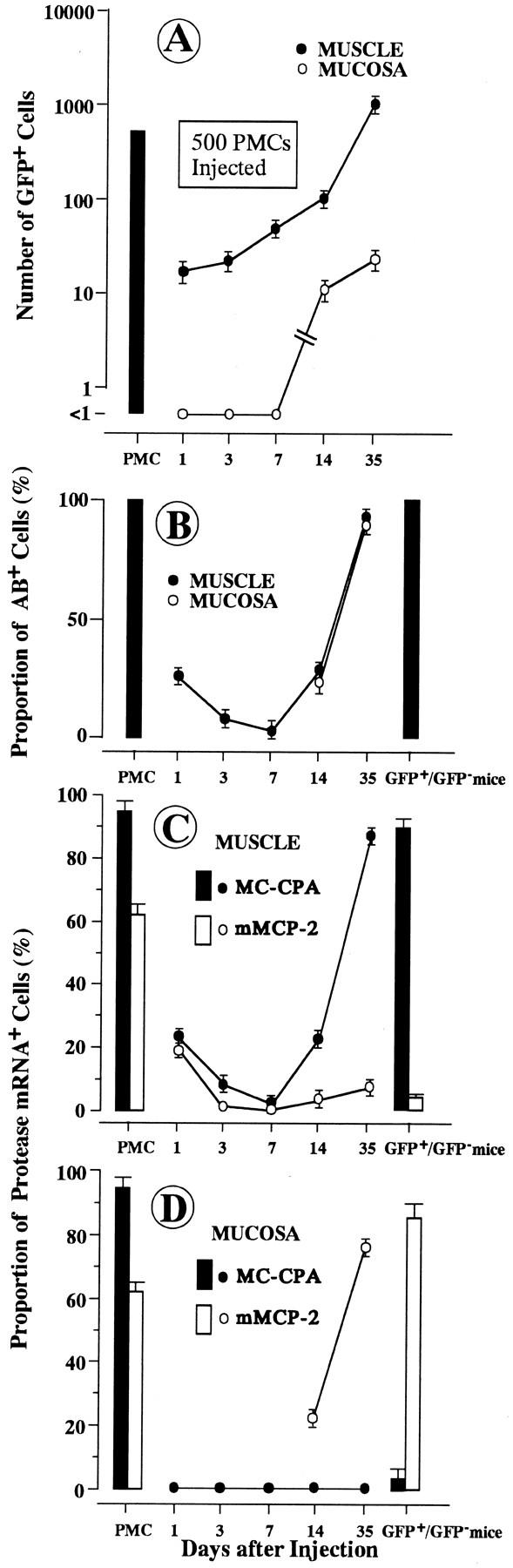
Changes of various parameters after injection of 500 PMCs from WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. A: Number of GFP+ cells. B: Proportion of AB+ cells to CFP+ cells. C: Proportion of MC-CPA mRNA+ or mMCP-2 mRNA+ cells to GFP+ cells in the muscularis propria. D: Proportion of MC-CPA mRNA+ or mMCP-2 mRNA+ cells to GFP+ cells in the mucosa. Eachpoint represents the mean of 8 to 28 injection points. Bar shows the SE. Column represents the value observed in the injected PMCs (mean ± SE of four experiments) or the value observed in each tissue of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice (mean ± SE of four mice).
Practically all PMCs purified from the peritoneal fluid were stained with AB. Such a high proportion of AB+ cells was maintained throughout the observation period in both the muscularis propria and mucosa of the stomachs when 10 4 PMCs were injected into the muscularis propria of the stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice (Figure 1B) ▶ . In contrast, when 500 PMCs were injected, the proportion of AB+ cells dropped to 3% on day 7 and then increased in the muscularis propria of stomachs of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice (Figure 2B) ▶ . AB− but GFP+ cells were observed on days 1, 3, and 7 after the injection (Figure 3) ▶ . Such AB− cells were considered to be the injected and degranulated PMCs. AB+ cells were detectable in the mucosa only after day 14 (Figure 2B) ▶ .
Figure 3.
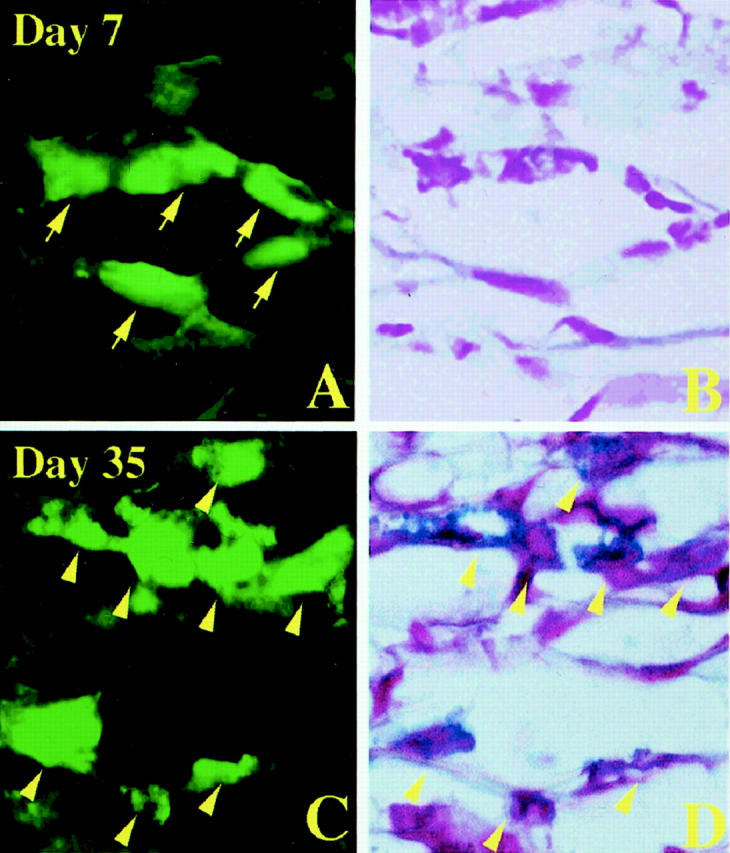
Granulated and degranulated mast cells after injection of 500 PMCs of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. A: GFP+ cells in the muscularis propria on day 7 after the injection. B: Same section of A stained with AB and nuclear fast red. GFP+ cells shown by arrows in A were not stained with AB. C: GFP+ cells in the muscularis propria on day 35 after the injection. D: Same section of C stained with AB and nuclear fast red. GFP+ cells shown by arrowheads in C were also stained with Alcian blue. Original magnification, ×1000.
When 10 4 PMCs were injected, mast cells that appeared in both the muscularis propria and mucosa of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice kept the original protease expression phenotype, (MC-CPA+/mMCP-2+; Figure 1, C and D ▶ ). In contrast, when 500 PMCs were injected, the protease expression phenotype changed. The protease expression phenotype of the mast cells that appeared in the muscularis propria of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice was similar to that of the mast cells observed in the muscularis propria of intact WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice (ie, MC-CPA+/mMCP-2−) (Figure 2C) ▶ , and the protease expression phenotype of the mast cells that appeared in the mucosa was similar to that of the mast cells observed in the mucosa of intact WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice (ie, MC-CPA−/mMCP-2+) (Figure 2D) ▶ . In other words, the protease expression phenotype adapted to new environments when a small number of PMCs was injected.
As already mentioned, the original protease expression phenotype was kept even in the stomach mucosa when 10 4 PMCs were injected into WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. In one experiment, we stained with berberine sulfate the sections of stomachs that were removed 35 days after the injection of 10 4 PMCs, which were originally berberine sulfate+. Unexpectedly, the binding ability of PMCs with berberine sulfate was lost in the mucosa despite of the maintenance of protease expression phenotype (MC-CPA+/mMCP-2+; Figure 4 ▶ ). In contrast, the berberine sulfate-binding ability was kept in the muscularis propria (Figure 4) ▶ .
Figure 4.
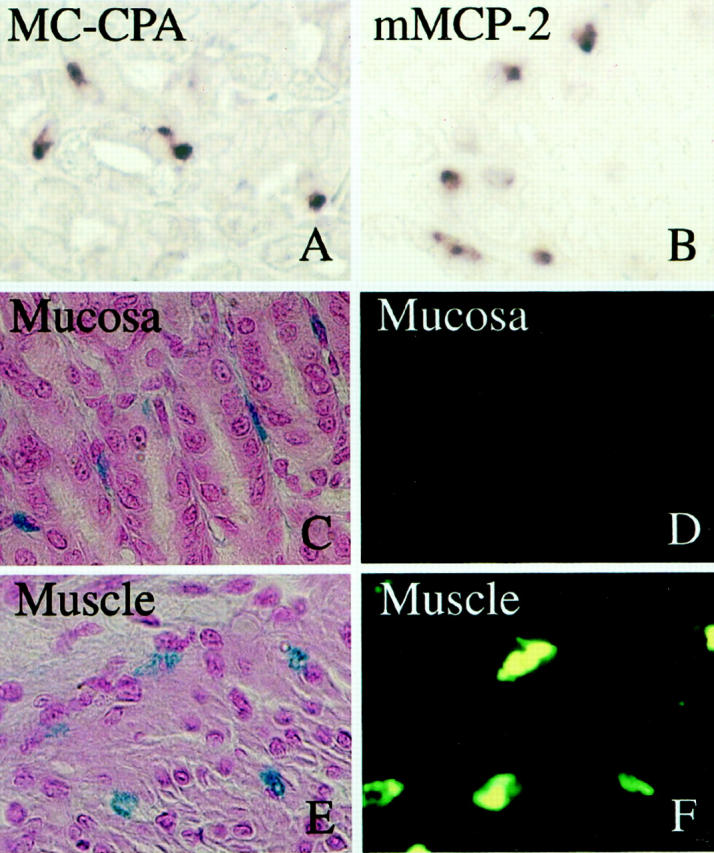
Loss of berberine sulfate-binding ability of mast cells in the mucosa 35 days after injection of 10 4 PMCs of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice, despite of the maintenance of the original protease expression phenotype (MC-CPA+/mMCP-2+). A: Expression of MC-CPA mRNA by the mast cells in the mucosa was demonstrated by in situ hybridization. B: Expression of mMCP-2 mRNA by the mast cells in the mucosa. C: Mast cells in the mucosa stained with AB and nuclear fast red. D: An adjacent section of C. Mast cells in the mucosa were not stained with berberine sulfate. E: Mast cells in the muscularis propria stained with AB and nuclear fast red. F: An adjacent section of E. Mast cells in the muscularis propria were stained with berberine sulfate. Original magnification, ×300.
Discussion
We injected 500 or 10 4 PMCs of WBB6F1-c-kit+/c-kit+; GFP+/GFP− mice into the stomach of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice. When 10 4 PMCs were injected, the protease expression phenotype of PMCs did not change in both the muscularis propria and mucosa (ie, MC-CPA+/mMCP-2+). On the other hand, when 500 PMCs were injected, the protease expression phenotype changed to MC-CPA+/mMCP-2− in the muscularis propria and to MC-CPA−/mMCP-2+ in the mucosa. The difference in protease expression phenotype among serosal, connective tissue, and mucosal mast cells are consistent with the previous result of Stevens and colleagues 29 and that of ourselves. 21,22 The fact that the protease expression phenotype changed only when a relatively small number of PMCs was injected was also consistent with our previous result. 4,22
When 10 4 PMCs were injected into the stomach wall of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice, the berberine sulfate-binding ability was lost in the mucosa despite of the maintenance of the original protease expression phenotype (MC-CPA+/mMCP-2+). We had reported that the berberine sulfate-binding ability of PMCs was lost in the mucosa after injection of a single PMC. 4 Although the dose effect of injected PMCs was observed in the protease expression phenotype, it was not detectable in the proteoglycan phenotype. In the next study, we will examine the staining characteristics of developing mast cells at various times after injections of a small or large dose of PMCs. This might simply reflect the fact that the protease expression phenotype is not consistent with the glycosaminoglycan phenotype. For example, although both mucosal and cultured mast cells are berberine sulfate−, mucosal mast cells are MC-CPA−/mMCP-2+/mMCP-4−/mMCP-6− whereas cultured mast cells are MC-CPA+/mMCP-2+/mMCP-4+/mMCP-6+. 21,22
When 10 4 PMCs were injected, the number of GFP+ cells reached to the maximum level on day 1 after the injection in both the muscularis propria and mucosa. The number did not significantly change thereafter. The proportions of AB+, MC-CPA+, and mMCP-2+ cells also did not change. This suggested that the injected PMCs settled soon after the injection. The PMCs kept the original protease expression phenotype throughout the observation period.
When 500 GFP+ PMCs were injected, only ∼20 GFP+ cells were detected in the injection site, ie, muscularis propria, on day 1 after the injection. Then the number increased and exceeded the number of injected PMCs on day 35. Therefore, most GFP+ cells that were observed in the muscularis propria on day 35 appeared to newly develop from the injected PMCs. The proportions of AB+ and MC-CPA+ cells decreased until day 7 and increased thereafter. PMCs possessing the MC-CPA+/mMCP-2+ phenotype may die or lose the original phenotype during these 7 days. The mast cells that developed in the muscularis propria from the surviving GFP+ cells showed the new phenotype, ie, MC-CPA+/mMCP-2−. Although our previous report suggested this, the present result indicated it much more clearly because we examined the fate of injected PMCs sequentially and because we traced the injected PMCs using GFP as a marker.
No GFP+ cells were detected in the mucosa of WBB6F1-c-kitW/c-kitWv; GFP−/GFP− mice on days 1, 3, and 7 after the injection of 500 PMCs. The mast cells of MC-CPA−; mMCP-2+ phenotype appeared to be originated from a small number of GFP+ PMCs that migrated from the muscularis propria. The original MC-CPA+/mMCP-2+ phenotype may be lost during the migration, and newly developing mast cells may acquire the MC-CPA−/mMCP-2+ phenotype. This also confirmed our previous result. 21,22 Because we did not examine the fate of injected PMCs sequentially and we identified mast cells only by AB staining in the previous experiment, 4 the present data showed the changing process of protease expression phenotype of PMCs more convincingly.
The present experiment clearly showed the usefulness of the GFP transgenic mice for studying the fate of transplanted mast cells. By using GFP-labeled PMCs, we could discriminate the loss of injected PMCs and their degranulation. Both processes occurred after the transplantation, but at least a small number of degranulated PMCs did remain in the injection site. Probably, such degranulated PMCs may acquire the new phenotype and adapt the new environment.
Footnotes
Address reprint requests to Yukihiko Kitamura, M.D., Department of Pathology, Osaka University Medical School, Yamada-oka, 2-2, Suita 565-0871, Japan. E-mail: kitamura@patho.med.osaka-u.ac.jp.
Supported by grants from the Ministry of Education, Science and Culture, the Ministry of Health and Welfare, and the Organization for Pharmaceutical Safety and Research.
References
- 1.Enerback L: The gut mast cell. Monogr Allergy 1981, 17:222-232 [Google Scholar]
- 2.Kitamura Y: Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol 1989, 7:59-76 [DOI] [PubMed] [Google Scholar]
- 3.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ: Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice: evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med 1985, 162:1025-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonoda S, Sonoda T, Nakano T, Kanayama Y, Kanakura Y, Asai H, Yonezawa T, Kitamura Y: Development of mucosal mast cells after injection of a single connective tissue-type mast cells in the stomach mucosa of genetically mast cell-deficient W/Wv mice. J Immunol 1986, 137:1319-1322 [PubMed] [Google Scholar]
- 5.Otsu K, Nakano T, Kanakura Y, Asai H, Katz HR, Austen KF, Stevens RL, Galli SJ, Kitamura Y: Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med 1987, 165:615-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanakura Y, Thompson H, Nakano T, Yamamura T, Asai H, Kitamura Y, Metcalfe DD, Galli SJ: Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood 1988, 72:877-885 [PubMed] [Google Scholar]
- 7.Yurt RW, Leid RW, Austen KF, Silbert JE: Native heparin from rat peritoneal mast cells. J Biol Chem 1977, 252:518-521 [PubMed] [Google Scholar]
- 8.Stevens RL, Lee TDG, Seldin DC, Austen KF, Befus D, Binebstok J: Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol 1986, 137:291-295 [PubMed] [Google Scholar]
- 9.Kitamura Y, Go S, Hatanaka K: Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood 1978, 52:447-452 [PubMed] [Google Scholar]
- 10.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE: Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci USA 1990, 87:3220-3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE: Isolation and molecular cloning of mast cell carboxypeptidase A: a novel member of the carboxypeptidase gene family. J Biol Chem 1989, 264:20094-20099 [PubMed] [Google Scholar]
- 12.Serafin WE, Reynolds DS, Rogelj S, Lane WS, Conder GA, Johnson SS, Austen KF, Stevens RL: Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J Biol Chem 1990, 265:423-429 [PubMed] [Google Scholar]
- 13.Serafin WE, Sullivan TP, Condar GA, Ebrahimi A, Marcham P, Johnson SS, Austen KF, Reynolds DS: Cloning of the cDNA and gene for mouse mast cell protease 4: demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J Biol Chem 1991, 266:1934-1941 [PubMed] [Google Scholar]
- 14.McNeil HP, Austen KF, Somerville LL, Gurish MF, Stevens RL: Molecular cloning of the mouse mast cell protease-5 gene: a novel secretory granule protease expressed early in the differentiation of serosal mast cells. J Biol Chem 1991, 266:20316-20322 [PubMed] [Google Scholar]
- 15.Reynolds DS, Gurley DS, Austin KF, Serafin WE: Cloning of the cDNA and gene of mouse mast cell protease-6: transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem 1991, 266:3847-3853 [PubMed] [Google Scholar]
- 16.Huang R, Blom TM, Hellman L: Cloning and structural analysis of mMCP-1, mMCP-4, and mMCP-5, three mouse mast cell specific serine proteases. Eur J Immunol 1991, 21:1611-1621 [DOI] [PubMed] [Google Scholar]
- 17.McNeil HP, Reynolds DS, Schiller Y, Ghildyal N, Gurley DS, Austen KF, Stevens RL: Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc Natl Acad USA 1992, 89:11174-11178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL: IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol 1992, 149:2123-2129 [PubMed] [Google Scholar]
- 19.Lützelschwab C, Huang MR, Kullberg MC, Aveskogh M, Hellman L: Characterization of mouse mast cell protease-8, the first member of a novel subfamily of mouse mast cell serine proteases, distinct from both the classical chymases and tryptases. Eur J Immunol 1998, 28:1022-1033 [DOI] [PubMed] [Google Scholar]
- 20.Hunt JE, Friend DS, Gurish MF, Feyfant E, Sali A, Huang C, Ghildyal N, Stechschulte S, Austen, Stevens RL: Mouse mast cell protease 9, a novel member of the chromosome 14 family of serine proteases that is selectively expressed in uterine mast cells. J Biol Chem 1997, 272:29158-29166 [DOI] [PubMed] [Google Scholar]
- 21.Jippo T, Tsujino K, Kim HM, Kim DK, Lee YM, Nawa Y, Kitamura Y: Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. Am J Pathol 1997, 150:1373-1382 [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YM, Jippo T, Kim DK, Katsu Y, Tsujino K, Morii E, Kim HM, Adachi A, Nawa Y, Kitamuta Y: Alteration of protease expression phenotype of mouse peritoneal mast cells by changing the microenvironment as demonstrated by in situ hybridization histochemistry. Am J Pathol 1998, 153:931-936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC: Green fluorescent protein as a marker for gene expression. Science 1994, 263:802-805 [DOI] [PubMed] [Google Scholar]
- 24.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y: ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 1997, 407:313-319 [DOI] [PubMed] [Google Scholar]
- 25.Niwa H, Yamamura K, Miyazaki J: Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 193–199 [DOI] [PubMed]
- 26.Jippo T, Ushio H, Hirota S, Mizuno H, Yamatodani A, Nomura S, Matsuda H, Kitamura Y: Poor response of cultured mast cells derived from mi/mi mutant mice to nerve growth factor. Blood 1994, 84:2977-2983 [PubMed] [Google Scholar]
- 27.Nomura S, Wills AJ, Edwards DR, Heath JK, Hogan BLM: Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol 1988, 106:441-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enerback L: Berberine sulfate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry 1974, 42:301-313 [DOI] [PubMed] [Google Scholar]
- 29.Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF: Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci USA 1994, 91:128-132 [DOI] [PMC free article] [PubMed] [Google Scholar]


