Abstract
Extracellular matrix metalloproteinase inducer (EMMPRIN), a glycoprotein present on the cancer cell plasma membrane, enhances fibroblast synthesis of matrix metalloproteinases (MMPs). The demonstration that peritumoral fibroblasts synthesize most of the MMPs in human tumors rather than the cancer cells themselves has ignited interest in the role of EMMPRIN in tumor dissemination. In this report we have demonstrated a role for EMMPRIN in cancer progression. Human MDA-MB-436 breast cancer cells, which are tumorigenic but slow growing in vivo, were transfected with EMMPRIN cDNA and injected orthotopically into mammary tissue of female NCr nu/nu mice. Green fluorescent protein was used to visualize metastases. In three experiments, breast cancer cell clones transfected with EMMPRIN cDNA were considerably more tumorigenic and invasive than plasmid-transfected cancer cells. Increased gelatinase A and gelatinase B expression (demonstrated by in situ hybridization and gelatin substrate zymography) was demonstrated in EMMPRIN-enhanced tumors. In contrast to de novo breast cancers in humans, human tumors transplanted into mice elicited minimal stromal or inflammatory cell reactions. Based on these experimental studies and our previous demonstration that EMMPRIN is prominently displayed in human cancer tissue, we propose that EMMPRIN plays an important role in cancer progression by increasing synthesis of MMPs.
Extracellular matrix metalloproteinase inducer (EMMPRIN) was originally designated tumor collagenase stimulating factor (TCSF) by Biswas et al 1 after isolation and purification of the 58-kd glycoprotein from the plasma membrane of cancer cells and demonstration of its function in stimulating fibroblast synthesis of collagenase-1 (MMP-1). The subsequent finding that EMMPRIN also induced fibroblast synthesis of gelatinase A (MMP-2) and stromelysin-1 (MMP-3) indicated a more general effect on the production of MMPs. 2 Recent studies have documented the capacity of recombinant EMMPRIN or EMMPRIN purified from cancer cells to stimulate fibroblast production/secretion of stromelysin-1, collagenase-1, and gelatinase A in vitro. 2,3 After secretion from fibroblasts, collagenase-1 is able to bind to EMMPRIN on the tumor cell surface. 4 The demonstration by in situ hybridization (mRNA localization) that peritumoral fibroblasts synthesize most of the MMPs (collagenases, gelatinases, stromelysins, and membrane type-MMPs) in human tumors rather than the cancer cells themselves has ignited interest in the role of EMMPRIN in tumor dissemination. 5,6 The association of intense EMMPRIN expression in neoplastic cells within invasive human tumors 7,8 further supports a role for EMMPRIN in cancer dissemination. These data are consistent with a central function for EMMPRIN in stimulating stromal cell production of MMPs which, after pericellular activation, directly degrade the extracellular matrix. 1
Peptide sequencing and cDNA isolation of EMMPRIN from tumor cells 1,9 led to the recognition that EMMPRIN is identical to human basigin 10 and M6 antigen, 11 proteins of previously unknown function that were identified by other investigators in embryonic and inflammatory tissues. A knockout mouse has been produced in which the murine homologue of basigin/EMMPRIN is lacking. 12 The null mutant is, in most cases, unable to undergo oocyte implantation, presumably due to the requirement for MMPs in this process. It is apparent that although many embryonic and adult tissues express EMMPRIN, the level of EMMPRIN expression and glycosylation in tumors is much greater than in corresponding normal tissues. 7,13-15
In the current study we have examined the function of EMMPRIN in a cancer model in immunodeficient mice. Human MDA-MB-436 breast cancer cells that are tumorigenic, estrogen independent, and moderately invasive in vitro, but slow growing in vivo, 16 were transfected with EMMPRIN cDNA and injected orthotopically into the mammary fat pad of nude mice. We took advantage of the observation that the 29-kd green fluorescent protein (GFP) of the jellyfish Aequoria victora retains its fluorescent properties when recombinantly expressed in eukaryotic cells 17 along with EMMPRIN cDNA and can be used as a powerful marker for gene expression and cancer dissemination in vivo. Cancer cells transfected with both EMMPRIN cDNA and GFP cDNA were compared with cancer cells transfected with GFP cDNA alone for tumorigenic behavior. The results demonstrated that tumor growth in nude mice was considerably enhanced by EMMPRIN/GFP- transfected breast cancer cells as compared to cells transfected with GFP alone.
Materials and Methods
Reagents
Restriction enzymes were purchased from Stratagene (La Jolla, CA). EMMPRIN was purified from LX-1 lung cancer cells using affinity column chromatography. 18 Monoclonal antibodies to EMMPRIN (clone 1G6.2) were produced in collaboration with Dr. D. Dembro at Chemicon International, Inc. (Temecula, CA). The F4/80 rat anti-mouse macrophage antibody was purchased from Serotec (Raleigh, NC). Thrombin was a kind gift from Dr. J. Jesty. Phorbol 12- myristate-13 acetate (PMA) was purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Lines and Culture Conditions
Human MDA-MB-436 breast cancer cells were maintained in Richter’s improved minimal essential medium supplemented with 10% donor calf serum. 16 Immunostaining of MDA-MB-436 cells was performed using a primary mouse monoclonal antibody to EMMPRIN (1G6.2) and a secondary goat anti-mouse IgG (H&L) horseradish peroxidase-labeled antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD).
Construction of Plasmids and Transfection into Cells
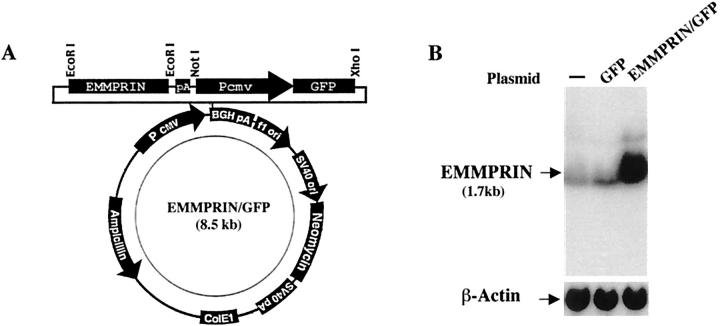
A 1.6-kb cDNA 1 representing the entire EMMPRIN sequence encoding 269 amino acid residues was placed at an EcoRI site under the control of the CMV promoter in pcDNA3 (Invitrogen, Carlsbad, CA). To facilitate identification of transfected cells in vitro and metastases in vivo, GFP (GFPmut1 variant) cDNA (Clontec Laboratory, Inc., Palo Alto, CA) was inserted into the EMMPRIN-containing plasmid. The GFP cDNA, along with a separate upstream cytomegalovirus promoter from pEGFP-C1 plasmid (Clontec), was inserted into the EMMPRIN expression vector between NotI and XhoI sites as shown in Figure 1A ▶ . An additional polyadenylation signal from pSG5 (Stratagene) was placed downstream of the EMMPRIN gene to provide balanced expression of both recombinant genes under control of CMV promoters. The resulting plasmid was named EMMPRIN/GFP. As a control plasmid, GFP cDNA alone was subcloned into pcDNA3 without EMMPRIN cDNA. In experiment 2, EMMPRIN cDNA was subcloned into pcDNA3 without GFP; the control plasmid was pcDNA3 alone.
Figure 1.
A: Schematic illustration of the EMMPRIN/GFP plasmid. A 1.6-kb cDNA representing the entire EMMPRIN sequence was placed at an EcoR 1 site under the control of the CMV promoter in pcDNA 3. GFP cDNA was inserted along with an upstream CMV promoter into the EMMPRIN expression vector between NotI and XhoI sites. A polyadenylation (PA) signal was placed downstream. B: Northern blot analysis of EMMPRIN. Approximately 20 μg of total cellular RNA from plasmid alone-transfected, GFP-transfected, and EMMPRIN/GFP-transfected MDA-MB-436 breast cancer cells (from experiment 3) was size fractionated in a 1% denaturing agarose gel, transferred to a nylon membrane, and incubated with 1.7 kb of 32P-radiolabeled EMMPRIN cDNA as a probe. Blots were analyzed by autoradiography. A single 1.7-kb mRNA transcript corresponding to the known EMMPRIN band was detected at ∼20× greater intensity in EMMPRIN/GFP-transfected cells as compared to plasmid alone or GFP-transfected cells.
The human MDA-MB-436 breast cancer cell line was stably transfected using the calcium phosphate precipitation method. 19 Selected G418-resistant clones were screened by fluorescent appearance using a Nikon microscope equipped with a xenon lamp power supply and a GFP filter set. Fluorescent positive clones were further analyzed by Northern blot analysis probed with an EMMPRIN cDNA fragment.
RNA Isolation and Northern Blot Hybridization
Total RNA was extracted from MDA-MB-436 cells stably transfected with desired plasmids by guanidine solubilization, phenol/chloroform extraction, and serial precipitation. 1,20 Approximately 20 μg of total RNA was resolved by denaturing gel electrophoresis followed by Northern transfer to nylon membranes (Schleicher and Schuell, Keene, NH). Blots were hybridized to 32P-radiolabeled EMMPRIN cDNA (1.7 kb) at 42°C as described 20 and analyzed after overnight exposure with an intensity screen at −80°C. The amount of the samples applied to the lanes was normalized by β-actin RNA.
Labeling of RNA Probes
Antisense and sense digoxigenin-labeled RNA probes for EMMPRIN, gelatinase A, and gelatinase B were synthesized by reverse-transcribing 1 μg of cDNA from a polymerase chain reaction that had used gene-specific primers that contain the T7 or T3 phage promoter sequence followed by 20–25 bases of the mRNA sequence. 21 The probes for human EMMPRIN (bases 319–701), human gelatinase A (bases 42–436), and mouse gelatinase B (bases 56–361) were designed based on published nucleotide sequences (GenBank accession numbers AH007299, J03210, and Z27231, respectively). Homology between the human and mouse nucleotide sequences for gelatinase A and gelatinase B are 91 and 78%, respectively, as determined by BLAST 2 sequence alignment (www.ncbi.nlm.nih.gov/gorf/bl2.html). In vitro transcription of the amplified DNA template was performed using the digoxigenin RNA labeling kit (Roche Molecular Biochemicals, Indianapolis, IN). Labeled probes were purified and sequences were verified.
In Situ Hybridization
Serial sections of paraffin-embedded mouse tumors were prepared for in situ hybridization according to the method of Komminoth. 22 Slides were processed for immunodetection using anti-digoxigenin alkaline phosphatase conjugate antibody (Roche Molecular Biochemicals) and then incubated with substrate solution (Wash and Block Set; Roche Molecular Biochemicals).
Cell Proliferation in Vitro
Cell proliferation assays were performed by plating MDA-MB-436 cells at 4 × 10 4 cells per well (Costar, Corning, NY) and then switched to serum-free medium. After 48 hours, serum-enriched medium was added again and cells were cultivated for 4 additional days. Cell counts were performed daily.
Tumor Formation in Mice and Preparation of Tissue Extracts
Four-week-old female athymic NCr nu/nu mice were obtained from Taconic Farms (Germantown, NY). Cancer cells (1 × 106) were injected into the mammary fat pad of nude mice. Tumor growth was monitored weekly. Tumor volume was calculated using the formula: (length) (width2)/2. At termination of experiments, mice were sacrificed, and autopsied; and tissue sections of the primary tumor, lungs, liver, lymph nodes, gastrointestinal tract, and other suspicious areas were prepared for histological/microscopic examination (hematoxylin and eosin staining of paraffin-embedded sections). Tissue sections were also stored in liquid nitrogen for subsequent in situ hybridization (see above) and extraction of MMPs. The extraction procedure for tumor tissue involved detergent and heat-extraction steps. 23
Zymography and Immunohistochemistry
Primary cell cultures were transferred to serum-free medium and cultivated for 18 hours with or without the addition of thrombin or PMA. Serum-free spent medium was then collected and tested by gelatin zymography. Gelatin substrate zymography was preformed in 10% polyacrylamide gels that had been cast in the presence of 0.1% gelatin (NOVEX, San Diego, CA). 24,25 Protein determinations were made using the bicinchoninic acid reagent (Pierce, Rockford, IL).
Immunohistochemistry for mouse macrophages, monocytes, and dendritic cells was performed using the rat anti-mouse F4/80 antibody (Serotec) as described by Tsuruga et al. 26 A biotinylated rabbit anti-rat IgG was used as the secondary antibody. Immunoreactivity was visualized by the avidin-biotin peroxidase complex method (Vectastain ABC kit; Vector Laboratories, Burlingame, CA).
Analysis of variance and Student’s t-test were used to compare differences between groups in various experiments; P < 0.05 was considered significant. Survival experiences between groups were compared by the Wilcoxen χ 2 test.
Results
Cell Transfection and Proliferation
Northern blot analysis using EMMPRIN cDNA as a probe detected ∼20-fold enhanced EMMPRIN expression by EMMPRIN/GFP-transfected cells as compared to GFP or non-transfected cells (Figure 1B) ▶ . Similar results were achieved with each of the three transfected clones used in experiments 1 to 3 (see below). Immunostaining of MDA-MB-436 cells using specific mouse monoclonal antibodies to EMMPRIN documented intense staining of EMMPRIN/GFP-transfected cells and infrequent weak staining of GFP-transfected or vector transfected cells (data not shown).
There were no significant differences in cell doubling times between GFP and EMMPRIN/GFP cDNA-transfected cells (∼18 hours) in medium with or without serum. These data are inconsistent with EMMPRIN acting as an autocrine growth factor for tumor cells in vitro.
Tumor Growth in Nude Mice
Three independent experiments, each using a different clone of EMMPRIN-transfected MDA-MB-436 cells, were performed. In experiments 1 and 2, the GFP-alone or vector-transfected clones did not form palpable tumors by the time of the experiment’s termination at 12 weeks; however, ∼0.01-cm 3 noninvasive tumors were identified at autopsy in 18 of 18 mice. In contrast, the EMMPRIN/GFP- or EMMPRIN (alone)- transfected clones formed palpable breast tumors at the site of mammary injection by week 6 in 18 of 18 mice which grew progressively to >1.7 cm 3 in diameter by week 12, at which time the animals were sacrificed. Histological examination of tissue sections revealed local cancer invasion, but no metastases.
In experiment 3, groups of 10 mice were injected with transfected MDA-MB-436 cells into mammary tissue. The tumors emanating from the EMMPRIN/GFP cDNA-transfected MDA-MB-436 cells grew relatively rapidly, and all mice expired or had to be sacrificed within 12 weeks (Figure 2A) ▶ . Extensive metastases to the liver, mediastinum, pleura, spleen, lymph nodes, and mesentery were present in 3 of 10 mice. In contrast, injection of the GFP cDNA-transfected tumor cells into mice resulted in tumors that grew considerably more slowly than EMMPRIN/GFP expressing tumors. Tumor diameter was <0.3 cm 3 and no metastases were noted at week 15 in 9 of 10 GFP-transfectant mice. One mouse in the GFP-transfected group developed a 1.4 cm 3 primary tumor by week 12. EMMPRIN/GFP and GFP expressing tumors (primary tumors and metastases) were readily visible by their expression of green fluorescence when examined grossly with fluorescent light (Figure 2B) ▶ . The enhancement effect of GFP on tumor visualization has been previously described. 27
Figure 2.
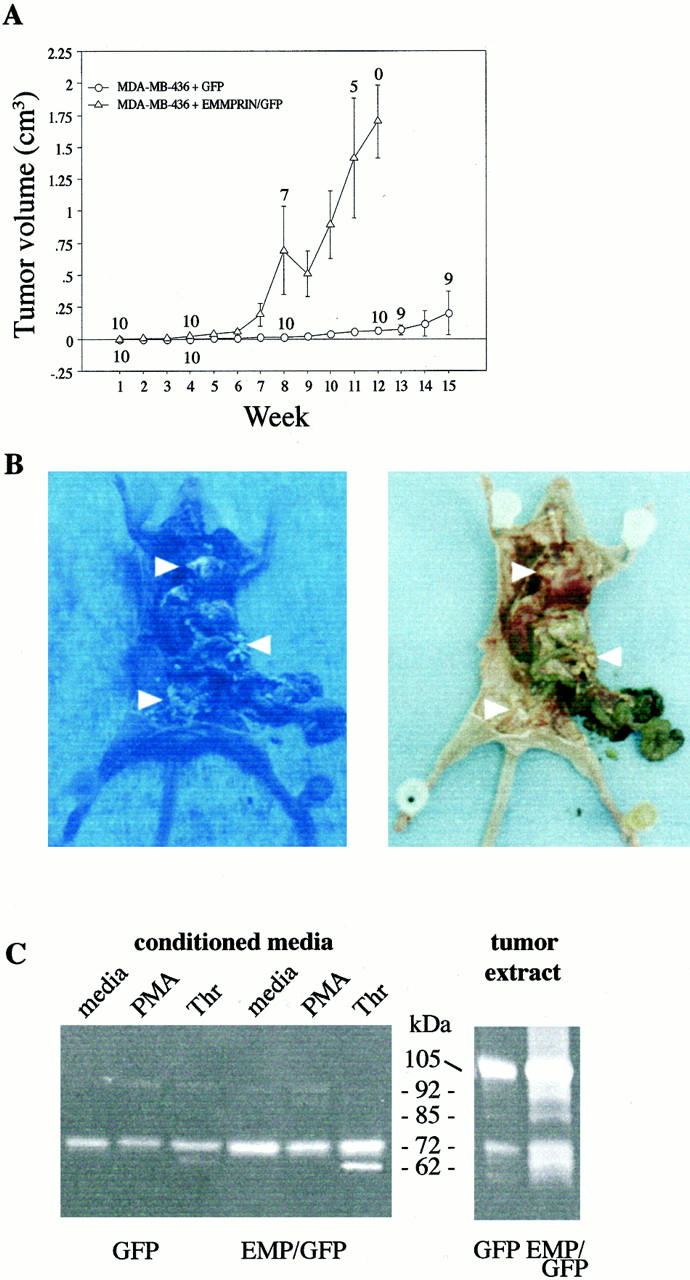
A: MDA-MB-436 breast cancer cells transfected with EMMPRIN/GFP cDNA resulted in enhanced rate of tumor growth after tumor cell implantation into the mammary fat pad of nude mice as compared to GFP-transfected cells. The tumorigenicity of transfected cells was assessed by weekly measurement of tumor size. The data represent the mean ± SE observed in 10 animals in each group injected with 1 × 10 6 transfected cancer cells. The numbers associated with each symbol refer to the number of mice alive at each time point (ie, at week 8, three mice in the EMMPRIN/GFP group had large tumors and were sacrificed, hence the number seven is listed). B: GFP-transfected tumors are readily visible under fluorescent light. EMMPRIN/GFP-transfected MDA-MB-436 breast cancer cells were injected into the mammary tissue of a female NCr nu/nu mouse. Eight weeks later, the mouse was sacrificed and extensive green colored metastatic tumors in the peritoneum, liver, spleen, and mediastinum were visible under fluorescent light (left photo). The photo on the right demonstrates the same tumors visualized by bright light (arrowheads identify tumors). C: Comparison of gelatinases secreted by MDA-MB-436 cells cultivated in serum-free medium and extracts of nude mouse tumors. Spent serum-free conditioned medium from primary cells cultivated for 18 hours with vehicle (medium), PMA, and thrombin (left) and tumor cell extracts (right) were assessed by gelatin substrate zymography. Protein concentration (15 μg/well) of tissue samples was equalized within each group. Conditioned medium and tumor extracts from the EMMPRIN/GFP group displayed more gelatinolytic activity than did the GFP-alone group of mice. The displayed extract from the GFP-alone tumor is from the largest tumor (1.4 cm 3 ) in this group of mice. The intensity of tumor gelatinolytic activity demonstrated in each group of mice did not correlate with tumor size (data not shown). Molecular weights were calculated using protein standards. The conditioned medium of HT-1080 cells was used to confirm the molecular weight of human gelatinase A and gelatinase B (data not shown).
Gelatinolytic Activity Extracted from Tumor Tissues and Cells
Gelatin zymograms of conditioned medium from 18-hour cultivated MDA-MB-436 tumor cells (Figure 2C ▶ , left panel) revealed that cells transfected with EMMPRIN/GFP cDNA secreted more than threefold more progelatinase A (72 kd) than did GFP cDNA-transfected cells. Treatment of both sets of transfected cells with thrombin (20 nmol/L) enhanced both secretion and activation of progelatinase A (more prominently displayed in the EMMPRIN/GFP-transfected cells). Treatment of cells with PMA (100 nmol/L) resulted in the appearance of weak gelatinolytic bands at 92 kd consistent with human progelatinase B.
Extracts of tumors derived from EMMPRIN/GFP cancer cell injections in mice displayed intense gelatinolytic bands localized at 105, 92, 85, 72, and 64–62 kd. Figure 2C ▶ (right panel) is representative of both groups of mice in experiment 3; the primary tumors from mice with metastases did not display higher levels of gelatinases than the nonmetastatic group (data not shown). The 105-kd band is consistent with mouse latent gelatinase B; human latent gelatinase B and activated mouse gelatinase B migrates at ∼92 kd. 28 The 72-kd and 62-kd gelatinolytic bands could represent human or mouse latent and activated gelatinase A, respectively. Tumor extracts from GFP alone-injected mice revealed weaker gelatinolytic bands (with minimal activated gelatinolytic bands) than EMMPRIN/GFP-injected mice.
Histochemistry and in Situ Hybridization
Hematoxylin and eosin staining of resected breast masses revealed extensive replacement of normal mammary tissue with carcinoma in tumors originating from mice injected with EMMPRIN/GFP- or GFP cDNA-transfected MDA-MB-436 cells; other than size of the tumor masses, the EMMPRIN-transfected and vector-transfected tumors were indistinguishable by routine staining. Minimal fibrosis and inflammatory cell infiltration were noted in tumor tissue and surrounding normal-appearing mammary tissue. The sparsity of inflammatory cells in the tumors was confirmed using an antibody (F4/80) that recognizes mouse macrophages, monocytes, and dendritic cells (data not shown).
In situ hybridization of tumor tissue from six mice injected with EMMPRIN/GFP-transfected cells revealed widely distributed, specific staining for EMMPRIN mRNA in cancer cells (Figure 3 ▶ , panel 2). Surrounding normal-appearing mammary ductal cells and scattered periductal cells also expressed EMMPRIN mRNA (panel 6). Gelatinase A mRNA was found in both cancer cells (panel 3) and the surrounding non-malignant tissue, including normal-appearing mammary ducts and adipose cells (panel 7). There was specific staining for gelatinase B mRNA in the tumor sections (panel 4), but not as widely distributed as gelatinase A. By counting the number of stained cells in serial sections of EMMPRIN cDNA-transfected tumors, the ratio of cells immunotyped as macrophages (F4/80 antibody) versus gelatinase B mRNA-expressing cells was ∼1:70. Intense staining for gelatinase B was also noted in small aggregates of cells (negative staining for mouse macrophages using F4/80 antibody) scattered around normal-appearing ducts (panel 8). Similar in situ hybridization results were found on examination of metastatic tumors in the EMMPRIN/GFP-treated mice (data not shown). Similar results were achieved using either human or mouse gelatinase A and gelatinase B mRNA probes. Specific staining was abolished by pretreatment of tissues with RNase (data not shown). No staining was detected in any of the tumor tissues that were hybridized with EMMPRIN, gelatinase A, or gelatinase B sense probes (data not shown).
Figure 3.
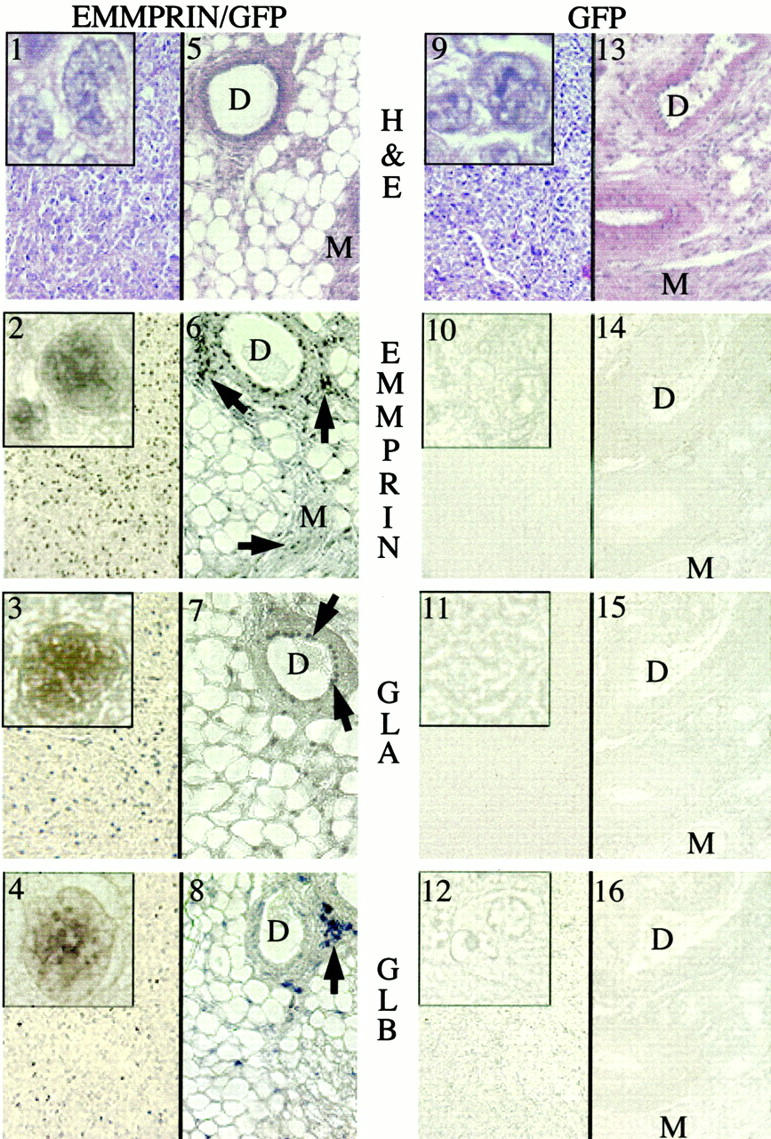
In situ hybridization of primary tumors from mice injected with EMMPRIN/GFP and GFP-transfected MDA-MB-436 breast cancer cells. Serial sections from tumor tissue (panels 1–4, and 9–12) and surrounding nonmalignant tissue (panels 5–8 and 13–16) were examined. Panels 1 and 9 represent H&E staining of cancer tissues from EMMPRIN/GFP and GFP tumors, respectively; panels 5 and 13 represent H&E staining of non malignant mammary tissues (tumor cells not identified) adjacent to the primary EMMPRIN/GFP and GFP tumors, respectively. Cells in the primary tumor mass from mice injected with EMMPRIN/GFP-transfected cells revealed widely distributed, specific staining with EMMPRIN, gelatinase A (GLA), and gelatinase B (GLB) antisense riboprobes (panels 2–4, respectively). Minimal cell staining for EMMPRIN, gelatinase A, and gelatinase B was seen in cancer cells from GFP-transfected MDA-MB-436 cells (panels 10–12). Nonmalignant tissues adjacent to the primary tumors from EMMPRIN/GFP mice demonstrated focal staining for EMMPRIN, gelatinase A, and gelatinase B in mammary ducts (D) and myocytes (M) (panels 6–8: arrows identify EMMPRIN-expressing cells). Nonmalignant tissue from GFP mice revealed no discernable staining for EMMPRIN, gelatinase A, or gelatinase B (panels 14–16). The insets in panels 1–4 display higher magnifications of mRNA-stained tumor cells; prominent nuclear staining is noted. Comparable cells (but minimally stained) in the GFP-transfected tumors are demonstrated in panels 10–12.
In the GFP-alone-transfected tumors (seven mice examined), virtually no EMMPRIN, gelatinase A, or gelatinase B mRNA was identified in the tumor cells or in the surrounding normal-appearing mammary tissue (Figure 3 ▶ , panels 10 to 12 and 14 to16).
Discussion
The current report describes a direct effect of EMMPRIN expression on tumorigenicity in an animal model. Transfection of EMMPRIN cDNA or EMMPRIN/GFP cDNA into human MDA-MB-436 breast cancer cells resulted in marked enhancement of tumor growth in nude mice after orthotopic injection of tumor cells as compared to injection of vector or GFP alone-transfected tumor cells. High levels of gelatinase A and gelatinase B mRNA expression were demonstrated by in situ hybridization in EMMPRIN-transfected tumors as compared to vector- or GFP-transfected tumors. Regardless of the size of the tumors, enhanced gelatinase B and gelatinase A levels were identified in zymograms from extracts of EMMPRIN/GFP-transfected tumors as compared to GFP tumors. The 105-kd gelatinolytic band represents mouse gelatinase B, 29 but the 92-kd band could be either activated mouse gelatinase B or latent human gelatinase B.
It is noteworthy that human MDA-MB-436 cells propagated in vitro readily secreted progelatinase A, but secreted minimal gelatinase B, whereas extracts of tumors removed from nude mice injected with these tumor cells contained higher levels of gelatinase B than gelatinase A. Treatment of these breast cancer cells with thrombin and PMA in vitro resulted in increased secretion and activation of progelatinase A and progelatinase B, respectively; this is consistent with the stimulatory effects of these agents described with other types of cells. 25,30 These observations are consistent with the concept that both mouse host cells and transplanted human cancer cells are responsible for the production of gelatinase A and gelatinase B in nude mouse tumors. An association between expression of EMMPRIN and gelatinase B in benign and malignant pigment cell skin lesions in humans has been reported, 31 but a direct stimulatory effect of EMMPRIN on gelatinase B expression or activation has not been previously described. EMMPRIN expression has also been linked to the activation of progelatinase A through a MT-MMP mechanism. 32
Another important observation in this study was that EMMPRIN-transfected MDA-MB-436 cancer cells secreted higher levels of gelatinase A in vitro than vector-transfected cells; this presumably represents autocrine stimulation. These cancer cells also displayed a more invasive phenotype than control transfectants when examined in a modified Boyden chamber (S. Caudroy, M. Polette, B. Nawrocki-Raby, B. Toole, S. Zucker, and P. Birembaut, submitted manuscript).
Our in situ hybridization data of tumors transplanted into nude mice differs from de novo human breast cancer. Gelatinase A mRNA was identified in EMMPRIN-transfected human cancer cells growing both in nude mice and in surrounding host stromal cells (Figure 3) ▶ . Previous studies in patients with breast cancer demonstrated the expression of gelatinase A and gelatinase B almost exclusively in peri-tumoral, stromal, and inflammatory cells, respectively. 6,8,33 However, a few reports have described gelatinase B expression in breast, 34 lung, 35 and liver carcinoma cells. 35,36 In comparing experimental cancer models to the human counterpart, it needs to be emphasized that cancer cell lines propagated in vitro (eg, MDA-MB-436) that are selected for their invasive properties generally express high levels of gelatinases. 37 Furthermore, by comparison to in situ human breast cancers, 38 transplanted human tumors in nude mice demonstrate sparse inflammatory and fibrotic reactions; this represents an important distinction that is often overlooked. These differences between human and animal models of cancer need to be considered in predicting human responses to novel therapies developed in experimental animal models.
A technical aspect of this study that needs explanation relates to quantitative differences in expression of EMMPRIN, gelatinase A, and gelatinase B using different methodologies (Figures 1B, 2C, and 3) ▶ ▶ ▶ . It should be noted that the nonradioactive digoxigenin-labeled RNA probes used in this study provide increased resolution and rapid detection of cellular messages, but are less sensitive than autoradiography 39 and substrate zymography.
In one of three sets of experiments, metastasis after orthotopic injection of tumor cells into nude mice occurred more frequently with EMMPRIN-transfected cells than with vector-transfected cells, but the overall rate was low. As we reported previously, 40 EMMPRIN expression did not affect tumor cell proliferation in vitro. Based on the established role of EMMPRIN in enhancing MMP synthesis by stromal cells, it would appear that increased degradation of extracellular matrix permits more rapid tumor growth in vivo. The higher rate of tumor growth with EMMPRIN-transfected cancer cells and the associated matrix degradation may also occur by favored neoplastic cell survival in a tissue stroma environment initially not permissive for tumor growth. Enhanced extracellular matrix degradation may also release growth factor-like fragments of matrix components, resulting in an indirect effect on cell proliferation. 41 A role for host-derived MMPs in tumor progression and angiogenesis has been supported by studies in gelatinase A-deficient (knockout) mice. 42 In contrast to these findings with EMMPRIN, stromelysin-3 (an MMP with minimal proteolytic activity on extracellular matrix proteins) expression in cancer cells promoted tumor take, but not tumor growth in nude mice. 43 These studies with EMMPRIN reinforce the notion that cancer dissemination is a multistep process and that extracellular matrix degradation contributes to the process but is insufficient in itself to account for tumor metastasis. 44 Continued exploration of genes responsible for the metastatic process is warranted. 45
Acknowledgments
This article is dedicated to the memory of our friend and colleague, Chitra Biswas, whose career was dedicated to the discovery and exploration of EMMPRIN. Dr. Biswas died in August 1993, but her inspiration continues to guide us in our studies of EMMPRIN. We thank Dr. Serge Lyubsky for his contribution to the histopathological studies and Dr. Philippe Birembaut for helpful discussions.
Footnotes
Address reprint requests to Stanley Zucker, Mail Code 151, VA Medical Center, Northport, NY 11768. E-mail: s_zucker@yahoo.com.
Supported by Department of Defense Breast Cancer grant DAMD 17-95-5017 and DAMD 17-99-9413 a REAP grant from the Department of Veterans Affairs, and National Institutes of Health grant RO1-CA79866.
References
- 1.Biswas C, Zhang Y, DeCastro R, Guuo H, Nakamura T, Kataoka H, Nabeshima K: The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 1995, 55:434-439 [PubMed] [Google Scholar]
- 2.Kataoka H, DeCastro R, Zucker S, Biswas C: The tumor cell-derived Collagenase Stimulating Factor, TCSP, increases expression of interstitial collagenase, stromelysin and 72 kDa gelatinase. Cancer Res 1993, 53:3155-3158 [PubMed] [Google Scholar]
- 3.Guo H, Zucker S, Gordon M, Toole BP, Biswas C: Stimulation of metalloproteinase production by recombinant EMMPRIN from transfected CHO cells. J Biol Chem 1996, 272:24-27 [PubMed] [Google Scholar]
- 4.Guo HL, Zucker S, Toole BP: EMMPRIN (CD147) an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer Res 2000, 60:888-891 [PubMed] [Google Scholar]
- 5.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P: A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 1990, 348:699-704 [DOI] [PubMed] [Google Scholar]
- 6.Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH: Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol 1996, 149:273-282 [PMC free article] [PubMed] [Google Scholar]
- 7.Polette M, Toole B, Tournier J-M, Zucker S, Birembaut P: TCSF expression and localization in human lung and breast cancer. J Histochem Cytochem 1997, 45:703-709 [DOI] [PubMed] [Google Scholar]
- 8.Dalberg K, Eriksson E, Enberg U, Kjellman M, Backdahl M: Gelatinase A, membrane type 1 matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: correlation with invasive growth of breast cancer. World J Surg 2000, 24:334-340 [DOI] [PubMed] [Google Scholar]
- 9.Nabeshima K, Lane WS, Biswas C: Partial sequencing and characterization of the rumor cell-derived collagenase stimulatory factor. Arch Biochem Biophys 1991, 285:90-96 [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi T, Masuzawa Y, Muramatsu T: The basigin group of the immunoglobulin superfamily: complete conservation of a segment in and around transmembrane domains of human and mouse basigin and chicken HT7 antigen. J Biochem 1991, 110:770-774 [DOI] [PubMed] [Google Scholar]
- 11.Kasinrerk W, Fiebiger E, Steffanova I, Baumruker T, Knapp W, Stockinger H: Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol 1992, 149:847-854 [PubMed] [Google Scholar]
- 12.Igakara T, Kadomatsu K, Kaname T, Muramatsu T, Fan Q-W, Yuasa S, Takahashi M, Senda T, Taguchi O, Yamamura KI, Arumura K, Muramatsu T: A null mutation in basigin, an immunoglobulin superfamily member, indicates important role in peri-implantation andspermatogenesis. Dev Biol 1998, 194:152-163 [DOI] [PubMed] [Google Scholar]
- 13.Caudroy S, Polette M, Tournier JM, Toole B, Zucker S, Birembaut P: Expression of extracellular matrix metalloproteinase-inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J Histochem Cytochem 1999, 47:1575-1580 [DOI] [PubMed] [Google Scholar]
- 14.Bordador LC, Li X, Toole B, Chen B, Regezi J, Zardi L, Hu Y, Ramos DM: Expression of EMMPRIN by oral squamous cell carcinoma. Int J Cancer 2000, 85:347-352 [PubMed] [Google Scholar]
- 15.Sameshima T, Nabeshima K, Toole BP, Yokogami K, Goya T, Koono M, Wakisaka S: Expression of EMMPRIN (CD147), a cell surface inducer of matrix metalloproteinases, in normal brain and gliomas. Int J Cancer 2000, 88:21-27 [DOI] [PubMed] [Google Scholar]
- 16.Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, Shima TB, Torri J, Konahue S, Lipman ME, Martin GM, Dickson RB: Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimetin in human breast cancer cell lines. J Cell Physiol 1992, 150:534-544 [DOI] [PubMed] [Google Scholar]
- 17.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC: Green fluorescent protein as a marker of gene expression. Science 1994, 263:802-805 [DOI] [PubMed] [Google Scholar]
- 18.Ellis SM, Nabishima K, Biswas C: Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulating factor. Cancer Res 1989, 49:3385-3391 [PubMed] [Google Scholar]
- 19.Cao J, Sato J, Takino T, Seiki M: The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for progelatinase A activation. J Biol Chem 1995, 270:801-805 [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Rehemtulla A, Bahou W, Zucker S: Membrane type matrix metalloproteinase 1 (MT-MMP1) activates progelatinase A without furin cleavage of the N-terminal domain. J Biol Chem 1996, 271:30174-30180 [DOI] [PubMed] [Google Scholar]
- 21.Birk PE, Grimm PC: Rapid nonradioactive in situ hybridization for interleukin-2 mRNA with riboprobes generated using the polymerase chain reaction. J Immunol Methods 1994, 167:83-89 [DOI] [PubMed] [Google Scholar]
- 22.Komminoth P: Detection of mRNA in tissue sections using DIG-labeled RNA and oligonucleotide probes. Nonradioactive in Situ Hybridization Application Manual. 1996, :pp 126-135 J Keesey, M Leous, R van Miltenburg, C Schroeder. Edited by S Grunewald-Jahno [Google Scholar]
- 23.Woessner JF: Quantification of matrix metalloproteinases in tissue samples. Barrett AJ eds. Proteolytic Enzymes: Aspartic and Metallopeptidases. 1995, :pp 510-528 Academic Press, San Diego [DOI] [PubMed] [Google Scholar]
- 24.Zucker S, Mirza H, Conner C, Lorenz A, Drews M, Bahou WF, Jesty J: Vascular endothelial growth factor induces a tissue factor cascade in endothelial cells: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer 1998, 75:780-786 [DOI] [PubMed] [Google Scholar]
- 25.Zucker S, Conner C, DiMassimo BI, Ende H, Drews M, Seiki M, Bahou WF: Thrombin induces the activation of progelatinase A in vascular endothelial cells: physiologic regulation of angiogenesis. J Biol Chem 1995, 270:23730-23738 [DOI] [PubMed] [Google Scholar]
- 26.Tsuruga E, Sakakura Y, Yajima T, Shide N: Appearance and distribution of dendritic cells and macrophages in dental pulp during early postnatal morphogenesis of mouse mandibular first molars. Histochem Cell Biol 1999, 112:193-204 [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Jiang P, Sun F-X, Hasegawa S, Baranov E, Chishima T, Shimada H, Moossa AR, Hoffman RM: A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res 1999, 59:781-786 [PubMed] [Google Scholar]
- 28.Bourguignon LYW, Gunja-Smith Z, Iida N, Zhu HB, Young LJT, Muller WJ, Cardiff RD: CD44v3, 8–10 is involved in cytoskeleton-mediumted tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol 1998, 176:206-215 [DOI] [PubMed] [Google Scholar]
- 29.Tanaka H, Hojo K, Yoshida H, Yoshioka T, Sugita K: Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun 1993, 190:732-740 [DOI] [PubMed] [Google Scholar]
- 30.Foda HD, George S, Conner C, Drews M, Tompkins DC, Zucker S: Activation of human umbilical vein endothelial cell progelatinase A by phorbol myristate acetate (PMA): A protein kinase C-dependent mechanism involving a membrane-type matrix metalloproteinase. Lab Invest 1996, 74:538-545 [PubMed] [Google Scholar]
- 31.van den Oorg JJ, Paemen L, Opdenakker G, De Wolf-Peeters C: Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am J Pathol 1997, 151:665-670 [PMC free article] [PubMed] [Google Scholar]
- 32.Toole BP, Zucker S: EMMPRIN, a tumor cell surface inducer of matrix metalloproteinase production in stroma cells. Cancer Res Alert 2000, 2:13-24 [PubMed] [Google Scholar]
- 33.Polette M, Birembaut P: Matrix metalloproteinases in breast cancer. Breast J 1996, 2:209-220 [Google Scholar]
- 34.Soini Y, Hurskainen T, Hoyhtya M, Oikarinen A, Autio-Harainen H: 72 kD and 92 kD type IV collageanse, type IV collagen and laminin mRNAs in breast cancer: a study by in situ hybridization. J Histochem Cytochem 1994, 42:945-951 [DOI] [PubMed] [Google Scholar]
- 35.Canete-Soler R, Litzky L, Lubenski I, Muschel RJ: Localization of the 92 kd gelatinase mRNA in squamous cell and adenocarcinomas of the lung using in situ hybridization. Am J Pathol 1994, 144:518-527 [PMC free article] [PubMed] [Google Scholar]
- 36.Ashida K, Nakatsukasa H, Higashi T: Cellular distribution of 92-kDa typeIV collagenase/gelatinase B in human hepatocellular carcinoma. Am J Pathol 1996, 149:1803-1818 [PMC free article] [PubMed] [Google Scholar]
- 37.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, Klebe RJ: Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol 1998, 16:483-496 [DOI] [PubMed] [Google Scholar]
- 38.Noel A, Munaut C, Nusgens B, Lapiere CM, Foidart JM: Different mechanisms of extracellular matrix remodeling by fibroblasts in response to human mammary neoplastic cells. Invasion Metastasis 1993, 13:72-81 [PubMed] [Google Scholar]
- 39.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K: In situ hybridization and immunohistochemistry. Curr Protocols Mol Biol 1995, 2(Supp 13): 14.13.11
- 40.Zucker S, Biswas C: Tumor collagenase stimulating factor (TCSF): A paracrine stimulating factor of fibroblast production of matrix metalloproteinases in cancer. Bull Inst Pasteur 1994, 92:282-290 [Google Scholar]
- 41.Chambers AF, Matrisian L: Changing view of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997, 89:1260-1270 [DOI] [PubMed] [Google Scholar]
- 42.Itoh T, Tanioka M, Yoshida H, Nishimoto H, Itohara S: Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 1998, 58:1048-1051 [PubMed] [Google Scholar]
- 43.Noel AC, Lefebre O, Maquoi E, Van Hoorde L, Chenard MP, Mareel M, Foidard J-M, Basset P, Rio M: Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest 1996, 97:1924-1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM: Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vitro. Cancer Res 1994, 54:4805-4812 [PubMed] [Google Scholar]
- 45.Clark EA, Golub TR, Lander ES, Hynes RO: Genomic analysis of metastasis reveals an essential role for RhoC. Nature 2000, 406:532-535 [DOI] [PubMed] [Google Scholar]