Abstract
FE65, a protein expressed in the nervous system, has the ability to bind the C-terminal domain of the amyloid precursor protein. This suggests a role for FE65 in the pathogenesis of Alzheimer’s disease (AD). The present study was conducted to find out if the distribution of FE65 immunoreactivity was affected during the course of AD, and to determine the degree of co-localization of FE65 with other proteins known to be involved in AD. Single immunoperoxidase-labeling experiments, conducted on six sporadic AD patients and six nondemented age-matched controls, showed that the proportion of volume occupied by FE65 immunoreactivity was not modified in the isocortex of AD patients. However, in hippocampal area CA4, increased FE65 immunoreactivity seemed to be associated with the severity of the disease. Double-immunofluorescent labeling did not show any clear co-localization of FE65 with the amyloid precursor protein. FE65 immunoreactivity was also absent from focal and diffuse deposits of the β-amyloid peptide. Unexpectedly double labeling experiments showed a co-localization of FE65 and tau proteins in intracellular tangles. Ultrastructural observations confirmed that FE65 was associated with paired helical filaments.
Alzheimer’s disease (AD) is a degenerative dementia that is characterized by the presence of senile plaques and neurofibrillary alterations. The core of the senile plaques is mainly composed of the β-amyloid peptide, the accumulation of which seems to be a major causative factor of the disease. The β-amyloid peptide is a proteolytic product of the amyloid precursor protein (APP), a cell surface protein with a large N-terminal extracellular domain, a single transmembrane segment, and a short C-terminal cytoplasmic tail. Because pathological accumulation of β-amyloid peptide in AD seems to result from a disregulation of the cleavage of APP, recent research efforts have been directed toward understanding the role of proteins interacting with APP that could act on the regulation of its processing. Internalization signals have been characterized in the cytoplasmic domain of APP. 1 Proteins interacting with this domain and possibly involved in the intracellular trafficking of APP have been recently identified using the yeast two-hybrid system. 2,3
It was recently found that FE65, a putative adaptor protein, 3 binds to the cytoplasmic domain of APP. 4-8 FE65 is a protein expressed in the nervous tissue, 9 and particularly in the hippocampus and the isocortex, 5 the areas principally affected in AD. Furthermore, the interaction of APP and FE65 has been shown in vitro to potentiate the translocation of APP to the cell surface and to dramatically increase the secretion of β-amyloid peptide. 8,10,11 The role of FE65 in the pathogenesis of AD is moreover strengthened by observations suggesting the association of a FE65 gene polymorphism with sporadic AD 12,13 although other data argue against such an association. 14,15
Until now, no attempts have been made to characterize FE65 in normal and pathological human tissue (although alterations at the level of mRNA expression have been described 16 ). One goal of the present study was to determine whether the distribution of FE65 immunoreactivity was affected during the course of AD. To explore this possibility the proportion of volume occupied by FE65 immunoreactive material was assessed in hippocampal and isocortical samples derived from two populations: normal aged nondemented patients and sporadic AD patients.
The second aim of this study was to better characterize the interaction of FE65 with proteins known to be involved in AD pathology. APP and FE65 have been found to be co-localized in cell cultures 10 but demonstration of the association of these two proteins in human nervous tissue is still lacking. Co-localization of FE65 and AD-related proteins (APP, β-amyloid, tau) in the human brain was looked for by means of double-immunofluorescent labeling that were subsequently analyzed using confocal laser scanning microscopy. Ultrastructural immunocytochemistry studies were finally performed to elucidate the possible association of FE65 with AD histopathological lesions.
Materials and Methods
Primary Antibodies
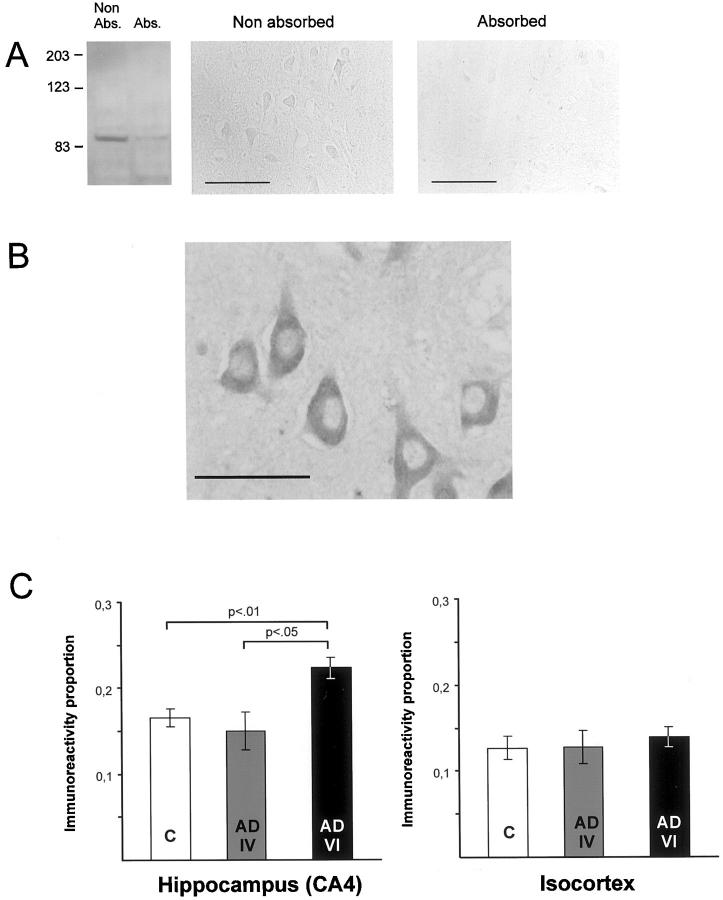
A polyclonal antibody directed against recombinant FE65 expressed in Escherichia coli was used at a 1:6000 dilution for single immunostainings. To perform Western blot, hippocampus of a control and two AD patients were homogenized. In the three investigated cases the antibody against FE65 recognized a major band at 90 kd that corresponds to the molecular weight of FE65 (Figure 1A ▶ ; left, first column). Immunoabsorption control experiments were conducted in parallel on brain homogenates (Figure 1A ▶ , left) as well as on paraffin-embedded sections of human hippocampi (Figure 1A ▶ , center and right). Preabsorption of the FE65 antiserum with the recombinant protein used for immunization strongly diminished the immunoreactivity in both preparations.
Figure 1.
FE65 immunostaining. A: Western blot of FE65 immunoreactivity in human brain homogenates (left, Non Abs. column). Absorption of the antibody (Abs. column) strongly diminished immunoreactivity. Molecular size markers are shown on the left (kd). On human brain paraffin-embedded sections, absorption of the antibody leads to similar results [compare “Non absorbed” (middle) and “Absorbed” (right)]. B: FE65 immunoreactivity was observed in AD and control cases in all types of neurons such as pyramidal cells of the hippocampus as illustrated on this photograph. Neuronal nuclei were devoid of immunostained material whereas a dense cytoplasmic FE65 immunoreactivity was noted. Scale bar, 50 μm. C: Proportion of FE65 immunoreactivity (mean ± SEM) in allo- (left) and isocortical (right) samples in control (C), AD with Braak stage IV (AD IV), and AD with Braak stage VI (AD VI) cases. FE65 immunoreactivity in the hippocampus (CA4) was higher in AD cases with severe neuropathological lesions as compared to patients with less advanced pathology and nondemented/age-matched cases. No differences were observed in the isocortex between AD and control cases.
As some of our results suggested an association between FE65 and neurofibrillary tangles, an immunostaining against human tau (polyclonal antibody A0024 used at a 1:500 dilution; DAKO, Glostrup, Denmark) was performed to assess the density of neurofibrillary tangle immunoreactivity in the studied brain areas.
To perform double-labeling experiments we used, in association with the anti-FE65 polyclonal antibody (used at a 1:1500 dilution for double-immunofluorescent labeling) 1) a monoclonal antibody that recognizes an N-terminal epitope on the APP protein (22C11 1:10; Boehringer, Mannheim, Germany); 2) a monoclonal antibody directed against residues 8 to 17 of the β-amyloid peptide (6F/3D 1:100; DAKO); 3) a monoclonal antibody against phosphorylated tau (AD2, 1:1000 for double labeling using the immunoperoxidase method and 1:500 for immunofluorescent double labeling, a gift from A. Delacourte, INSERM, Lille, France). 17
Cases
Six cases of sporadic AD (age range, 80 to 90 years) and six age-matched/sex-matched control cases (age range, 82 to 87 years) were used in this study. Sporadic AD cases were diagnosed according to the National Institute of Aging and Reagan Institute criteria 18 and were scored Braak’s stages IV (n = 2) or VI (n = 4). Control cases were nondemented patients who died without any known neurological disorders.
Immunohistochemistry and Microscopical Analysis
Single Labeling and Morphometric Analysis
Tissue samples were taken from Brodmann’s area 40 (supramarginal gyrus) and from the hippocampal formation (hippocampus proper and adjacent temporal cortices). Paraffin-embedded tissues were cut in serial 6-μm-thick slices on a microtome and classical immuno-histochemical procedures based on the avidin-biotin horseradish peroxidase method were applied, after microwave pretreatment (citrate buffer, pH 6, 400 W, 2× 10 minutes), to reveal FE65 antigenicity in brain samples.
Morphometric analysis in the hippocampal formation were restricted to the CA4 field of the hippocampus. As neuronal loss because of AD pathology is mild in this sector as compared to other fields of the hippocampus, 19 the estimate of FE65 immunoreactivity in the hippocampus was therefore not biased. We also controlled that no atrophy had taken place in the CA4 sector, the surface area of which was measured for each patient using the histogram function of Photoshop 5 software (Adobe, San Jose, CA) [AD patients versus control cases: t(10) = 0.293; ns].
To determine whether FE65 distribution was altered in AD patients, the proportion of area occupied by FE65 immunoreactive material, which corresponds to the proportion of volume according to Delesse principle, 20 was assessed by the standard stereological point-counting method. 21 Each counting field, examined with a ×40 objective, was visualized through a camera connected to a microscope, on a computer screen on which a grid with 108, 2-cm interspaced, points was applied. Points falling onto positive immunoreactive structures were summed up for each field and divided by the total number of points to give the proportion of FE65 immunoreactivity. For each type of samples (hippocampal and isocortical), the optimal number of fields was first determined by a pilot study to obtain a coefficient of error <10%.
In CA4, 25 nonoverlapping counting fields were investigated for every individual brain sample (one section per case). In the isocortex, four contiguous columns oriented perpendicularly to the pial surface and covering the whole thickness of the cortex were evaluated (one section per case). The variable number of fields in each column was standardized for each patient on a 10 level scale, using an algorithm that allows to establish equivalence between the 10 standardized levels and actual cortical layering. 22
An automated immunostaining station (Nexes station; Ventana Medical Systems, Illkirch, France) was used to obtain single immunolabeling of tau protein. A formic acid pretreatment was applied before immunohistochemistry and sections were finally counterstained with Harris hematoxylin. The same morphometric procedures as used for FE65 labeling were applied to assess the density of tau immunostained neurofibrillary tangles (one section of hippocampus and isocortex per case) with the following differences: 1) a ×25 objective and a 300-point counting grid were used, and 2) 10 fields were sampled in the CA4 sector. Only points falling onto intracellular immunostained tangles were counted.
Double Labeling
Double labeling of FE65 and tau proteins, using the immunoperoxidase method, were performed in one AD case. Primary antibodies were sequentially incubated and revealed, with diaminobenzidine and nickel-enhanced diaminobenzidine as chromogens.
Double-immunofluorescent stainings were performed in three of the six AD cases to analyze the degree of co-localization of FE65 and other proteins. As both the hippocampus and the isocortex of AD patients were investigated, two sections were examined per case. FE65 antigenicity was revealed by a streptavidin-cyanine 2 complex (Jackson Immunoresearch, West Grove, PA) whereas an anti-mouse IgG antibody conjugated to the fluorophore cyanine 3 (Jackson Immunoresearch) was used to visualize APP, β-amyloid, or tau proteins. Determination of co-localization was rendered difficult by autofluorescence in tissue samples, mainly caused by accumulation of lipofuscin granules. We therefore tested several series of pretreatments to extinguish autofluorescence and, in accordance with two recent studies 23,24 found that Sudan black was efficient to eliminate lipofuscin-like fluorescence without affecting the fluorophores’ intensities. Finally, double-immunofluorescent stainings were analyzed by means of a confocal laser-scanning microscope (TCS, Leica, Rueil Malmaison, France).
Ultrastructural Immunocytochemistry
To assess in more detail the ultrastructural localization of FE65 and its possible association with characteristic histopathological lesions, immunolabeling using the avidin-biotin-peroxidase method was performed on 50-μm-thick vibratome sections from a hippocampal sample in an AD case. After immunostaining, tissues were processed in accordance with classical pre-embedding procedures 25 and analyzed with an electron microscope (CM100; Phillips Limeil-Brévannes, France).
Results
Morphometric Analysis of FE65 Immunoreactive Material
The first aim of this study was to determine the topographical and cellular distribution of FE65 in human brain samples. Immunoreactivity of FE65 was detected exclusively in neurons of control and AD cases. A granular positive staining was found in the cytoplasm of neurons (Figure 1B) ▶ and exceptionally in the nucleus. In the hippocampal formation FE65 immunoreactivity was widely distributed in the dentate gyrus and among the various fields of the Ammon’s horn as well as in the subicular complex and adjacent rhinal cortices. In the supramarginal gyrus, FE65 immunoreactivity was observed in all cortical layers and concerned all types of neurons (granular, pyramidal).
In CA4, morphometric analyses indicated that the proportion of volume occupied by FE65 immunoreactivity was slightly higher in AD patients (mean, 0.199) as compared to normal cases (mean, 0.165; Figure 1C ▶ , left). This trend just failed to reach statistical significance [t-test; t(10) = 2.018; P > 0.071]; however when comparisons were restricted to the six control cases and the four stage VI cases a significant group effect was noted [t(8) = 3.619; P < 0.01]. Hence, Braak’s stages seemed to be linked with FE65 immunostaining in AD samples: the two stage IV cases showed a significantly lower proportion of FE65 immunoreactivity as compared to the four stage VI cases [t(4) = 3.147; P < 0.05], suggesting that FE65 immunoreactivity increases in the hippocampus in relation with the severity of the disease.
Morphometric analyses in isocortical samples did not reveal any significant differences in the number of positive FE65 neurons in control and AD patients [t-test; t(10) = 1.08; ns; Figure 1C ▶ , right]. No significant within-group effect was observed in AD patients in relation to Braak’s stages [t(4) = 0.74; ns]. Laminar distribution of FE65 was compared in the two populations and results showed no difference between normal and AD patients in the proportion of FE65-positive neurons whatever the cortical depth [not illustrated; t-tests; t(10) < 1.422; ps > 0.185].
Co-Localization of FE65 with Other Proteins
The second goal of the study was to determine whether FE65 was associated in the human nervous system with proteins classically described as being involved in AD pathology.
Double-immunofluorescent stainings for FE65 and APP did not show any co-localization between these two proteins. APP and FE65 were intermingled in the cytoplasm of cell bodies (Figure 2A) ▶ with no clear evidence of overlapping signals. APP-positive dilated neurites of the crown of the senile plaques were found to be devoid of FE65 immunoreactivity (Figure 2B) ▶ .
Figure 2.
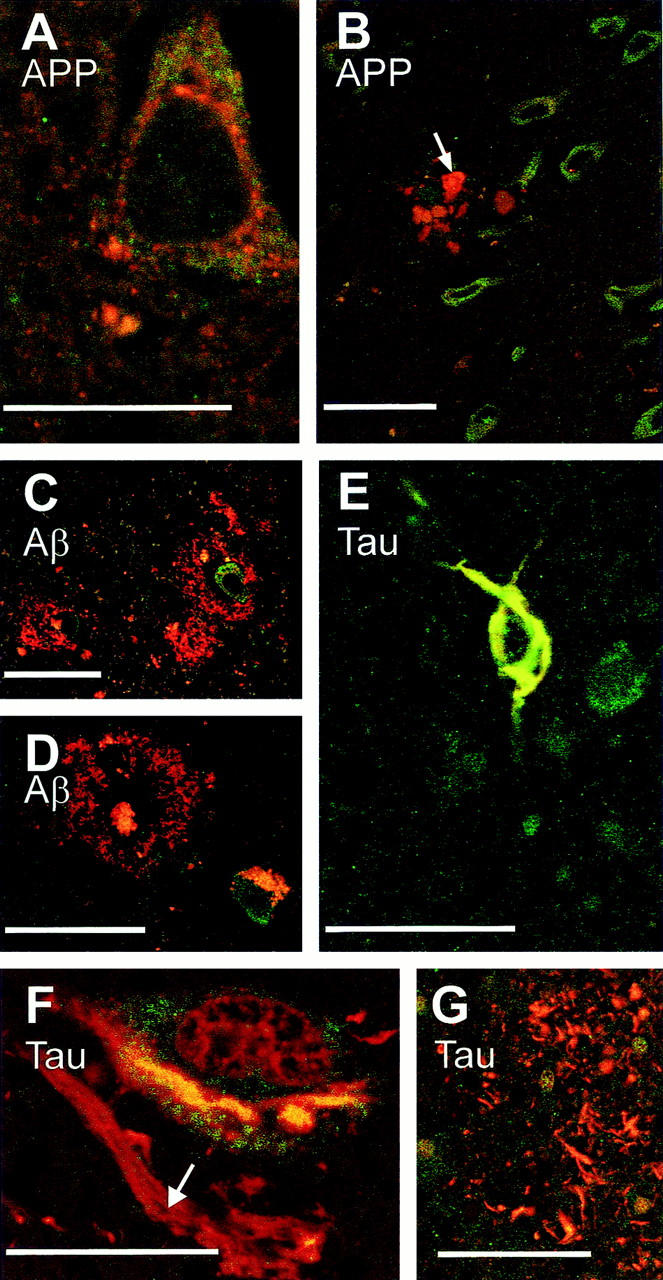
Immunofluorescence of FE65, APP, β-amyloid, and tau proteins. Double-immunofluorescent labeling (green, FE65; red, APP, β-amyloid, or tau) did not reveal co-localization of FE65 and APP in the cell body of neurons (A) nor was FE65 detected in APP-positive dilated neurites surrounding plaques (B, arrow indicates dilated neurites; note the vicinity of numerous green labeled FE65 immunopositive neurons), in diffuse β-amyloid deposits (C) and classical senile plaques (D, note that, in this material that was not treated with Sudan black, lipofuscine accumulated in a neuron). Double-tau-FE65 labeling underlines a co-localization of these two proteins in neurofibrillary tangles, an example of which is shown in E. FE65 and tau, however, were not co-localized in other neurofibrillary alterations (F, ghost tangle indicated by an arrow in the neighborhood of a neuron bearing a yellow double-labeled tangle; G, neurites of the senile crown). Scale bars, 25 μm (A and F) and 50 μm (B–E and G).
A series of double-staining experiments was conducted to determine whether FE65 was present in β-amyloid deposits. Diffuse amyloid deposits as well as dense core plaques were found to be free of FE65 immunoreactivity (Figure 2, C and D) ▶ .
Finally we looked for an association of FE65 and tau protein in neurofibrillary alterations. That possibility was raised when we found, in single-labeled material, that some positively immunostained FE65 neurons had the typical morphology of cells with neurofibrillary tangles. Double immunostaining using the immunoperoxidase method confirmed that some neurons contained both FE65 and tau in their cytoplasm (not illustrated). To determine whether the two proteins were co-localized in such neurons, immunofluorescent double stainings were subsequently performed and examined with confocal microscopy. We observed, in Sudan black-pretreated material, that tau and FE65 co-localized in most of the neurofibrillary tangles that were observed (Figure 2E) ▶ . Such a result was not because of an optical cross-talk between the two fluorophores (cyanine 2 and 3) as co-localization between tau and FE65 was only observed in neurofibrillary tangles located in cell bodies but not in ghost tangles (Figure 2F) ▶ nor in tau-positive neurites present at the periphery of senile plaques (Figure 2G) ▶ .
Relationships between FE65 and Neurofibrillary Tangles
To establish the presence of FE65 on neurofibrillary tangles, an ultrastructural study of FE65-immunostained material was conducted. FE65 immunoreactivity was found in cytosolic cellular compartments and labeling was often associated with lysosomal complexes (not illustrated). As shown in Figure 3 ▶ , FE65-positive immunostaining was also detected on some paired helical filaments.
Figure 3.
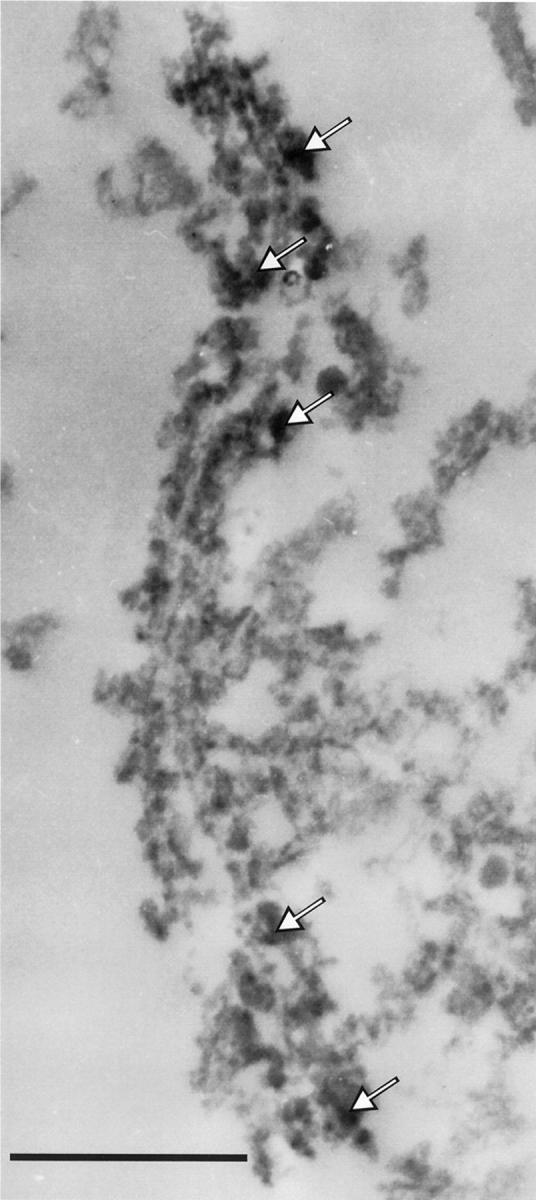
Immunocytochemistry of FE65 and neurofibrillary tangles. FE65-immunostained material (avidin-biotin-peroxidase method) was analyzed at the ultrastructural level. Electron microscopy observations showed that in regions rich in neurofibrillary tangles, FE65 is associated with paired helical filaments (examples of diaminobenzidine precipitates are indicated by arrows). Scale bar, 500 nm.
The relationship between FE65 and tau immunoreactivities was further explored using correlation analysis. The proportion of volume occupied by intracellular neurofibrillary tangles (the only tau-positive lesions that we found to be associated with FE65) was, as expected, increased in AD cases as compared to normal patients [CA4: t(10) = 8.345; P < 0.001; supramarginal gyrus: t(10) = 2.697; P < 0.05]. However no significant correlations were observed between the proportions of volume occupied by FE65-positive neurons and by tau immunostained intracellular tangles, either in the CA4 sector (r = 0.565; ns) or in the isocortex (r = 0.576; ns).
Discussion
The first aim of this study was to determine the immunohistochemical distribution of FE65 in the human central nervous system. Dense FE65-immunostaining material was mainly observed in the cytoplasm of neurons. Recent work of Sabo and collaborators 10 has also underlined the presence of FE65 in cytoplasmic organelles of MDCK and H4 human neuroglioma cells and we made similar observations in PC12 clones expressing FE65 (unpublished observations).
FE65 immunoreactivity was principally distributed in neurons in isocortical and hippocampal samples. No clear evidence supported the contention that FE65 immunoreactivity could be affected in the isocortex during the process of AD as no difference was observed between demented and age-matched control cases. However, in the hippocampus, a region early affected in AD patients, increased FE65 immunoreactivity seemed to be associated with the severity of histological alterations. First, the proportion of FE65 immunoreactivity tended to be higher in AD patients than in control cases. This trend did not reach statistical significance, a result that could be because of the small number of patients analyzed or, conversely, to the fact that AD cases were heterogeneous in terms of Braak’s stages. This last hypothesis gained support from the double evidence that 1) stage VI cases had a significantly increased hippocampal FE65 immunoreactivity when compared to control cases; and 2) stage IV patients had a significantly lower amount of FE65 immunoreactivity in the hippocampus than cases with advanced pathology. Increased FE65 immunoreactivity in AD patients with severe histopathological lesions could not be accounted either by atrophy of the CA4 sector (as the surface area of this hippocampal field was similar in AD patients and normal age-matched cases) or by neuronal loss (which is known, in this area, to be limited in AD, 19 and would have on the contrary decreased FE65 immunoreactivity).
An increase in FE65 mRNA level in human nervous tissue has recently been reported to be associated with AD. 16 Hu and colleagues, 16 using reverse transcriptase-competitive polymerase chain reaction, showed that FE65 mRNA was increased in regions described as being free of classical AD lesions (cerebellum, caudate nucleus). The same authors suggested that a similar increase in mRNA levels could also occur in AD-related cerebral regions before neuronal loss. A definite answer to that question would certainly be given by further immunohistochemical investigations involving larger cohorts of patients with different pathological status (from early stages to advanced AD pathology). Hu and colleagues 16 also showed that the level of neuronal FE65 mRNA was decreased in cortical samples, a result that contrasts with our own data indicating no significant alteration of FE65 immunoreactivity in the isocortex. This discrepancy could be explained by the difference in methods used (reverse transcriptase-polymerase chain reaction versus immunohistochemistry). Additionally, cortical samples were not taken from the same brain regions in the two studies (frontal and temporal cortices for Hu and collaborators 16 versus supramarginal gyrus in the present study).
The second aim of the present study was to characterize, in human nervous tissue, the association of FE65 with proteins classically described as being involved in AD. Recent studies have reported an association of FE65 and APP in cell culture models, 10 demonstrating co-localization of the two proteins in juxta-nuclear organelles (endoplasmic reticulum, Golgi apparatus, endosomes). Such an association was predicted on the basis of the existence of an interaction between the C-terminal phosphotyrosine-binding domain (PTB2) of FE65 and the APP cytoplasmic domain. 4-8 However, we failed to demonstrate a clear co-localization of FE65 and APP in human tissue samples, either in the cell body of the neurons or in the dilated neurites of the senile plaques. Some explanations may be proposed to account for these divergent results. It is possible that fixation used for autopsy material interferes with the integrity of the FE65-APP complex or alternatively one could suspect that FE65-APP interaction and resulting co-localization is a brief phenomenon that could not be demonstrated in our experimental conditions. Our results furthermore indicate that FE65 and the APP-derived β-amyloid peptide are also not co-localized in human nervous tissue. This observation was noted for all β-amyloid deposits (diffuse and focal deposits) for which no clear FE65 immunoreactivity was detected. Interestingly McLoughlin and collaborators 26 have recently demonstrated that X11, another protein that interacts with the cytoplasmic domain of APP, is also not detectable in the core of amyloid deposits. The same study however revealed that some neurites of the senile plaques are X11-positive, raising the possibility that FE65 and X11, two proteins that have opposite roles onto β-amyloid secretion, 27 might also differ in their degree of association with amyloid proteins in the human brain.
Finally, we demonstrated the unexpected co-localization of FE65 and tau proteins. First it appeared that neurons presenting the profile of cells with neurofibrillary tangles were FE65-positive. Further investigations using double-immunofluorescent labeling analyzed by means of confocal microscopy showed a tight co-localization of FE65 and tau in most of the intracellular tangles, suggesting the association of FE65 with AD-related cytoskeletal alterations. Ultrastructural observations confirmed that FE65 is closely associated with paired helical filaments. It is however noteworthy that FE65-tau co-localization was only observed in cell body neurofibrillary alterations and not in other tau-positive lesions (ghost tangles, dystrophic neurites), underlining the specificity of the interaction between these two proteins.
Although FE65 was co-localized with intracellular neurofibrillary tangles, there was no correlation between the proportions of volume occupied by tau and FE65 immunoreactivities. Our observations showed that FE65 was present in the cytoplasm of neurons with neurofibrillary tangles as in neurons free of them. Neurofibrillary tangles did not modify the proportion of volume occupied by FE65 immunoreactivity, and, therefore, did not correlate with it. We concluded that the increase in FE65 immunoreactivity did not result from a parallel increase in the number of tau-positive tangles but might only reflect an up-regulation of FE65 during the disease process.
Some evidence supports the contention that FE65, besides its relationships with APP, could act, directly and/or indirectly, on the cytoskeletal organization. We have recently found that stable expression of FE65 in differentiated PC12 cells induced cytoskeletal alterations that included modulations of the expression of phosphorylated neurofilaments as well as tau (unpublished observations). The role of FE65 on cytoskeletal elements is substantiated by the demonstration that this protein interacts via its WW domain with Mena, the mammalian homologue of the Drosophila-enabled protein, 28 which is known to participate to cytoskeleton dynamics through regulation of actin polymerization. 29 Sabo and collaborators 11 have recently demonstrated that FE65 and Mena are co-localized in growth cones and that this protein complex could participate to cone morphology and function. It has also been reported that the promoter of GSK-3β, a kinase involved in the phosphorylation of tau, could be indirectly controlled by FE65. 30,31 All these data strongly suggest that FE65 plays an active role (direct and/or indirect at the transcriptional level) in the modulation of cytoskeletal organization.
In summary, we gave some evidence that FE65 is a protein that could be crucially involved in the biochemical cascades that lead to AD. First, our results suggest that FE65 hippocampal immunoreactivity is modulated during the course of the disease. These preliminary observations will require further investigations to be more firmly established. Second, we demonstrated that FE65 and tau protein are associated in neurofibrillary tangles. This last result, added to the fact that FE65-APP complexes have been described in cell cultures models, suggest that FE65 is a key candidate to play the role of missing link between amyloid and neurofibrillary alterations that together constitute the main histopathological lesions of Alzheimer’s dementia.
Acknowledgments
We thank J.-J. Hauw for continuous support and advice; A. Delacourte for providing the AD2 antibody; C. Nze for expert technical help on confocal microscope hardware/software management; and the technical staff of the Escourolle Laboratory for stimulating discussion and experimental guidance.
Footnotes
Address reprint requests to Pr. Charles Duyckaerts, Laboratoire de Neuropathologie, Université Paris VI, Hôpital La Salpêtrière, 47 Bd de l’Hôpital, 75651 Paris, Cedex 13, France. E-mail: charles.duyckaerts@psl.ap-hop-paris.fr.
Supported by the “Réseau de recherche Alzheimer” (Aventis Pharma).
References
- 1.Lai A, Sisodia SS, Trowbridge IS: Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. J Biol Chem 1995, 270:3565-3573 [PubMed] [Google Scholar]
- 2.McLoughlin DM, Irving NG, Miller CCJ: The Fe65 and X11 families of proteins: proteins that interact with the Alzheimer’s disease amyloid precursor protein. Biochem Soc Trans 1998, 26:497-500 [DOI] [PubMed] [Google Scholar]
- 3.Russo T, Faraonio R, Minopoli G, De Candia P, De Renzis S, Zambrano N: Fe65 and the protein network centered around the cytosolic domain of the Alzheimer’s β-amyloid precursor protein. FEBS Lett 1998, 434:1-7 [DOI] [PubMed] [Google Scholar]
- 4.Fiore F, Zambrano N, Minopoli G, Donini V, Duilio A, Russo T: The regions of Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of the Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J Biol Chem 1995, 270:30853-30856 [DOI] [PubMed] [Google Scholar]
- 5.Bressler SL, Gray MD, Sopher BL, Hu Q, Hearn MG, Pham DG, Dinulos MB, Fukuchi K-I, Sisodia SS, Miller MA, Disteche CM, Martin GM: cDNA cloning and chromosome mapping of the human Fe65 gene: interaction of the conserved cytoplasmic domains of the human β-amyloid precursor protein and its homologues with the mouse Fe65 protein. Hum Mol Genet 1996, 5:1589-1598 [DOI] [PubMed] [Google Scholar]
- 6.Guénette SY, Chen J, Jondro PD, Tanzi RE: Association of a novel human Fe65-like protein with the cytoplasmic domain of the β-amyloid precursor protein. Proc Natl Acad Sci USA 1996, 93:10832-10837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLoughlin DM, Miller CC: The intracellular cytoplasmic domain of the Alzheimer’s disease amyloid precursor protein interacts with phosphotyrosine-binding domain proteins in the yeast two-hybrid system. FEBS Lett 1996, 397:197-200 [DOI] [PubMed] [Google Scholar]
- 8.Mercken L, Bock M, Pradier L, Franco X, Paul MF, Menager J, Fournier A: FE65 et COFE65: Deux Protéines Interagissant avec le Domaine Cytoplasmique de l’APP, Actualités sur la Maladie d’Alzheimer et les Syndromes Apparentés. Edited by M-C Gely-Nargeot, K Ritchie, J Touchon. Marseille, Solal, 1998, pp. 73–79>
- 9.Simeone A, Duilio A, Fiore F, Acampora D, De Felice C, Faraonio R, Paolocci F, Cimino F, Russo T: Expression of the neuron-specific FE65 gene marks the development of embryo ganglionic derivatives. Dev Neurosci 1994, 16:53-60 [DOI] [PubMed] [Google Scholar]
- 10.Sabo SL, Lanier LM, Ikin AF, Khorkova O, Sahasrabudhe S, Greengard P, Buxhaum JD: Regulation of β-amyloid secretion by Fe65, an amyloid protein precursor-binding protein. J Biol Chem 1999, 274:7952-7957 [DOI] [PubMed] [Google Scholar]
- 11.Sabo SL, Ikin AF, Buxbaum JD, Greengard P: Localization of APP and Fe65 in growth cones: possible role in membrane motility. Neurosci Abstr 1999, 25:1805 [Google Scholar]
- 12.Hu Q, Kukull WA, Bressler SL, Gray MD, Cam JA, Larson EB, Martin GM, Deeb SS: The human Fe65 gene: genomic structure and an intronic biallelic polymorphism associated with sporadic dementia of the Alzheimer type. Hum Genet 1998, 103:295-303 [DOI] [PubMed] [Google Scholar]
- 13.Lambert JC, Mann D, Goumidi L, Harris J, Pasquier F, Frigard B, Cottel D, Lendon C, Iwatsubo T, Amouyel P, Chartier-Harlin MC: A FE65 polymorphism associated with risk of developing sporadic late-onset Alzheimer’s disease but no risk with Aβ loading in brains. Neurosc Lett 2000, 293:29-32 [DOI] [PubMed] [Google Scholar]
- 14.Guénette SY, Bertram L, Crystal A, Bakondi B, Hyman BT, Rebeck GW, Tanzi RE, Blacker D: Evidence against association of the FE65 gene (APBB1) intron 13 polymorphism in Alzheimer’s patients. Neurosci Lett 2000, 296:17-20 [DOI] [PubMed] [Google Scholar]
- 15.Papassotiropoulos A, Bagli M, Becker K, Jessen F, Maier W, Rao ML, Ludwig M, Heun R: No association between an intronic biallelic polymorphism of the FE65 gene and Alzheimer’s disease. Int J Mol Med 2000, 6:587-589 [DOI] [PubMed] [Google Scholar]
- 16.Hu Q, Jin LW, Starbuck MY, Martin GM: Broadly altered expression of the mRNA isoforms of FE65, a facilitator of beta amyloidogenesis, in Alzheimer cerebellum and other brain regions. J Neurosci Res 2000, 60:73-86 [DOI] [PubMed] [Google Scholar]
- 17.Buee-Scherrer V, Condamines O, Mourton-Gilles C, Jakes R, Goedert M, Pau B, Delacourte A: AD2, a phosphorylation-dependent monoclonal antibody directed against tau proteins found in Alzheimer’s disease. Brain Res Mol Brain Res 1996, 39:79-88 [DOI] [PubMed] [Google Scholar]
- 18.Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, Hansen L, Hyman B, Jellinger K, Markesberg W, Perl D, Powers J, Price J, Trojanowski JQ, Wisniewski H, Phelps C, Khachaturian Z: Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging 1997, 18:S1-S2 [PubMed] [Google Scholar]
- 19.West MJ, Coleman PD, Flood DG, Troncoso JC: Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet 1994, 344:769-772 [DOI] [PubMed] [Google Scholar]
- 20.Delesse MA: Procédé mécanique pour déterminer la composition des roches. C R Acad Sci 1847, 25:544-545 [Google Scholar]
- 21.Weibel ER: Stereological methods. Practical Methods for Biological Morphometry. 1979, Academic Press, London
- 22.Duyckaerts C, Hauw JJ, Bastenaire F, Piette F, Poulain C, Rainsard V, Javoy-Agid F, Berthaux P: Laminar distribution of neocortical senile plaques in senile dementia of the Alzheimer type. Acta Neuropathol 1986, 70:249-256 [DOI] [PubMed] [Google Scholar]
- 23.Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW: Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem 1999, 47:229-236 [DOI] [PubMed] [Google Scholar]
- 24.Schnell SA, Staines WA, Wessendorf MW: Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 1999, 47:719-730 [DOI] [PubMed] [Google Scholar]
- 25.Priestly JV, Cuello AC: Electron Microscopic Immunohistochemistry for CNS Transmitters and Transmitters Markers, Immunohistochemistry. 1983:pp 273-322 Wiley, Edited by AC Cuello. New York
- 26.McLoughlin DM, Irving NG, Brownlees J, Brion JP, Leroy K, Miller CC: Mint2/X11-like colocalizes with the Alzheimer’s disease amyloid precursor protein and is associated with neuritic plaques in Alzheimer’s disease. Eur J Neurosci 1999, 11:1988-1994 [DOI] [PubMed] [Google Scholar]
- 27.Sastre M, Turner RS, Levy E: X11 interaction with β-amyloid precursor protein modulates its cellular stabilization and reduces amyloid β-protein secretion. J Biol Chem 1998, 273:22351-22357 [DOI] [PubMed] [Google Scholar]
- 28.Ermekova KS, Zambrano N, Linn H, Minopoli G, Gertler F, Russo T, Sudol M: The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J Biol Chem 1997, 272:32869-32877 [DOI] [PubMed] [Google Scholar]
- 29.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P: Mena, a relative of VASP and Drosophila enabled, is implicated in the control of microfilament dynamics. Cell 1996, 87:227-239 [DOI] [PubMed] [Google Scholar]
- 30.Lau KF, Miller CC, Anderton BH, Shaw PC: Molecular cloning and characterization of the human glycogen synthase kinase-3beta promoter. Genomics 1999, 60:121-128 [DOI] [PubMed] [Google Scholar]
- 31.Lambert JC, Goumidi L, Vrieze FW, Frigard B, Harris JM, Cummings A, Coates J, Pasquier F, Cottel D, Gaillac M, St Clair D, Mann DM, Hardy J, Lendon CL, Amouyel P, Chartier-Harlin MC: The transcriptional factor LBP-1c/CP2/LSF gene on chromosome 12 is a genetic determinant of Alzheimer’s disease. Hum Mol Genet 2000, 9:2275-2280 [DOI] [PubMed] [Google Scholar]