Abstract
Cutaneous lymphomas of the cytotoxic phenotype, including CD8+ and CD56+ lymphomas, have only recently been recognized. To characterize the phenotypic profile of these lymphomas, we investigated the expression of both cytotoxic molecules and killer cell inhibitory receptors by immunohistochemistry techniques. Frozen sections from four CD8+ and from three CD56+ cutaneous lymphomas were stained for the cytotoxicity markers including T-cell restricted intracellular antigen-1, perforin, granzyme B, and for expression of the inhibitory receptors including p58.1, p58.2, p70, p140, CD94, NKG2, and leukocyte immunoglobulin-like receptor (LIR-1). Apart from LIR-1, the CD8+ lymphomas in our series express p70 and p140 from the inhibitory receptors and only one or two of the cytotoxic proteins. The CD56+ lymphomas, on the other hand, express only LIR-1 of the set of inhibitory receptors and the whole panel of cytotoxic antigens. Various subtypes of cytotoxic cutaneous lymphomas (CD8+ and CD56+) differ in regard to their phenotypic and functional profile, which may explain differences in their biological behavior.
The majority of cutaneous lymphomas belong to the groups of B-cell lymphomas or of T-cell lymphomas with T-helper phenotype. In recent years cutaneous lymphomas of cytotoxic phenotype have been recognized. 1-4 In those cases the malignant cells may have either a T-cytotoxic (CD8+) or a natural killer (NK)/T-cell (CD56+) phenotype.
NK cells and cytotoxic T cells are characterized by the presence of azurophilic cytoplasmic granules. These granules contain cytotoxic proteins, such as T-cell-restricted intracellular antigen (TIA-1), granzyme B, and perforin. TIA-1 induces apoptosis through fragmentation of the DNA. This protein is found independently of the state of activation of the cells and characteristically shows a granular, cytoplasmic staining in immunohistochemistry. 5 Granzyme B, member of the serine protease family, is directly involved in the lytic activity. Through activation of the proapoptotic cascade of caspases it promotes DNA fragmentation. 6 Additionally cell death can be triggered by granzyme B independently of caspase activation, through nonnuclear mechanisms. 7 Unlike TIA-1, granzyme B is only found in activated cytotoxic lymphocytes. Perforin is another molecule directly involved in the cascade of cytotoxic cell death. On granule release, perforin monomers insert into the plasma membranes of target cells and polymerize into pore-forming aggregates. 8 These perforin pores may lead to osmotic lysis of the target cells and also allow granzymes to come in contact with the cytosol of the target cell and induce apoptosis. 9
NK cells and T lymphocytes share various cell surface receptors. For example CD2, CD11c, CD57, CD69, and CD122 are expressed by both NK and T cells. Recently NK-receptors recognizing MHC class I molecules have been identified. These include killer cell immunoglobulin-like receptors (KIRs) and lectin-like dimers, which are composed of CD94 associated with NKG2 molecules. 10 Recognition of class HLA class I molecules on target cells by KIRs blocks the natural and the antibody-dependent cell cytotoxicity in NK cells and the CD3/T-cell receptor-dependent cytotoxicity of T cells. 11 Other receptors, virtually identical in their extracellular part to KIR, but possessing only a short intracytoplasmic tail lacking intracytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs) can deliver activation signals to cytotoxic cells. 12 KIRs and CD94/NKG2 are transmembrane glycoproteins that undergo phosphorylation in a tyrosine residue in the two tandem ITIMs that bind various phosphatases to induce dominant-negative signals. 13 They allow cytotoxic cells to distinguish normal cells from cells with impaired expression of HLA class I molecules. KIRs include receptors recognizing HLA-C molecules (p58.1 and p58.2), 14 HLA-B molecules (p70), 15 or HLA-A (p140). 16 KIRs have been detected not only on NK cells, but also on subsets of T cells. Covalently bound CD94 and NKG2 form heterodimers that are expressed by the majority of NK cells, and belong to the superfamily of C-type lectins. CD94/NKG2A recognizes HLA-E, a nonclassical HLA-class I molecule, the gene for which is transcribed in most tissues. 17 It provides an inhibitory signal, thus ensuring a broad spectrum protection against NK-mediated cell lysis. Leukocyte immunoglobulin-like receptor (LIR)-1 is a member of the immunoglobulin superfamily, which is expressed on the majority of B cells, monocytes, and dendritic cells and a small subset of NK and T cells. Unlike KIRs, which are allele-specific, LIR-1 binds to a broad range of classical and nonclassical class I molecules. 18
In this study we assessed by immunohistochemistry the phenotype of cutaneous lymphomas of cytotoxic phenotype (four cases of CD8+ T-cell and three CD56+ lymphomas), by analyzing TIA-1, granzyme B, perforin, as well as KIRs, CD94/NKG2, and LIR-1.
Materials and Methods
Patients
Criteria for inclusion in the study were: 1) diagnosis of a lymphoproliferative disorder of cytotoxic phenotype; 2) initial presentation in the skin; 3) polymerase chain reaction evidence of TCR rearrangement by the CD8+ lymphomas; and 4) absence of immunosuppression, HIV-associated or iatrogenic. Seven patients, registered in the Lymphoma Registry of the Department of Dermatology in Zürich, met those criteria and were included in the study. Three of them had been diagnosed with CD56+ NK/T cutaneous lymphomas, and four had CD8+ cytotoxic primary cutaneous T-cell lymphomas.
Immunohistochemistry
Both paraffin-embedded and frozen material were available from all seven patients. Immunohistochemical stainings were performed with monoclonal IgG mouse antibodies specifically binding human CD2, CD3, CD4, CD5, CD7, CD8, CD16, CD30, CD34, CD43, CD45Ro, CD56, CD68, CD79a, MAC387, chloroacetate, and Ki67. The specimens were additionally examined for TIA-1 (Coulter Clone; Coulter Co., Hialeah, FL), granzyme B (Serotec, Oxford, UK) and perforin (Endogen, Woburn, MA) molecules. Stainings were also performed with monoclonal antibodies recognizing the NK receptors p58.1 (EB6), p58.2 (GL183), p70 (Z27), p140 (Q66), NKG2A (Z270), CD94 (XA185), and LIR-1/ILT2 (F278), from the Advanced Biotechnology Center (Genova, Italy) (Table 1) ▶ .
Table 1.
Monoclonal Antibodies Detecting Inhibitory Receptors Used in the Study
| Reactive mAbs | Isotype | Putative ligands | Function | Protein denomination |
|---|---|---|---|---|
| E B6 | IgG1 | HLA-Cw2,-Cw4,-Cw5,-Cw6 | Inhibitory | p58.1 |
| GL183 | IgG1 | HLA-Cw1,-Cw3,-Cw7 | Inhibitory | p58.1 |
| Z27 | IgG1 | HLA-Bw4 | Inhibitory | p70 |
| Q66 | IgM | HLA-A3/-A11 | Inhibitory | p140 |
| F278 | IgG1 | HLA-A,-B,-C,-G | Inhibitory | LIR-1 |
| Z270 | IgG1 | HLA-E | Inhibitory | CD94/NKG2 |
| XA185 | IgG1 |
Immunohistochemistry was performed by the alkaline-phosphatase anti-alkaline phosphatase technique after antigen retrieval, as previously reported.
Briefly, 3- to 5-μm-thick tissue sections adherent to slides coated with 0.1% (w/v) poly-l-lysine were deparaffinized with xylene and rehydrated. Antigen retrieval was performed by microwave pretreatment (ethylenediaminetetraacetic acid buffer, pH 8.0), initially for 2 minutes at 600 W and then three times for 5 minutes at 100 W. Nonspecific binding sites were blocked by incubating slides with normal rabbit serum for 15 minutes at room temperature. Tissue sections were incubated with an excess of mAb for 60 minutes. This was followed by three cycles of sequential incubations with rabbit anti-mouse IgG xenoantibodies and alkaline-phosphatase anti-alkaline phosphatase complexes. In the first cycle the incubations were of 30-minutes duration, in the second and the third they were 10 minutes each. All of the incubations were performed at room temperature in a moist chamber. The immunoreaction was visualized with a developing solution, containing neufuchsin (DAKO, Glostrup, Denmark). Finally, sections were counterstained with 1% hematoxylin.
In Situ Hybridization
All specimens were investigated for Epstein-Barr virus by in situ hybridization detecting Epstein-Barr virus-encoded RNA (DAKO).
Results
Four patients (one male, three females) had CD8+ primary cutaneous lymphomas and three (two males, one female) had CD56+ cutaneous lymphomas. Clinical data concerning the onset, type of lesions, course, therapy, and follow-up are presented in Table 2 ▶ .
Table 2.
Clinical Data Concerning Onset, Type of Lesions, Course, Therapy, and Follow-Up
| Case | Gender/ age | Initial stage | Time to progression to stage IV | Type of lesions | Skin involvement | Treatment | Progression | Outcome follow-up |
|---|---|---|---|---|---|---|---|---|
| CD56+-I | F /39 | IVb | Not applicable | Patches, plaques, tumors, hemorrhagic lesions | Widespread (head, body, extremities) | Prednisone, radiotherapy, EPOCH, Ifosfamid/Ara-C/VP-16, CHOP, CVB, caelyx | Skin, oral mucosa, systemic involvement (bone marrow) | Alive with disease (36 months) |
| CD56+-II | M /70 | IIb | 9 months | Plaques | Right breast, right shoulder, chin | Excision, radiotherapy, IFN-α | Skin, lymph nodes, systemic involvement (bone marrow) | Alive with disease (36 months) |
| CD56+-III | F /49 | IIb | 12 months | Patches, plaques, tumors, hemorrhagic lesions | Wide spread (head, trunk, extremities) | Radiotherapy, INF-α,+ PUVA, CHOP, LVP, CEOP, VACOP B | Skin, oral mucosa, lymph nodes, systemic involvement (bone marrow, CNS) | Dead from disease (27 months) |
| CD8+-I | F /66 | Ib | No progression | Patches | Widespread | UVB-phototherapy | Skin | Alive with disease (60 months) |
| CD8+-II | M /36 | Ib | No progression | Patches, plaques | Thighs | Topical hexadecyl-phosphocholine, Elocom-creme, PUVA | Skin | Alive with disease (72 months) |
| CD8+-III | M /42 | Ib | No progression | Patches, papules | Widespread | Neotigason, topical steroids, PUVA | Skin | Alive with disease (30 months) |
| CD8+-IV | F /45 | Ia | No progression | Patches | Widespread (trunk, thighs) | No | Skin | Alive with disease (6 months) |
EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; Ifosfamid/Ara-C/VP16, ifosfamid, cytarabine, etoposide; CVB, cyclophosphamide, etoposide, carmustine; LVP, l-asparginase, vincristine, prednisone; CEOP, cyclophosphamide, epirubicin, vincristine, prednisone; VACOP-B, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin.
Detailed results of the immunohistochemical analysis are presented in Table 3 ▶ . The NK/T lymphomas showed a persistent expression of CD4, CD43, and CD56. Most of the other T-lymphocyte markers, including CD2, CD3, CD5, CD7, CD8, CD30, and NK cell markers such as CD16 and CD57 were negative. Myelomonocytic cell markers (CD68 and myeloperoxidase) were also negative. The phenotype of the CD8+ primary cutaneous T-cell lymphomas was characterized by the expression of CD2, CD3, CD8, and CD43. CD4, CD5, CD7, CD16, CD30, CD34, CD45Ro, CD56, CD57, CD68, CD79a, MAC383, and chloroacetate were negative. The proliferation rate as estimated by the Ki67 stain was definitely lower (20%, three of four; 10%, one of four) than that of the CD56+ lymphomas (90%, two of three; 50%, one of three). In situ hybridization failed to detect Epstein-Barr virus in all of the seven specimens.
Table 3.
Immunohistochemistry Results
| Case no. | NK lymphomas | CD8+ lymphomas | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | IV | |
| CD2 | − | − | − | − | + | + | − |
| CD3 | − | − | − | + | + | + | + |
| CD4 | + | + | + | − | − | − | − |
| CD5 | − | − | − | − | − | − | − |
| CD7 | − | − | − | − | − | − | − |
| CD8 | − | − | − | + | + | + | + |
| CD16 | − | − | − | nd | nd | nd | nd |
| CD30 | − | − | − | − | − | − | − |
| CD34 | − | − | − | − | − | − | − |
| CD43 | + | + | + | + | + | + | + |
| CD45Ro | − | − | − | − | − | − | − |
| CD56 | + | + | + | − | − | − | − |
| CD57 | − | − | − | − | − | − | − |
| CD68 | − | − | − | − | − | − | − |
| CD79a | − | − | − | − | − | − | − |
| MAC383 | − | − | − | − | − | − | − |
| Chloroac | − | − | − | − | − | − | − |
| Ki67 | 90% | 90% | 50% | 20% | 20% | 20% | 10% |
| TIA1 | + | + | + | + | + | + | + |
| Perforin | + | + | + | − | + | + | − |
| Granz. B | + | + | + | + | − | + | + |
| P70 | − | − | − | + | − | + | + |
| P140 | − | − | − | + | + | + | + |
| NKG2A | − | − | − | − | − | − | − |
| LIR1 | + | + | + | + | + | + | + |
| CD94 | − | − | − | − | − | − | − |
| P58.1 | − | − | − | − | − | − | − |
| P58.2 | − | − | − | − | − | − | − |
Regarding the markers of cytotoxicity, TIA-1, perforin, and granzyme B were expressed in all three CD56+ lymphomas. TIA-1 was expressed also in all four CD8+ lymphomas. Two of the four CD8+ lymphomas expressed perforin and three expressed granzyme B. Thus although all CD56+ lymphomas expressed the whole set of cytotoxic molecules, only one of four CD8+ lymphomas (case III), expressed simultaneously TIA-1, perforin, and granzyme B.
There was a major difference between CD56+ NK/T lymphomas and CD8+ lymphomas concerning the expression of KIRs. Expression of p70 was detected in three of four CD8+ lymphomas, but not in CD56+ lymphomas. Also, p140 was expressed in all four CD8+ lymphomas, whereas it was absent in all CD56+ lymphomas. LIR-1 was detected in all specimens analyzed. In contrast, no expression of p58.1, p58.2, CD94, and NKG2 could be detected (Figure 1) ▶ .
Figure 1.
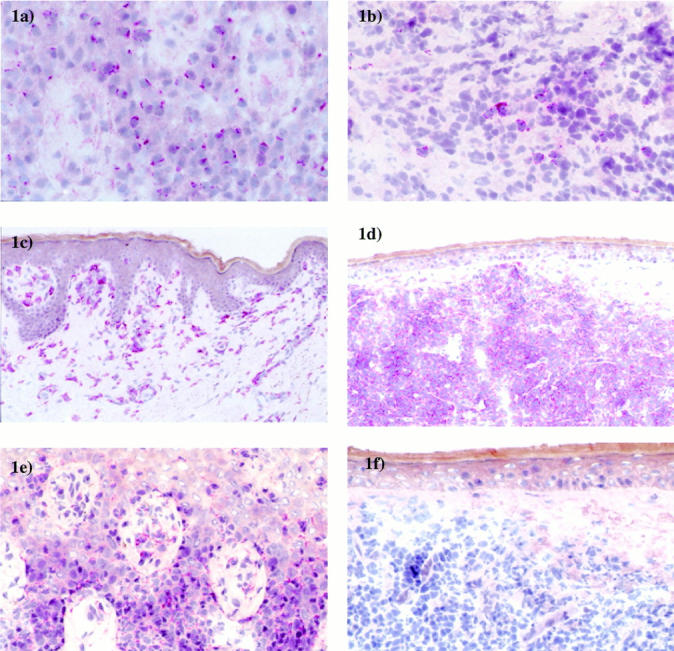
a: Positive TIA-1 staining in a CD8+ primary cutaneous lymphoma. b: Positive TIA-1 staining in a CD56+ cutaneous lymphoma. c: Positive LIR-1 staining in a CD8+ primary cutaneous lymphoma. d: Positive LIR-1 staining in a CD56+ cutaneous lymphoma. e: Positive p70 staining in a CD8+ primary cutaneous lymphoma. f: Negative p70 staining in a CD56+ cutaneous lymphoma. Original magnifications: ×63 (a and b), ×20 (c and d), ×40 (e and f).
Discussion
Both CD8+ primary cutaneous T-cell lymphomas and CD56+ NK/T-cell lymphomas represent rare subtypes of cutaneous lymphomas that have only recently been identified. The number of reported cases is small and the phenotype was poorly characterized. In this study by the use of immunohistochemistry we investigated seven cases retrieved from the registry of cutaneous lymphomas at the Department of Dermatology in Zürich with particular accent on cytotoxic markers and NK cell inhibitory receptors.
The data available in the literature regarding the expression of cytotoxicity markers are contradictory. Thus, Petrella and colleagues 19 failed to detect TIA-1 or granzyme B in all seven cases of CD4+CD56+ cutaneous lymphomas analyzed. Similar results were reported by Bagot and colleagues 20 in a single case described. In contrast, Takeshita and colleagues 21 could detect expression of TIA-1, granzyme B, and perforin in 10 cases of cutaneous CD56+ NK/T cell lymphomas with angiodestruction and only weak expression of granzyme B and perforin in six cases without angiodestruction. Berti and colleagues 22 described 17 cases of CD8+ primary cutaneous T-cell lymphomas, 15 of which expressed TIA-1. Five of the investigated specimens were additionally found to express perforin and granzyme B. In our present study TIA-1 was detected in all lesions analyzed, including CD56+ and CD8+ lymphomas. Two of three CD56+ lymphomas expressed also perforin and granzyme B. At least two of them displayed features typical of angiodestruction. One of the CD8+ lymphomas in our study weakly expressed two cytotoxic molecules, one case expressed only granzyme B, and one only perforin.
Expression of TIA-1 is characteristic of cytotoxic cells regardless of their activation status, whereas expression of both perforin and granzyme B is up-regulated in activated cytotoxic cells and correlates with the levels of cytolytic activity.
Also the expression, or lack thereof, of HLA-class I-specific inhibitory receptors displays distinguishing features in CD56+ or CD8+ lymphomas. All specimens analyzed were negative for p58.1, 58.2, CD94, and NKG2, but expressed strongly LIR-1. The CD8+ lymphomas expressed in addition to LIR-1, also p70 (three of four) and p140 (four of four). Adversely, these receptors were not expressed by the CD56+ lymphomas.
LIR-1 is a member of the Ig superfamily, which has been shown to bind the human cytomegalovirus HLA-class I homologue UL-18 protein. Vitale et al 18 further showed that LIR-1 can function as a low affinity receptor for HLA-class I molecules, recognizing different HLA alleles, coded for by different HLA loci. It has been shown that LIR-1 interacts with the relatively nonpolymorphic α3 domain of class I proteins and the analogous region of UL-18 using its N-terminal immunoglobulin-like domain. LIR-1 recognition of class I molecules resembles the CD4-HLA-class II interaction more than the KIR-HLA-class I interaction, implying a functional distinction between LIR-1 and KIRs. Normally, LIR-1 is expressed on the majority of B cells, monocytes, and dendritic cells, but only on a small subset of normal NK and T cells. 23 The presence of LIR-1 in all investigated lesions of both CD8+ and in CD56+ lymphomas suggests that either LIR-1-positive cell populations carry a higher risk for malignant transformation, or that LIR-1 expression is associated to a growth/survival advantage to the dominant clonal cells. In contrast, p70 and p140 are selectively found in CD8+ lymphomas. In view of the high affinity of these inhibitory receptors for HLA-class I, their expression might account for the fact that no histological evidence for necrosis next to the tumor site was found in any of these samples, despite expression of cytotoxic molecules by the tumor cells.
The results of our study further suggest that the panel of antibodies useful for the immunohistochemical differentiation between the two types of cytotoxic cutaneous lymphomas should include CD2, CD3, CD4, CD8, CD56, Ki67, granzyme B, perforin, p70, and p140.
Acknowledgments
We thank Mrs. B. Müller for her excellent technical assistance.
Footnotes
Address reprint requests to Reinhard Dummer, M. D., Department of Dermatology, University Hospital–Zurich, 31, Gloriastr., CH-8091, Switzerland. E-mail: dummer@derm.unizh.ch.
References
- 1.Agnarsson BA, Vonderheid EC, Kadin ME: Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol 1990, 22:569-577 [DOI] [PubMed] [Google Scholar]
- 2.Adachi M, Maeda K, Takekawa M, Hinoda Y, Imai K, Sugiyama S, Yachi A: High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol 1994, 47:278-282 [DOI] [PubMed] [Google Scholar]
- 3.Dummer R, Potoczna N, Haffner AC, Zimmermann DR, Gilardi S, Burg G: A primary cutaneous non-T, non-B CD4+, CD56+ lymphoma. Arch Dermatol 1996, 132:550-553 [PubMed] [Google Scholar]
- 4.Willemze R, Kerl H, Sterry W, Berti E, Cerroni S, Chimenti JL, Diaz-Peréz LJ, Geerts ML, Goos M, Knobler R, Ralfkiaer E, Santucci M, Smith N, Wechsler J, van Vloten WA, Meijer CJLM: EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood 1997, 90:354-371 [PubMed] [Google Scholar]
- 5.Tian Q, Streuli M, Saito H: A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991, 67:629-639 [DOI] [PubMed] [Google Scholar]
- 6.Talanian RV, Yang XH, Turbov J, Seth P, Ghayur T, Casiano CA, Orth K, Froelich CJ: Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J Exp Med 1997, 186:1323-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greebberg AH: Granzyme B-induced apoptosis. Adv Exp Med Biol 1996, 406:219-228 [DOI] [PubMed] [Google Scholar]
- 8.Liu CC, Walsh CM, Young JD: Perforin: structure and function. Immunol Today 1995, 16:194-201 [DOI] [PubMed] [Google Scholar]
- 9.Froelich CJ, Orth K, Turbov J, Seth P, Babior BM, Gottlieb RA, Shah GM, Bleackley RC, Dixit VM, Hanna WL: New paradigm for lymphocyte granule mediated cytotoxicity: targets bind and internalize granzyme B but an endosomolytic antigen is necessary for cytosolic delivery and apoptosis. J Biol Chem 1996, 271:29073-29081 [DOI] [PubMed] [Google Scholar]
- 10.Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, Mingari MC, Moretta L: Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev 1997, 155:105-117 [DOI] [PubMed] [Google Scholar]
- 11.Ljunggren HG, Karre K: In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990, 11:237-244 [DOI] [PubMed] [Google Scholar]
- 12.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L: Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med 1995, 182:875-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, Wagtmann N, Winter CC: Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev 1997, 155:135-144 [DOI] [PubMed] [Google Scholar]
- 14.Colonna M, Samaridis J: Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science 1995, 268:405-408 [DOI] [PubMed] [Google Scholar]
- 15.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P: The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med 1995, 181:1133-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pende D, Biassoni R, Cantoni C, Verdiani S, Falco M, di Donato C, Accame L, Bottino C, Moretta A, Moretta L: The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J Exp Med 1996, 184:505-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braud VM, Allan DS, O’Callaghan CA, Soderstrom K, D’Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ: HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391:795-799 [DOI] [PubMed] [Google Scholar]
- 18.Vitale M, Castriconi R, Parolini S, Pende D, Hsu ML, Moretta L, Cosman D, Moretta A: The leukocyte Ig-like receptor (LIR)-1 for the cytomegalovirus UL18 protein displays a broad specificity for different HLA class I alleles: analysis of LIR-1 + NK cell clones. Int Immunol 1999, 11:29-35 [DOI] [PubMed] [Google Scholar]
- 19.Petrella T, Dalac S, Maynadie M, Mugneret F, Thomine E, Courville P, Joly P, Lenormand B, Arnould L, Wechsler J, Bagot M, Rieux C, Bosq J, Avril M, Bernheim A, Molina T, Devidas A, Delfau Larue MH, Gaulard P, Lambert D: CD4+ CD56+ cutaneous neoplasms: a distinct hematological entity? Groupe Francais d’Etude des Lymphomes Cutanes (GFELC). Am J Surg Pathol 1999, 23:137-146 [DOI] [PubMed] [Google Scholar]
- 20.Bagot M, Bouloc A, Charue D, Wechsler J, Bensussan A, Boumsell L: Do primary cutaneous non-T non-B CD4+CD56+ lymphomas belong to the myelo-monocytic lineage? J Invest Dermatol 1998, 111:1242-1244 [DOI] [PubMed] [Google Scholar]
- 21.Takeshita M, Yamamoto M, Kikuchi M, Kimura N, Nakayama J, Uike N, Daimaru H, Sawada H, Okamura T: Angiodestruction and tissue necrosis of skin-involving CD56+ NK/T-cell lymphoma are influenced by expression of cell adhesion molecules and cytotoxic granule and apoptosis-related proteins. Am J Clin Pathol 2000, 113:201-211 [DOI] [PubMed] [Google Scholar]
- 22.Berti E, Tomasini D, Vermeer MH, Meijer CJ, Alessi E, Willemze R: Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol 1999, 155:483-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu ML: A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 1997, 7:273-282 [DOI] [PubMed] [Google Scholar]


