Abstract
The serine proteases thrombin and trypsin are among many factors that malignant cells secrete into the extracellular space to mediate metastatic processes such as cellular invasion, extracellular matrix degradation, angiogenesis, and tissue remodeling. The degree of protease secretion from malignant cells has been correlated to their metastatic potential. Protease activated receptors (PAR)-1 and -2, which are activated by thrombin and trypsin respectively, have not been extensively characterized in human tumors in situ. We investigated the presence of PAR-1 and PAR-2 in human normal, benign and malignant tissues using immunohistochemistry and in situ hybridization. Our results demonstrate PAR-1 and PAR-2 expression in the tumor cells, mast cells, macrophages, endothelial cells, and vascular smooth muscle cells of the metastatic tumor microenvironment. Most notably, an up-regulation of PAR-1 and PAR-2 observed in proliferating, smooth muscle actin (SMA)-positive stromal fibroblasts surrounding the carcinoma cells was not observed in normal or benign conditions. Furthermore, in vitro studies using proliferating, SMA-positive, human dermal fibroblasts, and scrape-wounded human dermal fibroblasts demonstrated the presence of PAR-1 and PAR-2 not detected in quiescent, SMA-negative cultures. PAR-1 and PAR-2 in the cells forming the tumor microenvironment suggest that these receptors mediate the signaling of secreted thrombin and trypsin in the processes of cellular metastasis.
Malignant cells solicit the help of other cell types, such as stromal fibroblasts, mast cells, monocytes, and vascular cells, to facilitate their invasion into the surrounding tissue 1 because unrestrained growth of the tumor, by itself, does not result in invasion and metastasis. 2 The interface between the invading malignant cells and the hosting stromal cells, referred to as the tumor microenvironment (TME), 3 possesses a vast array of well-orchestrated cell signaling molecules which function to facilitate the ability of the proliferating tumor front to invade the stroma, as well as to degrade and remodel the extracellular matrix. 1 Of the many factors secreted by tumor cells, the two proteolytic enzymes, thrombin and trypsin, have been correlated to the stage and type of carcinoma and are associated with cell invasion and extracellular matrix degradation. 4,5 Furthermore, the ratio of proteases to their inhibitors in the TME can favor capillary sprout elongation and lumen formation during angiogenesis. 2
Thrombin is known to influence the behavior of all of the cells identified within the TME. For instance, thrombin activates platelets to adhere to other cells or extracellular matrix, increases vascular permeability and expression of adhesion molecules, attracts monocytes, stimulates mitogenic activity of endothelial cells and fibroblasts, and degranulates mast cells. 6-9 Thrombin also influences the rate of deposition of connective tissue proteins and the development of tissue fibrosis during normal wound healing; a process similar to cellular metastasis. 10,11 Many of thrombin’s effects are mediated through the seven transmembrane G-protein coupled receptors, protease-activated receptor (PAR)-1, via proteolytic cleavage of the amino-terminal extension unveiling a new amino terminus that activates the receptor through a tethered peptide ligand mechanism. 12 In vitro studies have demonstrated increased tumor cell adhesion to endothelium, extracellular matrix and platelets, enhanced metastatic capacity of tumor cells, and activated cell growth and stimulation of angiogenesis in response to thrombin and PAR-1 agonist peptides. 13-18 PAR-1 has been localized in smooth muscle cells, 19 pancreas tumor cells, 20-21 carcinoma and melanoma cell lines 16 and recently in human mast cells. 22 In breast carcinoma cells, the level of PAR-1 expression has been correlated to the degree of invasiveness. 23 Furthermore, B16F10 melanoma cells, transfected with PAR-1, enhanced thrombin-treated tumor cell adhesion to fibronectin 2.5-fold in vitro and pulmonary metastasis as high as 39-fold in vivo compared to the control thrombin-treated tumor cells. 24
Trypsin can stimulate fibroblasts to secrete procollagen, stimulate mast cells to degranulate, and is secreted by numerous tumor cell lines that are correlated with the stage and histological type of carcinoma. 4,25-27 Some of the actions of trypsin are mediated by a second protease-activated receptor known as PAR-2. 8,28-30 PAR-2 has been described in human tissues and tumor cell lines 20,29-33 as well as in human mast cells. 22 The ability of trypsin to degrade matrix proteins suggests it may participate in the processes of invasion, adhesion and metastasis; however, the presence of trypsin in tumors also suggests that PAR-2 may mediate these processes. 34 Although it is clear that tumor-derived trypsin-like enzymes could directly regulate growth in an autocrine and/or paracrine manner via PAR-2 activation, 30 the function of PAR-2 activation remains to be fully characterized.
The expression of PAR-1 and PAR-2 in malignant and benign human tumor tissues has not been extensively described in their histological context among the surrounding cell types forming the TME. The aim of this study was to characterize the expression of PAR-1 and PAR-2 protein and mRNA in normal, benign and malignant human tissues using immunohistochemistry and in situ hybridization, respectively. In addition, in vitro studies were used to investigate the presence of PAR-1 and PAR-2 in quiescent (hyperconfluent), proliferating (subconfluent), and wounded cultured fibroblasts. The results from these studies highlight the changes in expression of PAR-1 and PAR-2 in supporting cells in the TME as tumors gain metastatic potential.
Materials and Methods
Reagents
Primary antibodies used in these experiments include the following: desmin (Dako, Carpinteria, CA), endothelial cell (CD31; Dako), fibroblast (prolyl 4-hydroxylase) (Dako), macrophage (CD68; Dako), mast cell tryptase (Dako), non-immuno serum (Vector Laboratories, Burlingame, CA), PAR-1 (The Robert Wood Johnson Pharmaceutical Research Institute (RWJPRI), Spring House, PA), 35-37 PAR-2 (RWJPRI), 33,35,38 smooth muscle actin (Dako), DNA topoisomerase IIα (TOPO IIα) (Pharmingen, San Diego, CA) 39 and vimentin (Dako).
3′-Biotinylated molecular probes used for in situ hybridization include the following: PAR-1 (5′ TTC ATT TTT CTC CTC CTC CTC CTC ATC C) (Research Genetics, AL), 36-37 PAR-2 (5′ CAA TAA TGT AGA CGA CCG GAA GAA AGA) (Research Genetics, Huntsville, AL), 38 glyceraldehyde-3-phosphate dehydrogenase (GAP-DH) (5′ GAC GCC TGC TTC TCC TCC TTC TTG) (Ransom Hill, Ramona, CA), poly d(T) (5′ TTT TTT TTT TTT TTT TTT TTT TTT) (Research Genetics), lac Z (5′ CAC AGC GGA TGG TTC GGA TAA TG) (Ransom Hill).
Immunohistochemistry
Commercial human checkerboard tissue slides (Dako; Biomeda, Foster City, CA) representing normal breast tissues (n = 26), benign breast fibroadenomas (n = 14), malignant breast carcinomas (n = 46), and six other non-breast human carcinomas (n = 4 to 6 of each) were deparaffinized, hydrated and processed for routine immunohistochemistry (IHC) as previously described. 33 Briefly, slides were microwaved in Target buffer (Dako), cooled, placed in phosphate-buffered saline (PBS; pH 7.4) and treated with 3.0% H2O2 for 10 minutes. Slides were processed through an avidin-biotin blocking system according to the manufacturer’s instructions (Vector Laboratories) and then placed in PBS. All subsequent reagent incubations and washes were performed at room temperature.
Normal blocking serum (Vector Laboratories) was placed on all slides for 10 minutes. After briefly rinsing in PBS, primary antibodies were placed on slides for 30 minutes. The slides were washed and biotinylated secondary antibodies, goat anti-rabbit (polyclonal antibodies) or horse anti-mouse (monoclonal antibodies) were placed on the tissue sections for 30 minutes (Vector Laboratories). After rinsing in PBS, the avidin-horseradish peroxidase-biotin complex reagent (ABC; Vector Laboratories) was added for 30 minutes. Slides were washed and treated with the chromogen 3,3′-diaminobenzidine (DAB, Biomeda) twice for 5 minutes each, then rinsed in dH2O, and counterstained with hematoxylin, dehydrated in graded ethanols, cleared in xylene, coverslipped in Permount (Fisher Scientific, Pittsburgh, PA) and photographed with an Olympus BX50 light microscope. The negative controls included replacement of the primary antibody with pre-immune serum or with the same species IgG isotype non-immune serum.
Analysis of PAR-1 and PAR-2 Immunoreactivity
The tissues were scored for the intensity of PAR-1 and PAR-2 immunoreactivity to compare the relative amounts of PAR-1 and PAR-2 in the stromal fibroblasts and epithelial cells in the normal (n = 26), benign (n = 14) and malignant (n = 46) breast tissues. For each tissue, the presence of PAR-1 and PAR-2 immunoreactivity in the stromal fibroblasts were ranked under a 20× objective according to the following criteria: 1) no immunoreactivity (N); 2) weak, light brown immunoreactivity (W); 3) moderate brown immunolabeling (M), and 4) intense, dark brown immunoreactivity (S) (Table 1) ▶ . The negative controls did not produce observable labeling.
Table 1.
Human Breast Tissues Ranked by the Degree of PAR Immunolabeling in Stromal Fibroblasts
| Antibody | Rank | Malignant | Benign | Normal |
|---|---|---|---|---|
| PAR-1 | N | 0 | 14 | 26 |
| W | 7 | 0 | 0 | |
| M | 17 | 0 | 0 | |
| S | 22 | 0 | 0 | |
| PAR-2 | N | 0 | 14 | 26 |
| W | 5 | 0 | 0 | |
| M | 16 | 0 | 0 | |
| S | 25 | 0 | 0 |
N, absence of immunoreactivity (IR); W, weak, light brown IR; M, moderate, brown IR; S, strong, dark brown IR.
Double Immunohistochemistry
To help characterize the PAR-1 and PAR-2 positive stromal cells, we used double immunohistochemical methods (IHC:IHC) to simultaneously detect PAR-1 or PAR-2 expression with detection of a proliferation marker Topo IIα, 39 a fibroblasts marker (prolyl 4-hydroxylase), an endothelial marker (CD31), and smooth muscle actin (SMA). Protocols for IHC:IHC have been previously described. 40 Briefly, slides were first processed for single IHC labeling protocols for detection of each marker antibody as described above, except that the chromogen was SG (Vector Laboratories). Without processing the slides for hematoxylin, PAR-1 or PAR-2 antibodies were placed on the tissues for 30 minutes. After several PBS washes, the biotinylated horse anti-mouse secondary antibodies (Vector Laboratories) were similarly incubated. The presence of PAR-1 or PAR-2 positive cells was visualized using an alkaline phosphatase detection system through incubation with alkaline phosphatase conjugated ABC followed by development using the Fast Red chromogen (Sigma Chemical Co., St. Louis, MO). Slides were then routinely counterstained and coverslipped with a water-based mounting media (Dako).
In Situ Hybridization
Slides were routinely dewaxed, rehydrated, placed in 3% H2O2 for 10 minutes at room temperature, and processed for in situ hybridization (ISH) as previously described. 36-38 Briefly, after a 5-minute wash in water, slides were placed in Universal buffer (Research Genetics) and the tissue sections were digested with pre- diluted pepsin (Research Genetics) for 10 minutes at 42°C. Sections were washed and then dehydrated in 100% alcohol for 1 minute. Each probe was diluted to 1.0 μg/ml in commercially formulated hybridization buffer (Biomeda) and heated for 5 minutes at 103°C in a microcentrifuge tube on a heat block. The ISH probes were maintained at 42°C in a water bath until placement onto the tissue sections. Ten microliters of probe was added to each section, and a coverslip was gently placed to cover the solution and prevent evaporation. Slides were placed in a humid chamber and incubated at 42°C for 2 hours. After hybridization, they were then immediately placed into a low stringency wash (2 × SSC) for 5 minutes at 42°C, followed by a high stringency wash (0.1 × SSC) for 5 minutes at 42°C. Sections were washed in PBS and treated with ABC for 1 hour at room temperature. After washing, sections were placed in DAB for 2 × 5 minutes, washed, briefly stained with hematoxylin, then coverslipped. Positive controls included two biotinylated mRNA oligonucleotide probes: GAPDH mRNA and a poly d(T) probe that hybridizes non-specifically to all mRNA. Negative controls included 1) the absence of probe in the probe cocktail; 2) a biotinylated probe that hybridizes to lac Z operon mRNA; and 3) pre-digestion of the tissues with RNase, DNase free (10 μg/μl, Boehringer Mannheim, Indianapolis, IN) for 2 hours at 42°C before probe hybridization.
Cell Culture
Human neonatal dermal fibroblasts and their culture media were obtained from Clonetics/BioWhittaker (Walkersville, MD). Cell suspensions (5 × 104/ml) were seeded in 4-well chamber slides (NUNC, Naperville, IL) for immunocytochemistry. Cells were incubated for either 2 days (subconfluent, proliferative conditions) or 9 days (hyperconfluent, quiescent conditions) before evaluation without serum exchange. To mimic the in vivo activation of differentiated, quiescent fibroblasts in vitro, 9-day quiescent cultured cells were subjected to scrape wounding, which was induced by the end of a pipette, and then cultured for 5 additional days without medium exchange (wound conditions). As a control, other 9-day cultures without scrapes continued to grow in parallel.
Immunocytochemistry
Four-chambered culture slides were routinely fixed with 10% neutral buffered saline for 10 minutes at room temperature, rinsed in PBS, and then assayed for ICC as previously described. 24,35 Hyperconfluent (quiescent), subconfluent (proliferating), and wounded cultures were processed for ICC using antibodies to PAR-1, PAR-2, smooth muscle actin (SMA), Topo IIα and pre-immune serum. Before processing, the chambers were carefully removed from the slides. All washing steps were performed using Automation Buffer with Tween-20 (Research Genetics). Primary antibodies were added to the wells for 30 minutes at room temp. After washes, the secondary antibodies were similarly incubated on the cells. Subsequently, the presence of the primary antibodies were detected using the ABC followed by DAB development for 2 × 5 minutes each. The slides were then counterstained using hematoxylin and coverslipped.
Results
In Situ PAR-1 and PAR-2 Protein Expression
PAR-1 and PAR-2 proteins were localized in formalin-fixed, paraffin-embedded tissues. Normal (n = 26), benign (n = 14), and malignant (n = 46) human breast tissues and six non-breast carcinomas (n = 4–6 of each) were assayed simultaneously in a multitissue format to eliminate potential staining artifacts such as slide-to-slide and run-to-run variability. Marginal increases of PAR-1 and PAR-2 expression were observed in the malignant cells as compared to the normal and benign epithelial cells. Striking changes in PAR-1 and PAR-2 expression were noted in the stromal fibroblasts surrounding the malignant cells as compared to the fibroblasts surrounding the normal and benign epithelial cells (Table 1) ▶ . No PAR-1 or PAR-2 immunolabeling was observed in the stromal fibroblasts of the benign (n = 14) or normal (n = 26) breast tissues. In contrast, most malignant tissues had prominent moderate to strong PAR-1 (n = 39/46) and PAR-2 (n = 37/46) labeling in the stromal fibroblasts.
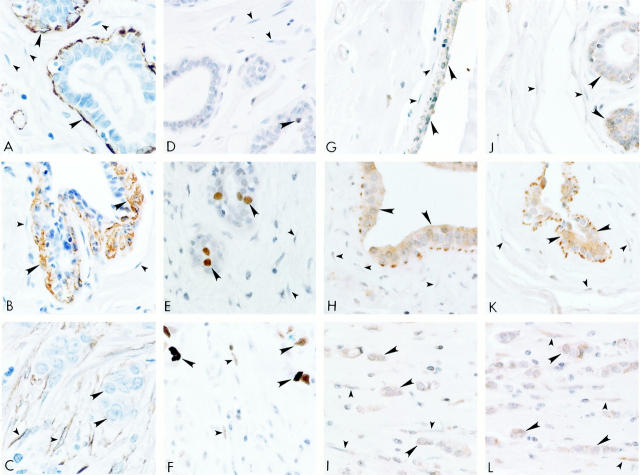
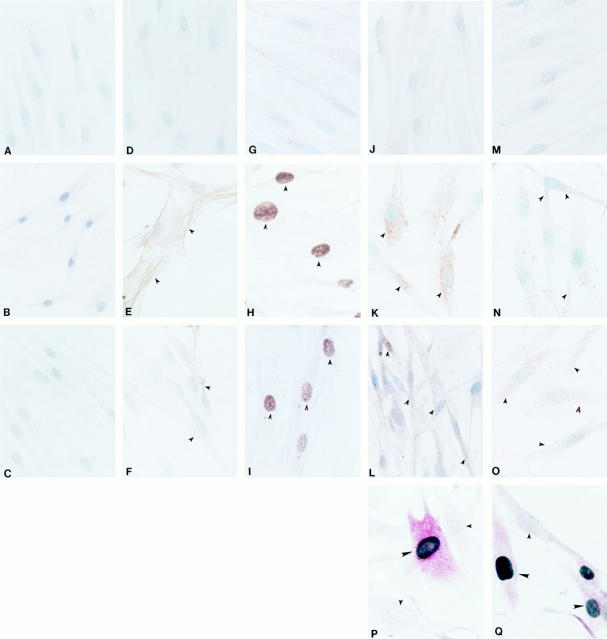
We applied additional immunohistochemical markers to further characterize these tissues (Figure 1) ▶ . No immunolabeling was detected using negative control antibodies in normal, benign and malignant breast tissue stromal fibroblasts or epithelial cells (data not presented). SMA-positive immunolabeling was localized in the myoepithelial cells (large arrowheads) around the epithelial ducts and in the vascular smooth muscle cells in the normal (Figure 1A) ▶ and benign fibroadenoma (Figure 1B) ▶ breast tissues. SMA immunolabeling was absent from stromal fibroblasts in the normal (Figure 1A) ▶ and benign (Figure 1B) ▶ tissues, which were immunoreactive to the fibroblast marker (data not presented). In the malignant breast carcinoma tissues, SMA immunolabeling (small arrowheads) was prominent in the stromal fibroblasts surrounding the tumor cells, in addition to the vascular smooth muscle cells (Figure 1C) ▶ . Carcinoma cells (large arrowheads) did not express SMA. Positive, nuclear Topo IIα immunolabeling (large arrowhead), a marker for proliferating cells, 39 was sparsely observed in normal breast epithelial cells (Figure 1D) ▶ and absent in stromal fibroblasts (small arrowheads). Topo IIα nuclear immunolabeling was observed in the benign, fibroadenoma cells (large arrowheads), but was similarly absent in the surrounding stromal fibroblasts (small arrowheads) in Figure 1E ▶ . In contrast, Topo IIα nuclear immunolabeling was observed in stromal fibroblasts (small arrowheads) and tumor cells (large arrowheads) of the malignant tissues (Figure 1F) ▶ . Furthermore, the stromal fibroblasts surrounding the malignant cells also expressed vimentin but did not express desmin (data not presented).
Figure 1.
Representative immunohistochemical micrographs for normal (left: A, D, G, and J), benign (center: B, E, H, and K), and malignant (right: C, F, I, and L) breast tissues. A–C: Smooth muscle actin antibodies; D–F: DNA topoisomerase IIα antibodies; G–I: PAR-1 antibodies; J–L: PAR-2 antibodies. Large arrowheads indicate normal, benign and malignant epithelial cells in the breast. Small arrowheads indicate the stromal fibroblasts. Original magnification, ×600.
Immunolocalization studies indicated that PAR-1 and PAR-2 were co-expressed in the different cell types in normal, benign, and malignant tissues. In normal breast tissues, PAR-1 (Figure 1G) ▶ and PAR-2 (Figure 1J) ▶ immunolabeling (large arrowheads) was confined to the normal breast ductal epithelial cells. PAR-1 (Figure 1H) ▶ and PAR-2 (Figure 1K) ▶ immunolabeling (large arrowheads) was also observed in the fibroadenoma cells. In both cases, normal and benign tissues, surrounding stromal fibroblasts (small arrowheads) did not express detectable PAR-1 (Figure 1, G and 1H ▶ , respectively) or PAR-2 (Figure 1, J and K ▶ , respectively). In the breast carcinoma tissues, PAR-1 and PAR-2 positive immunoreactivity was observed in many cell types forming the TME such as in the malignant cells (large arrowheads) and the stromal fibroblasts (small arrowheads) (Figure 1, I and L) ▶ . Although not present in these photomicrographs, PAR-1 and PAR-2 immunolabeling was also observed in endothelial cells, vascular smooth muscle cells, as well as in the mast cells and macrophages. The PAR-1 and PAR-2 mast cell labeling pattern was consistent with our previous report, 22 and was similarly localized to the plasma membrane and to the membranes of the secretory vesicles. PAR-1 and PAR-2 positive macrophages were also observed around these cancerous tissues. Labeling in the macrophages was observed in or on the plasma membrane as well as intracellularly (Figure 1, I and L) ▶ . The identity of the mast cells and macrophages were confirmed in these tissue sections using antibodies to mast cell tryptase and macrophages (CD68) (data not presented).
Co-Localization of PAR-1 and PAR-2 Expression in Proliferating-, Smooth Muscle Actin-, Fibroblastic-Positive Cells in Situ
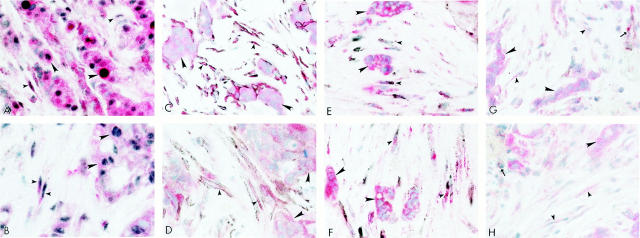
The results of double immunohistochemical labeling using antibodies to PAR-1 or PAR-2 with antibodies to Topo IIα demonstrated that proliferating stromal cells expressed PAR-1 and PAR-2 immunolabeling as represented in Figures 2A and 2B ▶ , respectively. Co-localization of PAR-1 (Figure 2A) ▶ or PAR-2 (Figure 2B) ▶ immunolabeled red with black, immunolabeled Topo IIα positive nuclei was observed in both malignant cells (large arrowheads) and stromal fibroblasts (small arrowheads) in the breast carcinoma tissues. The proliferating, PAR-1 and PAR-2 positive stromal cells surrounding the carcinoma cells also expressed SMA (small arrowheads, Figures 2C and 2D ▶ , respectively). This immunophenotype was not observed in normal and benign breast tissues (data not presented). Furthermore, these proliferating, SMA, PAR-1 and PAR-2 stromal cells also were immunolabeled with antibodies for prolyl 4-hydroxylase, a fibroblast marker (small arrowheads, Figure 2, E and F ▶ , respectively). These cells did not express the endothelial marker, CD31 (Figure 2, G and H ▶ , respectively; small arrowheads), which was only observed in endothelium of nearby vessels (arrows).
Figure 2.
Malignant breast tissues were processed for double immunohistochemical procedures. Malignant breast carcinoma cells (large arrowheads) and stromal fibroblasts (small arrowheads) co-express PAR-1 (red) and Topo IIα (black) in A; PAR-2 (red) and Topo IIα in B. Similarly, C and D show co-expression of red labeled PAR-1 and PAR-2 respectively with stromal cells expressing black labeled smooth muscle actin (arrowheads). Similarly, these proliferating, smooth muscle actin immunolabeled stromal cells also co-express (small arrowheads) a fibroblastic marker (black) and red labeled PAR-1 (E) and PAR-2 (F). These cells did not express an endothelial marker, CD31. Arrows indicate areas of CD31-positive (black) immunoreactivity in the vascular endothelial cells among red labeled PAR-1 (G) or PAR-2 (H) malignant (large arrowheads) and stromal cells (small arrowheads). Original magnification, ×600.
PAR-1 and PAR-2 Expression in Other Tumors
Other human non-breast malignant tumors demonstrated similar PAR-1 and PAR-2 expression in the tumor cells (large arrowheads), stromal fibroblasts (small arrowheads), mast cells and macrophages (arrows), as well as in the endothelial and vascular smooth muscle cells (Figure 3) ▶ . Figure 3 ▶ shows PAR-1 (Figure 3, A, C and E) ▶ and PAR-2 (Figure 3, B, D, and F) ▶ immunolabeling in tissues representing a gastric carcinoma (n = 4; Figure 3, A and B ▶ ), an undifferentiated carcinoma (n = 4, Figure 3, C and D ▶ ) and a lung adenocarcinoma (n = 4, Figure 3, E and F ▶ ). PAR-1 and PAR-2 immunoreactivity was similarly present in heptacarcinomas (n = 6), thyroid carcinomas (n = 4) and ovarian carcinomas (n = 6) (data not shown). Positive PAR-1 and PAR-2 immunoreactivity was also observed on surrounding endothelial and vascular smooth muscle cells, as well as in the stromal fibroblasts in contrast to the absence of PAR-1 and PAR-2 immunoreactivity in the stromal fibroblasts on the normal tissue counterparts (data not presented).
Figure 3.
Examples of PAR-1 (A, C, E) and PAR-2 (B, D, F) expression in human gastric carcinoma (A–B), undifferentiated carcinoma (C–D), and lung adenocarcinoma (E–F) tissues. Large arrowheads indicate positive immunolabeling in the tumor cells and small arrowheads indicate positive immunolabeling in the stromal fibroblasts. Arrows indicate PAR-1 or PAR-2 positive immunolabeling in macrophages. Original magnification, ×600.
In Situ PAR-1 and PAR-2 mRNA Expression
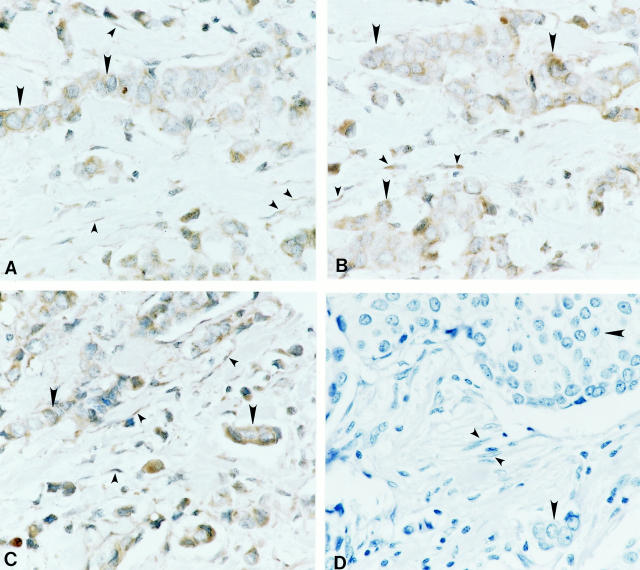
The PAR-1 and PAR-2 protein expression correlated well with their respective mRNA levels in the same tissues as determined by in situ hybridization. The localization patterns of PAR-1 (Figure 4A) ▶ and PAR-2 (Figure 4B) ▶ mRNA were observed in human breast carcinoma tissues (n = 48). Figure 4A ▶ shows the intracellular localization of PAR-1 mRNA in the malignant tumor cells (large arrowheads) and in the surrounding stromal fibroblasts (small arrowheads). PAR-1 mRNA was not present in the stromal cells of the normal (n = 26) and benign (n = 10) breast tissues (data not presented). Similar localization patterns were observed for PAR-2 in the same breast carcinoma tissues as shown in Figure 4B ▶ , and PAR-2 mRNA was also not present in the stromal cells of the normal and benign breast tissues (data not presented). As a positive control probe, cells also expressed GAPDH mRNA (Figure 4C) ▶ . When the same tissues were probed with the lac Z biotinylated mRNA probe (negative control), no observable labeling was observed in tumor cells (large arrowheads) or stromal fibroblasts (small arrowheads) (Figure 4D) ▶ .
Figure 4.
Expression of PAR-1 (A) and PAR-2 (B) mRNA through in situ hybridization in human malignant breast carcinoma tissues. The positive control probe, GAPDH (C), and the negative control probe lac Z (D) are also presented. Large arrowheads indicate tumor cells and small arrowheads indicate stromal fibroblasts. Original magnification, ×600.
In addition, PAR-1 and PAR-2 mRNA was similarly observed in the following tissues: gastric carcinomas (n = 4), undifferentiated carcinomas (n = 4), lung adenocarcinomas (n = 4), heptacarcinomas (n = 6), thyroid carcinomas (n = 4) and ovarian carcinomas (n = 6) (data not shown).
In Vitro PAR-1 and PAR-2 Expression
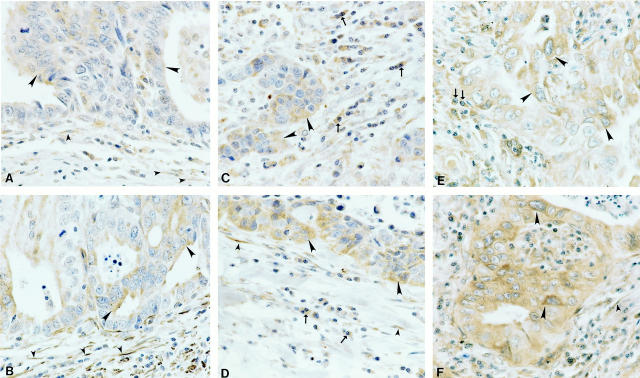
Our IHC and ISH results indicated that PAR-1 and PAR-2 expression was induced in stromal fibroblasts during the transition to a myofibroblast phenotype. We used ICC to determine whether this transition could be mimicked in vitro. Hyperconfluent, fibroblast cultures (quiescent conditions) were compared to 1) subconfluent cultures with visible mitotics (proliferative conditions), and 2) confluent cultures subjected to a mechanical scrape and allowed to recover for 5 days without changing the media (wound conditions). Figure 5 ▶ (Figure 5, A ▶ -C) shows the lack of observable immunolabeling using negative control antibodies in all three tissue culture conditions. SMA immunolabeling was present in the proliferating cells (Figure 5E ▶ , arrowheads) and in the cells migrating over the scraped area (Figure 5F ▶ , arrowheads), but was absent in the confluent cultured cells (Figure 5D) ▶ , suggesting that the confluent conditions produced quiescent, differentiated cells that were not myofibroblasts. Immunoreactivity to the proliferation marker, Topo IIα, was present in the nuclei of the proliferating cells in the subconfluent cultures (Figure 5H ▶ , arrowheads) and in the cells migrating over the scraped area in the wounded cultures (Figure 5I ▶ , arrowheads), but was absent in the cell nuclei of the quiescent cells (Figure 5G) ▶ , further confirming the quiescent, non-proliferating status of these differentiated fibroblasts when grown to confluency.
Figure 5.
Quiescent (A, D, G, J, and M), proliferating (B, E, H, K, and N), and wounded (C, F, I, L, and O) human dermal fibroblasts were processed for immunocytochemistry using negative control antibodies (A–C), antibodies to detect smooth muscle actin (SMA) (D–F), Topo IIα (G–I), PAR-1 (J–L), and PAR-2 (M–O). No observable labeling of cells using negative control antibodies. No SMA immunolabeling was observed in the quiescent cultured cells (D). SMA-positive cells (arrowheads) were observed in cultured cells in the proliferating and wounding conditions (E–F). Proliferating cells were detected by the presence of brown, Topo IIα-positive nuclei in the proliferating cells (H–I), but not in the quiescent cells (G). Positive intracellular and membrane PAR-1 (K–L) and PAR-2 (N–O) immunoreactive cells (arrowheads) were observed in the cells in the proliferating and wounding conditions, but were absent in the cells in the quiescent conditions. Cultured cells were also processed for double immunohistochemical procedures. Cultured cells in the wounding conditions co-express (large arrowheads) PAR-1 (red) and Topo IIα (black nuclei) (P). Similarly, wounding cells also co-express (large arrowheads) PAR-2 (red) and Topo IIα (black nuclei) (Q). Small arrowheads indicate non-proliferating cells without detectable PAR-1 (P), PAR-2 (Q), or Topo IIα (P and Q). Original magnifications: A-O, ×600; P, Q, ×900.
Positive intracellular and membrane PAR-1 and PAR-2 immunoreactivity (arrowheads) was not observed in the quiescent, non-proliferating cells (Figures 5J and 5M ▶ , respectively). However, positive PAR-1 and PAR-2 immunolabeling (arrowheads) was observed in the proliferating cells in the subconfluent (Figures 5K and 5N ▶ , respectively) and wounded (Figures 5, L and O ▶ , respectively) conditions.
PAR-1 and PAR-2 Expression Is Associated with Proliferating Cells in Vitro
In an effort to replicate our in situ findings in the in vitro condition, we similarly applied double immunohistochemical labeling using antibodies to PAR-1 or PAR-2 with antibodies to Topo IIα, and demonstrated that proliferating cells expressed PAR-1 and PAR-2 immunolabeling. Figure 5, P and Q ▶ , respectively, show representative examples of these results. Co-localization of red immunolabeled PAR-1 (Figure 5P) ▶ or red immunolabeled PAR-2 (Figure 5Q) ▶ with black immunolabeled Topo IIα positive nuclei was observed in proliferating cultured fibroblasts (large arrowheads). As an internal control, a nearby non-proliferating, Topo IIα-negative cells (small arrowheads) did not express obvious detectable levels of PAR-1 (Figure 5P) ▶ or PAR-2 (Figure 5Q) ▶ .
Discussion
One of the most important features in cell metastasis is the ability of tumor cells to produce extracellular conditions conducive to their growth through degradation and subsequent remodeling of the extracellular matrix. This study provides evidence for the presence of PAR-1 and PAR-2 not only on the malignant carcinoma cells, but also on the cell types forming the TME, including mast cells, vascular endothelial cells, smooth muscle cells, macrophages and most interestingly, on reactive stromal fibroblasts. By expressing PAR-1 and PAR-2, these cell types may act as proteolytic sensors to extracellular thrombin and trypsin, initiating a cellular response to tissue damage incurred through the processes of cell metastasis. The remodeling of the tumor stroma provides a permissive environment for tumor metastasis, relying on the interplay of all of the cells within the TME.
PAR-1 and PAR-2 expression has previously been shown on endothelial cells, vascular smooth muscle cells and mast cells. It is therefore not surprising to find similar results for these cell types within the TME. Activation of either PAR-1 or PAR-2 on these cells results in characteristic events associated with inflammatory responses such as generation of cytokines, expression of adhesion molecules and increased vascular permeability. However, little is known about the presence of PAR-1 and PAR-2 on macrophages. It has been reported that macrophages can secrete thrombin, 41 and that thrombin has been localized in pulmonary alveolar macrophages, 42 suggesting an association between macrophages and thrombin. The presence of PAR-1 and PAR-2 on human macrophages in malignant tumors in situ has not been reported previously, although PAR-2 immunoreactivity has been reported on macrophage-like cells in the adventitia of the mouse isolated ureter. 43 Here, we show that macrophages express both PAR-1 and PAR-2. PAR-1 and PAR-2 activation may provide a stimulus for macrophages to proliferate, migrate and/or phagocytize degraded stromal proteins, in addition to synthesizing and secreting thrombin and growth factors into the TME.
The most striking observation from our study is the presence of PAR-1 and PAR-2 on the stromal fibroblasts surrounding the metastatic tumor cells but not on the stromal fibroblasts surrounding the benign, non-metastatic, or normal epithelial cells. The exact origin of the PAR-1 and PAR-2 expressing stromal fibroblasts is unclear, ie, local dedifferentiated stromal fibroblasts, vascular smooth muscle cells, or migrating undifferentiated stem cells such as pericytes. 44 In breast cancer, it has been shown that primary fibroblasts convert to the myofibroblast phenotype when exposed to tumor cells; vascular smooth muscle cells and pericytes can also differentiate to myofibroblasts, but to a lesser extent. 44 The stromal fibroblasts associated with metastatic tumors in our study were characterized by the positive expression of SMA, prolyl 4-hydroxylase (a fibroblast marker), Topo IIα (a proliferation marker) and vimentin, as well as the absence of the vascular markers desmin and CD31, confirming the fibroblastic nature of these cells. 44-48
Reactive stromal myofibroblasts are frequently associated with cancers of epithelial origin, a process known as desmoplasia. 49 The induction of this phenotype has not been well characterized, however in vitro studies have indicated that diffusable signals, such as transforming growth factor-β, generated from primed or initiated carcinoma cells are involved. 44,50-52 The stromal myofibroblasts, in turn, influence the invasive and metastatic potential of carcinoma cells by an unidentified mechanism 1 once the carcinoma cells invade the basement membrane surrounding the epithelial cells. Elaboration of matrix degrading proteases, deposition of new extracellular matrix proteins to facilitate tumor cell adhesion, cell motility and cell proliferation, 1,11 and release of cytokines and growth factors by these myofibroblasts emphasize the importance of this phenotypic change to the invasiveness of the tumor. PAR-1 and PAR-2 activation results in many of these biochemical events, indicating that they are likely participants in the balance of tumor containment and/or metastasis. 53-58 Moreover, the expression of tissue factor, an essential co-factor for plasma coagulation factor VII/VIIa, was reported to be consistently observed in stromal cells of invasive breast carcinomas but not in the benign breast tumors. 58 The increased presence of tissue factor/factor VIIa within the TME, which in turn can generate thrombin via the extrinsic coagulation pathway on fibroblasts, parallels our observation of increased PAR-1 expression.
Benign proliferative disorders are characterized by a continuous basement membrane separating the epithelium from the stroma, similar to the normal tissue organization. 2 It is possible that the presence of a continuous basement membrane may actually quarantine any tumor-derived thrombin or trypsin from the stromal fibroblasts. Thus, the actions of thrombin and trypsin within the TME may be accentuated through up-regulation of PAR-1 and PAR-2 in the stromal fibroblasts as they de-differentiate (ie, SMA-negative to SMA-positive). The activation of PAR-1 and PAR-2 on tumor cells contributes to migration by increasing their adhesive properties and releasing urokinase, both of which are early changes during the initiation of metastasis. 14,59-60
We were able to mimic our in situ observations in vitro using cultured human dermal fibroblasts. Quiescent, SMA-negative, non-proliferating (Topo IIα-negative) cell cultures did not express detectable PAR-1 or PAR-2, similar to those of the stromal fibroblasts in normal and benign human tissues in situ. Most notably, we were able to mimic the transformation of PAR-1 and PAR-2-negative to PAR-1 and PAR-2-positive fibroblasts in vitro, after the quiescent cells were subjected to scrape wounding indicating that cell damage relays a signal for PAR induction.
In summary, this is the first in situ histological comparative report describing the presence of PAR-1 and PAR-2 protein and mRNA in human malignant tumor cells and local mast cells, macrophages, endothelium, and vascular smooth muscle cells of the TME. More importantly, we observed PAR-1 and PAR-2 immunolabeling in the stromal fibroblasts immediately surrounding the malignant cells that was absent in the surrounding stromal fibroblasts of the normal and benign breast epithelial cells. The presence of both PARs and their activating proteases within the TME suggests an autocrine and/or paracrine cascade in the processes of cellular metastasis, perhaps as natural mechanisms of tissue injury. It will be important to investigate if there is a correlation between the relative amounts of PAR-1 or PAR-2 in the tumors cells and in the stromal fibroblasts with tumor grade, and to expand our investigations into the expression of all of the members of the PARs into other pathological tissues. Because the degree of tumor cell malignancy has been classified by the amounts of secreted thrombin or trypsin, theoretically, the amounts of PAR-1 and PAR-2 in the TME cells may also be a valid predictor of metastatic activity, thereby acquiring diagnostic and prognostic value. More importantly, these data suggest attractive targets for therapeutic approaches, whereby PAR-1 and PAR-2 antagonists and anti-thrombin and anti-tryptase agents may be directed to disrupt some of the processes of cell metastasis.
Acknowledgments
We are grateful to Patti A. Reiser, BS, MT, HT (ASCP), Norah A. Gumula, HT (ASCP), Brenda M. Hertzog, BS, MT (ASCP), and Deborah A. Polkovitch, BS, MT (ASCP) of MorphoMetrics, Drug Discovery, RWJPRI for their excellent histological and immunohistochemical expertise. The authors want to acknowledge Dr. Robert G. Nagele, of School of Osteopathic Medicine, University of Medicine and Dentistry of New Jersey, Stratford, NJ and Dr. Paul Farber of Temple Medical School, Philadelphia, PA for their invaluable advice and review of the manuscript.
Footnotes
Address reprint requests to Dr. Michael R. D’Andrea, The R. W. Johnson Pharmaceutical Research Institute, Welsh and McKean Rds., Spring House, PA 19477. E-mail: mdandrea@prius.jnj.com.
References
- 1.Gregoire M, Lieubeau B: The role of fibroblasts in tumor behavior. Cancer Metastasis Rev 1995, 14:339-350 [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA, Steeg PS, Stetler-Stevenson W: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 [DOI] [PubMed] [Google Scholar]
- 3.O’Meara RAQ: Coagulative properties of cancers. Ir J Med Sci 1958, 394:474-479 [PubMed] [Google Scholar]
- 4.Koivunen E, Saksela O, Itkonen O, Osman S, Huhtala M-L, Stenman U-F: Human colon carcinoma, fibrosarcoma and leukemia cell lines produce tumor-associated trypsinogen. Int J Cancer 1991, 47:592-596 [DOI] [PubMed] [Google Scholar]
- 5.Walz DA, Fenton JW: The role of thrombin in tumor cell metastasis. Invasion Metastasis 1995, 14:303-308 [PubMed] [Google Scholar]
- 6.Dennington PM, Berndt MC: The thrombin receptor. Clin Exp Pharmacol Physiol 1994, 21:349-358 [DOI] [PubMed] [Google Scholar]
- 7.Grand RJA, Turnell AS, Grabham PW: Cellular consequences of thrombin-receptor activation. Biochem J 1996, 313:353-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenberg MD: Protease-mediated signaling: new paradigms for cell regulation and drug development. Trends Pharmacol Sci 1996, 17:3-6 [DOI] [PubMed] [Google Scholar]
- 9.Razin E, Marx G: Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J Immunol 1984, 133:3282-3285 [PubMed] [Google Scholar]
- 10.Carney DH, Mann R, Redin WR, Pernia SD, Berry D, Heggers JP, Hayward PG, Robson MC, Christie J, Annable C: Enhancement of incisional wound healing and neovascularization in normal rats by thrombin and synthetic thrombin receptor-activating peptides. J Clin Invest 1992, 89:1469-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers RC, Dabbagh K, McAnulty RJ, Gray AJ, Blanc-Brude OP, Laurent GJ: Thrombin stimulates fibroblast procollagen production via proteolytic activation of protease-activated receptor 1. J Biochem 1998, 333:121-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vu TK, Hung DT, Wheaton VI, Coughlin SR: Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64:1057-1068 [DOI] [PubMed] [Google Scholar]
- 13.Nierodzik ML, Kajumo LR, Karpatkin S: Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res 1992, 52:3267-3272 [PubMed] [Google Scholar]
- 14.Nierodzik ML, Bain RM, Liu L-X, Shiviji M, Takeshita K, Karpatkin S: Presence of the seven transmembrane thrombin receptor in human tumour cells: effects of activation on tumour adhesion to platelets and tumour tyrosine phosphorylation. Br J Haematiology 1996, 92:452-457 [DOI] [PubMed] [Google Scholar]
- 15.Klementsen B, Jorgensen L: Mechanisms involved in the early interaction between HeLa cells, platelets and endothelial cells in vitro under the influence of thrombin. APMIS 1997, 105:391-401 [DOI] [PubMed] [Google Scholar]
- 16.Wojtukiewicz MZ, Tang DG, Ben-Josef E, Renaud C, Waltz DA, Honn KV: Solid tumor cells express functional tethered ligand thrombin receptor. Cancer Res 1995, 55:698-674 [PubMed] [Google Scholar]
- 17.Wojtukiewicz MZ, Tang DG, Ciarelli JJ, Nelson KK, Waltz DA: Thrombin increases the metastatic potential of tumor cells. Int J Cancer 1993, 54:793-806 [DOI] [PubMed] [Google Scholar]
- 18.Tsopanglou NE, Maragoudakis ME: On the mechanism of thrombin-induced angiogenesis: inhibition of attachment of endothelial cells on basement membrane components. Angiogenesis 1997, 1:192-200 [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Hayzer DJ, Corson MA, Runge MS: Molecular cloning of the rat vascular smooth muscle thrombin receptor. Evidence for in vitro regulation by basic fibroblast growth factor. J Biol Chem 1992, 267:16975-16979 [PubMed] [Google Scholar]
- 20.Kaufmann R, Schafberg H, Nowak G: Proteinase-activated receptor-2-mediated signaling and inhibition of DNA synthesis in human pancreatic cancer cells. Int J Pancreatol 1998, 24:97-102 [DOI] [PubMed] [Google Scholar]
- 21.Rudroff C, Schafberg H, Nowak G, Weinel R, Scheele J, Kaufmann R: Characterization of functional thrombin receptors in human pancreatic tumor cells (MIA PACA-2). Pancreas 1998, 16:189-194 [DOI] [PubMed] [Google Scholar]
- 22.D’Andrea MR, Rogahn CJ, Andrade-Gordon P: Localization of protease-activated receptors-1 and -2 in human mast cells: indications for mast cell amplification cascade. Biotech Histochem 2000, 75:85-90 [DOI] [PubMed] [Google Scholar]
- 23.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R: Thrombin Receptor overexpression in malignant and physiological invasion processes. Nat Med 1998, 4:909-914 [DOI] [PubMed] [Google Scholar]
- 24.Nierodzik ML, Chen K, Takeshita K, Li J-J, Huang Y-Q, Feng X-S, D’Andrea MR, Andrade-Gordon P, Karpatkin S: Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 1998, 92:3694-3700 [PubMed] [Google Scholar]
- 25.Koshikawa N, Yasumitsu H, Nagashima Y, Umeda M, Miyazaki K: Identification of one- and two-chain forms of trypsinogen 1 produced by a human gastric adenocarcinoma cell line. J Biochem 1994, 303:187-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koshikawa N, Yasumitsu H, Umeda M, Miyazaki K: Multiple secretion of matrix serine proteases by human gastric adenocarcinoma cell line. Cancer Res 1992, 52:5046-5053 [PubMed] [Google Scholar]
- 27.Hirahara F, Miyagi Y, Miyagi E, Yasumitsu H, Koshikawa N, Nagashima Y, Kitamura H, Minaguchi H, Umeda M, Miyazaki K: Trypsinogen expression in human ovarian carcinomas. Int J Cancer 1995, 63:176-181 [DOI] [PubMed] [Google Scholar]
- 28.Nystedt S, Emilsson K, Larsson A-K, Strombeck B, Sundelin J: Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem 1995, 232:84-89 [DOI] [PubMed] [Google Scholar]
- 29.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J: Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA 1994, 91:9208-9212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin S, Payan DG, Bunnett N: Molecular cloning, expression and potential functions of the human proteinase-activated receptor 2. J Biochem 1996, 314:1009-1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirza H, Yatsula V, Bahou WF: The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest 1996, 97:1705-1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J, Schecter N, Woolkalis MJ, Brass LF: Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem 1997, 277:4043-4049 [DOI] [PubMed] [Google Scholar]
- 33.D’Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P: Characterization of protease activated receptor (PAR-2) immunoreactivity in normal human tissues. J Histochem Cytochem 1998, 46:1-8 [DOI] [PubMed] [Google Scholar]
- 34.Miyata S, Koshikawa N, Yasumitsu H, Miyazaki K: Trypsin stimulates integrin a5b1-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of protease-activated receptor-2. J Biol Chem 2000, 275:4592-4598 [DOI] [PubMed] [Google Scholar]
- 35.Smith-Swintosky VL, Cheo-Isaacs CT, D’Andrea MR, Santulli RJ, Darrow AL, Andrade-Gordon P: Protease-activated receptor (PAR-2) is present in the rat hippocampus and is associated with neurodegeneration. J Neurochem 1997, 69:1890-1896 [DOI] [PubMed] [Google Scholar]
- 36.Cheung W-M, D’Andrea MR, Andrade-Gordon P, Damiano BP: Vascular injury response in thrombin receptor (PAR-1) deficient mice. Arterioscler Thromb Vasc Biol 1999, 19:3014-3024 [DOI] [PubMed] [Google Scholar]
- 37.Festoff BW, D’Andrea MR, Citron BA, Salcedo RM, Smirnova IV, Andrade-Gordon P: Motor neuron cell death in Wobbler mutant mice and over-expression of a novel G-protein-coupled, protease-activated receptor for thrombin. Mol Med 2000, 6:494-508 [PMC free article] [PubMed] [Google Scholar]
- 38.Damiano BP, D’Andrea MR, Cheung W-M, de Garavilla L, Andrade-Gordon P: Increased expression of protease activated receptor-2 (PAR-2) in proliferating cells of balloon-injured rat carotid artery. Thromb Haemost 1999, 81:808-814 [PubMed] [Google Scholar]
- 39.D’Andrea MR, Farber P, Foglesong PD: Immunohistochemical detection of DNA topoisomerase IIα and IIβ compared with detection of Ki-67, a marker of cellular proliferation, in human tumors. Appl Immunohist 1994, 2:177-185 [Google Scholar]
- 40.D’Andrea MR, Rogahn CJ, Damiano BP, Andrade-Gordon P: A simultaneous histochemical and immunohistochemical staining protocol to evaluate 4 differently stained cell types in restenosis. Biotech Histochem 1999, 74:172-180 [DOI] [PubMed] [Google Scholar]
- 41.Lindahl U, Peiler G, Bozgwald J, Seljelid R: A prothrominase complex of mouse peritoneal macrophages. Arch Biochem Biophys 1989, 273:180-188 [DOI] [PubMed] [Google Scholar]
- 42.Zacharski LR, Memoli VA, Morain WD, Schlaeppi J-M, Rousseau SM: Cellular localization of enzymatically active thrombin in intact human tissues by hirudin binding. Thromb Haemost 1995, 73:793-797 [PubMed] [Google Scholar]
- 43.Moffatt JD, Cocks TM: The role of protease-activated receptor-2 (PAR-2) in the modulation of beating of the mouse isolated ureter: lack of involvement of mast cells or sensory nerves. Br J Pharm 1999, 128:860-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bisell MJ: The origin of myofibroblasts in breast cancer. J Clin Invest 1995, 95:859-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webber MM, Trakul N, Thraves PS, Bello-DeOcampo D, Chu WW, Storto PD, Huard TK, Rhim JS, Williams DE: A human prostatic stromal myofibroblasts cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis 1999, 20:1185-1192 [DOI] [PubMed] [Google Scholar]
- 46.Chiavegato A, Scatena M, Roelofs M, Ferrareses P, Pauletto P, Passerini-Glazel G, Pagano F, Sartore S: Cytoskeletal and cytocontractile protein composition of smooth muscle cells in developing and obstructed rabbit bladder. Exp Cell Res 2078, 1993:310-320 [DOI] [PubMed] [Google Scholar]
- 47.Sappino AP, Schurch W, Gabbiani G: Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 1990, 63:144-161 [PubMed] [Google Scholar]
- 48.Babij P, Zhao J, White S, Woodcock-Mitchell J, Absher M, Baldor L, Periasamy M, Low RB: Smooth muscle myosin regulation by serum and cell density in culture rat lung connective tissue cells. Am J Physiol 1993, 265:L127-L132 [DOI] [PubMed] [Google Scholar]
- 49.Schmitt-Graff A, Desmouliere A, Gabbiani G: Heterogeneity of myofibroblast phenotypic features an example of fibroblastic cell plasticity. Virchows Arch 1994, 425:3-24 [DOI] [PubMed] [Google Scholar]
- 50.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tisty TD, Cunha GR: Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999, 59:5002-5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noel A, Foidart J-M: The role of stroma in breast carcinoma growth in vivo. J Mammary Gland Biol Neopl 1998, 3:215-225 [DOI] [PubMed] [Google Scholar]
- 52.Lieubeau B, Garrigue L, Barbieux I, Meflah K, Gregoire M: The role of transforming growth factor-β1 in the fibroblastic reaction associated with rat colorectal tumor development. Cancer Res 1994, 54:6526-6532 [PubMed] [Google Scholar]
- 53.Hung DT, Vu TKH, Nelken NA, Coughlin SR: Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol 1992, 116:827-832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vouret-Craviari V, Van Obberghen-Schilling E, Rasmussen UB, Pavirani A, Lecocq JP, Pouysségur J: Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell 1992, 3:95-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawes KE, Gray AJ, Laurent GJ: Thrombin stimulates fibroblast chemotaxis and replication. Eur J Cell Biol 1993, 61:126-130 [PubMed] [Google Scholar]
- 56.Grubber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren S, Schwartz LB, Korn JH: Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol 1997, 158:2310-2317 [PubMed] [Google Scholar]
- 57.Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, McAnulty RJ: Mast cell tryptase stimulates human lung fibroblast proliferation via proteinase-activated receptor-2. Am J Physiol Lung Cell Mol Physiol 2000, 278:L193-L201 [DOI] [PubMed] [Google Scholar]
- 58.Vrana JA, Stang MT, Grande JP, Getz MJ: Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor-β family. Cancer Res 1996, 56:5063-5070 [PubMed] [Google Scholar]
- 59.Nguyen DHD, Hussaini IM, Gonias SL: Binding of urokinase-type plasminogen activator to its receptor in MCF-7 cells activates extracellular signal-regulated kinase 1 and 2 which is required for increased cellular motility. J Bio Chem 1998, 273:8502-8507 [DOI] [PubMed] [Google Scholar]
- 60.Evans DM, Sloan-Stakeleff KD: Role of urokinase PAIs in the control of cancer invasion and metastasis. DN&P 1997, 10:85–88