Abstract
Recent evidence suggests that adult-derived stem cells, like their embryonic counterparts, are pluripotent. These simple, undifferentiated and uncommitted cells are able to respond to signals from their host tissue microenvironment and differentiate, producing progeny that display a phenotype characteristic of the mature cells of that tissue. We used a clonal stem cell line (termed WB-F344) that was derived from an adult male rat liver to investigate the possibility that uncommitted stem cells from a nonmyogenic tissue source would respond to the tissue microenvironment of the heart in vivo and differentiate into cardiac myocytes. Male WB-F344 cells that carry the Escherichia coli β-galactosidase gene were identified in the left ventricular myocardium of adult female nude mice 6 weeks after transplantation. We confirmed the presence of a rat Y-chromosome-specific repetitive DNA sequence exclusively in the β-galactosidase-positive myocytes by polymerase chain reaction and fluorescence in situ hybridization. Immunohistochemistry, using a cardiac troponin T-specific monoclonal antibody, and ultrastructural analysis confirmed a cardiac myocyte phenotype of the stem cell-derived myocytes. The β-galactosidase-positive myocytes ranged from <20 μm to 110 μm in length. The longer of these cells contained well-organized sarcomeres and myofibrils, and formed intercalated disks and gap junctions with endogenous (host-derived) myocytes, suggesting that WB-F344-derived myocytes participate in the function of the cardiac syncytium. These results demonstrate that adult liver-derived stem cells respond to the tissue microenvironment of the adult heart in vivo and differentiate into mature cardiac myocytes.
Recent studies have emphasized the dominance of the tissue microenvironment on the differentiation and functional properties of stem cells after their transplantation and engraftment. 1-16 Embryonic stem cells that home in a microenvironment have been shown to respond to cues in the surrounding niche and to acquire the phenotypic characteristics of cells native to that tissue microenvironment. Several lines of evidence suggest that adult-derived stem cells, like their embryonic counterparts, possess multipotent differentiation capacity. 1-6 Neural stem cells derived from adult donors differentiate into a hematopoietic lineage when engrafted into bone marrow. 4,9 Adult-derived bone marrow stem cells differentiate in vivo into neural 5,7,10,15,16 , skeletal muscle, 11,12 and hepatic cells 13,14 after their transplantation and engraftment into these tissue sites. The results from these studies suggest that undifferentiated stem cells derived from adult tissues are not determined progenitor cells with limited differentiation potential. Rather, these cells seem to possess a much broader capacity for cellular differentiation that is dependent on and responsive to the specific signals present in the microenvironment of the transplantation site. That adult-derived stem cells possess this form of reactive plasticity of differentiation capacity raises the intriguing possibility that stem cells isolated from extracardiac tissue in a patient could be used to repair the damaged heart of the same patient.
We tested the concept that adult-derived stem cells will differentiate into cardiac myocytes in the tissue microenvironment of the heart in vivo using a clonal stem cell line (WB-F344) derived from the adult liver. 17-22 The WB-F344 cell line was isolated and cloned from the liver of a young adult male Fischer 344 rat and established as a propagable cell line under conditions that excluded differentiated hepatocytes or biliary epithelial cells as the cells of origin. 17-22 WB-F344 cells are diploid, anchorage-dependent, express contact inhibition in vitro, and are not tumorigenic. They exhibit a poorly differentiated nonhepatocytic simplified epithelial cell phenotype in vitro. After exposure to differentiating stimuli in vitro, WB-F344 cells express phenotypes with characteristics of hepatocytes. 20 Most important, in the context of the present study, WB-F344 cells transplanted into syngeneic rat liver give rise to differentiated hepatocytes and biliary epithelial cells in vivo. 21,22,23 We transplanted WB-F344 cells into normal hearts of adult female nude mice to examine the engraftment and differentiation potential of this male adult-derived stem cell line in the cardiac microenvironment in vivo. We present evidence that the liver-derived WB-F344 stem cells modulate their lineage commitment after transplantation into the cardiac tissue microenvironment in the adult heart and differentiate into cardiac myocytes in vivo.
Materials and Methods
Cell Culture and Transplantation
Male WB-F344 cells were genetically tagged with the E. coli lac Z gene (β-galactosidase) by infection with the CRE BAG2 retrovirus. 21 WB-F344 cells were cultured in Richter’s minimal essential medium supplemented with 10% fetal calf serum, in a 5% CO2, 95% air environment, at 37°C. Cells intended for transplantation were harvested by trypsinization, washed in cell culture medium, and resuspended in serum-free medium at 2 × 10 5 cells/ml. Female homozygote nude mice (Tac:Cr:(NCr)-nufBR) were anesthetized with ketamine (50 mg/kg) and xylazine (2.5 mg/kg) injected intraperitoneally. Cell injections were performed with the needle attached directly to a tuberculin syringe or a butterfly infusion system and introduced over the subxyphoid area and directed cephalad toward the left lateral chest. When blood was aspirated from the left ventricle, the cells (50 μl of 2 × 10 5 cells/ml) were injected, presumably in the wall of the ventricle, as the needle was withdrawn. Control animals were handled identically, and received sham injections of serum-free medium. The mice were euthanized 6 weeks later by inhalation of isofluorane. National Institutes of Health guidelines for the usage of laboratory animals were strictly followed. The experimental protocol was approved by the Duke Institutional Animal Care and Use Committee.
Histochemistry and Immunohistochemistry
The hearts were dissected and sliced coronally into three blocks, which were immediately frozen in liquid nitrogen, and stored at −80°C. Five serial cryostat sections (8 μm) were obtained from each block and used to screen for β-galactosidase activity, using a β-galactosidase-staining kit from Boehringer Mannheim (Mannheim, Germany). For the β-galactosidase reaction, cryosections on glass slides were fixed for 10 minutes in 2% formaldehyde (Mallinckrodt, Paris, Kentucky), 0.2% glutaraldehyde (Fisher, Pittsburgh, PA), in 0.1 mol/L sodium phosphate buffer, pH 7.3. The sections were then extensively washed in this buffer and incubated at 37°C with the β-galactosidase substrate, 3-indolyl-β-d-galactopyranoside (X-gal; Boehringer Mannheim, Mannheim, Germany) for 1 to 4 hours. If β-galactosidase-positive cells were not found on the initial screen, the heart blocks were not sectioned further. Blocks that contained β-galactosidase-expressing cells were selected and serially sectioned obtaining 70 serial 8-μm sections. These sections were kept at −80°C until needed. The β-galactosidase expressed in the cytoplasm of WB-F344 cells is bacterial in origin and is optimally activated at pH 7 to 7.5, whereas mammalian (lysosomal) β-galactosidase is activated at lower pH (pH 3.0 to 6.0). We monitored the pH of all solutions used in the β-galactosidase reactions and strictly maintained the pH at 7.3. This rigorous control of the pH enhanced the possibility that only bacterial β-galactosidase activity was detected.
Demonstration of cardiac troponin T was achieved using a monoclonal antibody (mAb) (designated mAb 13-11) that recognizes a cardiac-specific troponin T epitope. 24 For this purpose, frozen tissue sections were fixed in 1% formaldehyde in phosphate-buffered saline permeabilized with 0.1% Triton, and incubated with mAb 13-11, as previously described. 25 A goat fluorescein-labeled anti-mouse antibody was used as a secondary antibody. Normal mouse serum was substituted for the primary antibody in control immunostaining reactions.
Electron Microscopy
Fresh frozen heart muscle sections were fixed in 1% formaldehyde while still adherent on the glass slides as described above for the β-galactosidase reaction. After performing the β-galactosidase reaction, the sections were postfixed in 1% osmium tetraoxide, dehydrated in a graded ethanol series, and infiltrated in L. R. White resin (Ted Pella, Redding, CA). After separating the glass from the resin, β-galactosidase-positive myocytes were selected and microsectioned at 70 nm. The thin sections were examined with a LEO EM 910 transmission electron microscope at 80 kV. No lead citrate or uranyl acetate was used for postfixation or counterstaining.
Laser Capture Microdissection (LCM)
After performing the β-galactosidase reaction, tissue sections were washed in phosphate-buffered saline and dehydrated according to the National Institutes of Health protocol for LCM ( Ref 26 ; http://dir.nichd.nih.gov/1 cm/LCMTAP.htm). Single β-galactosidase-expressing myocytes and endogenous (host) myocytes were independently microdissected from recipient heart sections using an Arcturus Pixcell II laser capture microdissection instrument and the instructions supplied with the instrument. Four WB-F344-derived β-galactosidase-positive myocytes were captured on one cap membrane and two cells on another (Figure 1, c and d) ▶ . Twenty myocytes that did not express β-galactosidase (host myocytes) were captured on a third membrane. The caps were inserted into Eppendorf tubes containing 50 μl of a digestion buffer suggested by the National Institutes of Health protocol (0.04% proteinase K, 1 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L Tris-HCl, pH 8.0, and 1% Tween-20). The digestion was allowed to continue overnight at 37°C. The resulting nucleic acid fraction served as a template for amplification of a rat Y-chromosome-specific repetitive DNA sequence using primers previously described: 27 5′-GGTTCTAGACTGTAAAACCCAGAC-3′ and 5′-ACTTAAAACTAAGCTTATTGGCCA-3′. A portion (10 μl) of the polymerase chain reaction (PCR) reactions were analyzed on 1.5% agarose gels (Figure 1e) ▶ and the remaining PCR product was cloned into a TA cloning vector (InVitrogen, Carlsbad, CA). The resulting clones were screened for the Y-chromosome-specific repetitive DNA sequence by PCR, and subsequently, positive clones were subjected to automated DNA sequencing.
Figure 1.
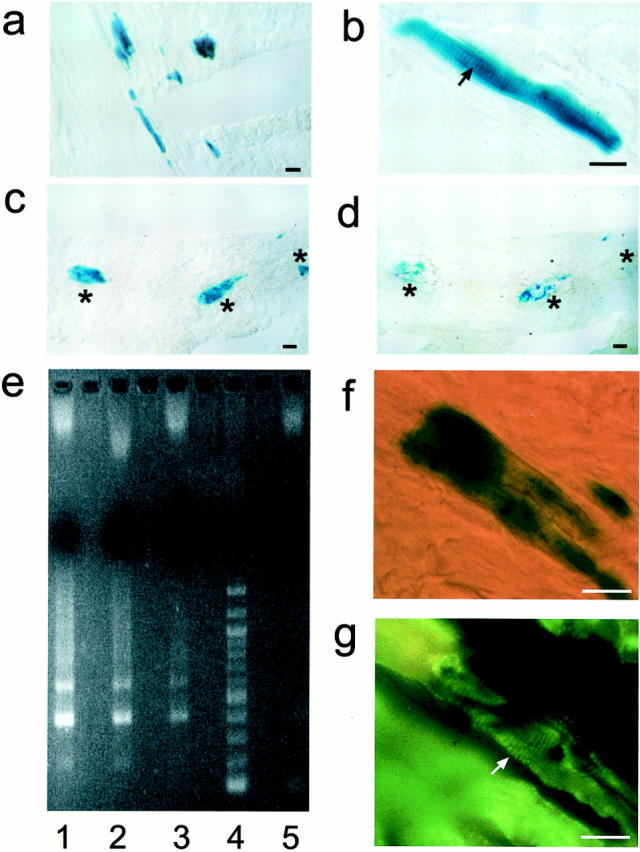
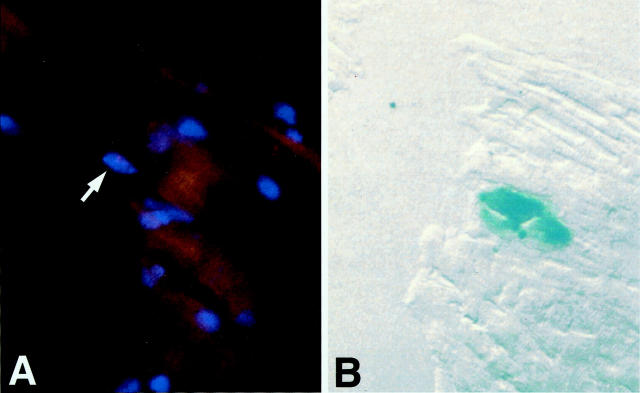
WB-F344 cells engraft in the myocardium. Engrafted WB-F344 cells express β-galactosidase (a–c) and range from small undifferentiated cells to long striated cells that measure up to 110 μm in length (b). β-galactosidase-expressing myocytes before dissection by LCM (c, asterisks) and after (d, asterisks) were used as a template to amplify by PCR a rat Y-chromosome-specific repetitive DNA sequence. e: Agarose electrophoresis of the PCR product. Unlike myocytes from control female mice (lane 5) but like WB-F344 cells grown in culture (lane 2), β-galactosidase-expressing myocytes (lanes 1 and 3) contain multimeters of the rat Y-chromosome repetitive 200-bp sequence (200, 400, 600 bp). e: lane 1, PCR product from four blue WB-F344-derived myocytes dissected by LCM from a female mouse donor heart section; lane 2, PCR product from WB-F344 cells in culture; lane 3, PCR product from two blue WB-F344-derived myocytes dissected by LCM from a female mouse donor heart section; lane 4, molecular weight standard (New England Biolabs, Beverly, MA); lane 5, PCR product from 20 random control native myocytes. f: A β-galactosidase-expressing myocyte demonstrates, after permeabilization and incubation with mAb 13-11, fluorescent sarcomeric striations (g, arrow) in the same cell. Scale bar, 25 μm.
Fluorescence in Situ Hybridization (FISH)
The FISH reaction was performed on fresh-frozen 8-μm tissue sections that immediately preceded or followed a section that demonstrated β-galactosidase-expressing myocytes. A rat Y-probe, 27 consisting of a Y-chromosome-specific repetitive DNA sequence in pUC18 plasmid (kindly provided to us by Dr. Barbara Hoebee, Bilthoven, The Netherlands) was used to test whether the β-galactosidase-expressing cells originated from the transplanted WB-F344 cells. The Y-probe-containing plasmid was labeled with digoxigenin by nick translation (Boehringer Mannheim). FISH was performed on fresh frozen tissue sections fixed in 1:1 (v/v) acetone-methanol solution. DNA was denatured in a solution of 70% formamide (Omnipur, Gibbstown, NJ) in 2× standard saline citrate (0.3 mol/L NaCl, 30 mmol/L sodium citrate) at 70°C for 12 minutes. 28 The probe was denatured at 72°C and applied to the tissue section overnight at 37°C in a humidified chamber. The sections were washed three times for 10 minutes each in 50% formamide in 2× standard saline citrate solution at 42°C, followed by two washes in 2× standard saline citrate for 5 minutes each and incubated with a sheep anti-digoxigenin-rhodamine-labeled antibody (Roche, Indianapolis, IN). After this incubation, the slides were washed, covered using a mounting medium containing 4,6-diamidino-2-phenylindole counter stain (Vectashield; Vector Laboratories, Inc., Burlingame, CA), and examined using a Zeiss confocal fluorescence microscope equipped with a triple-band pass filter.
Results
WB-F344-derived myocytes were found engrafted in the myocardium of host mice 6 weeks after cardiac injection. WB-F344-derived myocytes were identified among host myocytes based on their expression of histochemically detectable β-galactosidase activity (Figures 1 and 2) ▶ ▶ . WB-F344-derived myocytes were found on the initial screen in the left ventricular myocardium of two mice. Hearts from the other eight mice did not demonstrate β-galactosidase-expressing cells on the initial screen and were not sectioned further. It is conceivable that in these mice the injected cells entered the left ventricle and became scattered throughout the systemic circulation. Alternately, β-galactosidase-positive myocytes might be present within aspects of the heart tissues that were not sectioned and analyzed in the present study. Future studies will examine these possibilities. β-galactosidase expression was confirmed to originate exclusively from engrafted WB-F344 cells in the two mice with the following experiments. We monitored the timing for development of the blue precipitate during incubation with the X-gal substrate and found the blue color developed within the first 1 to 2 hours of incubation. We allowed the incubation to continue for an additional 2 to 3 hours for easier visualization by light microscopy. Taking advantage of the male origin of WB-F344 cells engrafted into female recipient hosts, we confirmed that the β-galactosidase expressing cells had a rat Y-chromosome-specific DNA sequence. Additionally, we ruled out the possibility that β-galactosidase activity might have originated from endogenous inflammatory cells, such as macrophages, which could have invaded the myocardium in response to the injection of WB-F344 cells. We elicited a cellular inflammatory response in a control mouse by injecting thioglycollate in the peritoneal cavity and examined the inflammatory cells for β-galactosidase activity. Although the inflammatory response was intense, no cells demonstrated β-galactosidase activity. Collectively, these results lead us to conclude that the β-galactosidase-positive myocytes in the recipient hearts originated from the engrafted donor WB-F344 stem cells.
Figure 2.
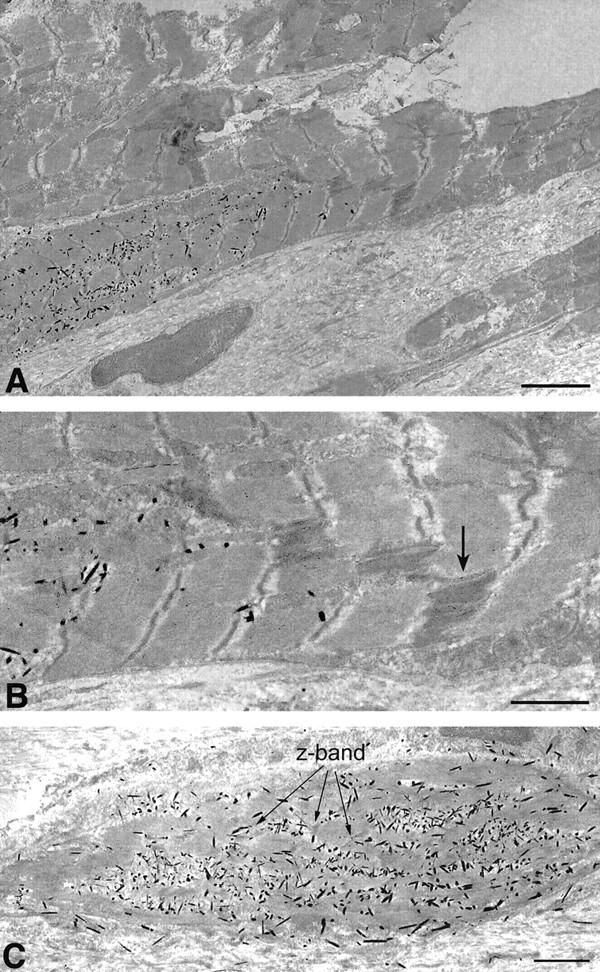
Electron micrographs of β-galactosidase-expressing WB-F344-derived myocytes. X-gal reaction product is the electron-dense crystalloid precipitate in the cytoplasm of well-differentiated (A) and differentiating (C) WB-F344-derived myocytes. A well-differentiated WB-F344-derived myocyte with well-ordered sarcomeres is anatomically coupled via an intercalated disk (magnified in B) and gap junctions (B, arrow) with an adjacent myocyte that does not contain X-gal product. C: Nascent early sarcomere striations present in a differentiating ∼20-μm myocyte (arrows, Z-band). B: High-power of intercalated disk seen in A. Arrow points to suspected gap junction. No counter fixation or staining with lead citrate and uranyl acetate. Scale bars: 4 μm (A); 2 μm (B and C).
The number of β-galactosidase-positive cells contained within an individual tissue section varied throughout the heart wall. β-galactosidase-positive cells present in one section would disappear with further serial sectioning and reappear 10 to 15 sections deeper into the block. These findings could have resulted from WB-F344 cells being showered throughout the myocardium by way of the coronary circulation when they were injected into the ventricular cavity, or the cells not being continuously injected into the myocardium, while the needle was being withdrawn. We confirmed that β-galactosidase-expressing myocytes were derived from WB-F344 cells by demonstrating that they contain a rat Y-chromosome through PCR and FISH analyses of a rat Y-chromosome-specific repetitive DNA sequence. Individual β-galactosidase-positive myocytes were microdissected from heart cryosections using laser capture microscopy (Figure 1, c and d) ▶ . PCR amplification of their DNA, cloning, and sequencing of the PCR product confirmed that the β-galactosidase-positive myocytes contained the rat Y-chromosome-specific repetitive DNA sequence (Figure 1e) ▶ . As a positive control, we harvested WB-F344 cells from culture and demonstrated that they contained this sequence (Figure 1e ▶ , lane 2). In contrast, endogenous (female) myocytes that were microdissected from tissue sites proximal to the β-galactosidase-positive myocytes did not contain the rat Y-chromosome-specific sequence (Figure 1e ▶ , lane 5). We used FISH to show that β-galactosidase-positive myocytes contained the rat Y-chromosome. The FISH reaction was performed on fresh frozen sections while the β-galactosidase reaction was performed on fixed tissue sections. To circumvent these differences in tissue preparation, we used tissue obtained from immediately adjacent 8-μm-thick serial sections, causing a shift in register of 8 μm or more. Using this serial section approach, the cytoplasmic β-galactosidase reaction product was demonstrated in one tissue section, and the rhodamine-stained Y-chromosome in the nucleus of the same myocyte in the adjacent section (see Figure 3 ▶ ). A cardiac phenotype was confirmed in β-galactosidase-expressing cells demonstrating that they expressed a cardiac-specific protein, using a monoclonal antibody that recognizes a cardiac-specific troponin T epitope, which is conserved across species. 24 The β-galactosidase-expressing cells demonstrated localization of the fluorescence to striations consistent with troponin T localization in the sarcomere (Figure 1, f and g) ▶ .
Figure 3.
Fluorescence in situ hybridization. The pink Y-chromosome (arrow) and the blue X-gal product are demonstrated in adjoining 8-μm serial sections of the same cell. A: Rhodamine staining of the Y-chromosome probed with a rat Y-chromosome-specific repetitive DNA sequence (arrow). B: Adjoining section of the same myocyte, the blue color is from the X-gal reaction. Superimposition of both signals is affected by an 8-μm shift in register of the two serial sections.
WB-F344-derived myocytes ranged from <20 to 110 μm in length (Figure 1 ▶ ; a, b, c, and f). To provide a measure of the size range among the engrafted WB-F344 cells, we grouped the cells into three sizes, <20 μm, 20 to 50 μm, and 50 to 110 μm in length. We counted 44 cells that were <20 μm in length, 34 cells that were 20 to 50 μm in length, and 53 cells that were 50 to 110 μm in length. Some of the shortest cells may have been captured in cross-section. This distribution is consistent with the possibility that WB-F344-derived cells were at different stages of maturation 6 weeks after injection, as suggested by the electron microscopic findings (Figure 1c) ▶ . The longest of the β-galactosidase-positive cells were well differentiated with clear sarcomere striations (Figure 1b) ▶ identified by light microscopy, whereas cells that measured 20 to 50 μm in length were less well differentiated, exhibiting less well-developed sarcomeres by light microscopy. Striations and sarcomeres in the latter group of cells were readily demonstrated by electron microscopy. WB-F344-derived myocytes were identified by the presence of the electron-dense crystalloid precipitates (β-galactosidase reaction product) in their cytoplasm (Figure 2) ▶ . Nascent striations were present in the small (∼20 μm, Figure 2c ▶ ) cells whereas larger cells demonstrated well-organized and differentiated sarcomeres (Figure 2a) ▶ . In the well-differentiated myocytes, intercalated disks and apparent gap junctions formed anatomical couplings between β-galactosidase-positive WB-F344-derived myocytes and endogenous (host) myocytes that did not contain any X-gal reaction precipitate (Figure 2, a and b) ▶ . The presence of anatomical couplings between stem cell-derived and host myocytes suggests strongly that these WB-F344-derived myocytes participate in the function of the cardiac syncytium.
Discussion
The goal of this study was to test whether the microenvironment of the heart can induce the differentiation of stem cells derived from the liver of an adult rat into a well-differentiated cardiac phenotype in vivo. Collectively, our results provide proof of this concept. We demonstrate that adult liver-derived stem cells, which differentiate into hepatic cells in the liver in vivo, can be reprogrammed in the heart into a cardiac lineage, producing cells with a well-differentiated cardiac myocyte phenotype. These findings are consistent with the hypothesis that reactivation of genetic programs in adult stem cells is possible in response to appropriate stimulation. Although this type of extreme reprogramming has been recently described in somatic cells isolated from other adult tissues, 29 this is the first study to demonstrate that cells derived from an adult liver stem cell line are capable of reprogramming their gene expression in the adult cardiac microenvironment in vivo and acquiring the phenotype of mature well-differentiated cardiac myocytes.
It is generally believed that adult mammalian cardiac myocytes have undergone terminal differentiation and can no longer divide to generate new myocytes. Instead, the residual myocytes respond to the cardiac workload after damage by undergoing hypertrophy. Depending on the extent and cause of injury, hypertrophied myocytes may not successfully support the cardiac output, and cardiac decompensation and heart failure result. The great impact of heart disease on mortality and quality of life and the scarcity of donor hearts for organ transplantation have led several investigations to examine transplantation of different types of cells into the heart in an attempt to achieve repair. 30-39 Cells used in these studies were precommitted to a muscle lineage before transplantation in vivo. Embryonic cardiomyocytes derived from embryonic stem cells 39 and fetal cardiomyocytes survive in the cardiac environment in vivo, and form primitive couplings with host cells. 30 Similarly, transplanted skeletal muscle myoblasts 31-37 develop into skeletal muscle myotubes and survive uncoupled with native cardiomyocytes for up to 3 months in recipient hearts. Cardiac myocyte cell lines have also been generated for potential therapeutic usage in the failing heart and for understanding myocyte function in vitro. 40,41 A cell line derived from mouse atrial myocytes and propagated in vitro maintained an adult cardiac myocyte phenotype in culture. 41 Bone marrow stromal cells have been transformed in vitro into a cardiomyogenic cell line with 5-azacytidine 40 treatment. This cell line has been suggested, 42 but has not been tested in vivo, as a source of cardiomyocytes for cell replacement therapy in cardiomyopathies. Our study goes further and demonstrates that cells from a stem cell line that is neither of embryonic origin nor committed to a muscle lineage will engraft in the adult heart in vivo and differentiate into well-organized mature cardiac myocytes.
Stem cell differentiation has been suggested to be regulated by two independent pathways. One pathway that allows their exit from the stem-cell state requires negatively active events that involve the silencing of regulators that repress the expression of specific gene. 43 A second pathway that is positively acting involves regulation by transcription factors that allow stem cells to enter commitment into a specific lineage. 43 The response of this WB-F344 liver-derived stem cell line in the heart in vivo as shown here, and a recent preliminary communication that reports that bone marrow stem cells differentiate in the ischemic heart into fetal myocytes (Orlic D, Kajstura J, Chimenti S, Li B, Anderson S, Bodine D, Pickel J, Leri A, Nadal-Ginard B, Anversa P: Exogenous hematopoietic stem cells can regenerate infarcted myocardium. Late Breaking Science Abstracts, American Heart Association, 2000, New Orleans), demonstrate that the adult heart tissue microenvironment expresses the appropriate signals that allow the exit of these extracardiac cells from their stem-cell state and differentiation into myocytes.
This raises the possibility that adult-derived human stem cells can be isolated from a patient, propagated in culture, and used to support the patient’s diseased heart. The potential therapeutic advantages of a clonal stem cell line such as WB-F344 would be the ability to expand the cell number in culture ex vivo and generate a well-characterized population of cells. These could be reintroduced into the patient or stored for future therapeutic interventions, avoiding the complications of allograft transplant rejection.
Acknowledgments
We thank Dr. Barbara Hoebee from the Laboratory of Carcinogenesis and Mutagenesis, National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands, for kindly providing the Y-chromosome specific probe (9.1 ES8); Dr. Katherine Pryzwansky for helpful discussions; Dr. Richard J. Rahija for helpful information about selection of animal model; Dr. Stephanie Cohen for guidance with the FISH; and Mrs. Phyllis Bason and Mrs. Annette Oakeley for preparing the manuscript.
Footnotes
Address reprint requests to Nadia N. Malouf, M.D., Department of Pathology, University of North Carolina at Chapel Hill, CB 7525, Brinkhous Bullitt Bldg., Chapel Hill, NC 27514. E-mail: malouf@med.unc.edu.
Supported in part by National Heart, Lung, and Blood Institute grants R01 HL42250, R01 HL20749, and R01 CA29323 from the National Cancer Institute.
References
- 1.Fuchs E, Segre JA: Stem cells: a new lease on life. Cell 2000, 100:143-155 [DOI] [PubMed] [Google Scholar]
- 2.Watt FM, Hogan BLM: Out of Eden: stem cells and their niches. Science 2000, 287:1427-1430 [DOI] [PubMed] [Google Scholar]
- 3.Slack JMW: Stem cells in epithelial tissues. Science 2000, 287:1431-1433 [DOI] [PubMed] [Google Scholar]
- 4.Gage FH: Mammalian neural stem cells. Science 2000, 287:1433-1438 [DOI] [PubMed] [Google Scholar]
- 5.van der Kooy D, Weiss S: Why stem cells? Science 2000, 287:1439-1441 [DOI] [PubMed] [Google Scholar]
- 6.Weissman IL: Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 2000, 287:1442-1446 [DOI] [PubMed] [Google Scholar]
- 7.Prockop D: Marrow stromal cells as stem cells for non-hematopoietic tissues. Science 1997, 276:71-74 [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284:143-147 [DOI] [PubMed] [Google Scholar]
- 9.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL: Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 1999, 283:534-537 [DOI] [PubMed] [Google Scholar]
- 10.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ: Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA 1998, 95:3908-3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari G, Custella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F: Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998, 279:1528-1530 [DOI] [PubMed] [Google Scholar]
- 12.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC: Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999, 401:390-394 [DOI] [PubMed] [Google Scholar]
- 13.Peterson BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP: Bone marrow as a potential source of hepatic oval cells. Science 1999, 284:1168-1170 [DOI] [PubMed] [Google Scholar]
- 14.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS: Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000, 31:235-240 [DOI] [PubMed] [Google Scholar]
- 15.Brazelton TR, Rossi FMV, Keshet GI, Blau HM: From marrow to brain expression of neuronal phenotypes in adult mice. Science 2000, 290:1775-1779 [DOI] [PubMed] [Google Scholar]
- 16.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR: Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 2000, 290:1779-1782 [DOI] [PubMed] [Google Scholar]
- 17.Tsao M-S, Smith JD, Nelson KG, Grisham JW: A diploid epithelial cell line from normal adult rat liver with phenotypic properties of “oval” cells. Exp Cell Res 1984, 154:38-52 [DOI] [PubMed] [Google Scholar]
- 18.Grisham JW, Thorgeirsson SS: Liver stem cells. Potten CS eds. Stem Cells. 1997, :pp 233-282 Academic Press, London [Google Scholar]
- 19.Coleman WB, Grisham JW: Epithelial stem-like cells of the rodent liver. Strain A Diehl AM eds. Liver Growth and Repair. 1998, :pp 50-99 Chapman & Hall, London [Google Scholar]
- 20.Coleman WB, Smith GJ, Grisham JW: Development of dexamethasone-inducible tyrosine aminotransferase activity in WB-F344 rat liver epithelial stemlike cells cultured in the presence of sodium butyrate. J Cell Physiol 1994, 161:463-469 [DOI] [PubMed] [Google Scholar]
- 21.Coleman WB, Wennerberg AE, Smith GJ, Grisham JW: Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol 1993, 142:1373-1382 [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, Grisham JW: Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol 1997, 151:353-359 [PMC free article] [PubMed] [Google Scholar]
- 23.Chapman L, Coleman WB, Hixson DC: Integration, survival, and differentiation of liver epithelial cells in hepatic and pancreatic ducts. FASEB J 1999, 13:A160 [Google Scholar]
- 24.Malouf NN, McMahon D, Oakeley AE, Anderson PAW: A cardiac troponin T epitope conserved across phyla. J Biol Chem 1992, 267:9269-9274 [PubMed] [Google Scholar]
- 25.McMahon DK, Anderson PAW, Nassar R, Bunting JB, Saba Z, Oakeley AE, Malouf NN: C2 C12 cells: biophysical, biochemical and immunocytochemical properties. Am J Physiol 1994, 266:C1795-C1802 [DOI] [PubMed] [Google Scholar]
- 26.Schütze K, Lahr G: Identification of expressed genes by laser-mediated manipulation of single cells. Nat Biotechol 1998, 16:737-742 [DOI] [PubMed] [Google Scholar]
- 27.Essers J, de Stoppelaar JM, Hoebee B: A new rat repetitive DNA family shows preferential localization on chromosome 3, 12 and Y after fluorescence in situ hybridization and contains a subfamily which is Y chromosome specific. Cytogenet Cell Genet 1995, 69:246-252 [DOI] [PubMed] [Google Scholar]
- 28.Gussoni E, Wang Y, Fraefel C, Miller RG, Blau HM, Geller AI, Kunkel LM: A method to codetect introduced genes and their products in gene therapy protocols. Nat Biotechnol 1996, 14:1012-1016 [DOI] [PubMed] [Google Scholar]
- 29.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH: Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385:810-813 [DOI] [PubMed] [Google Scholar]
- 30.Soonpaa MH, Koh GY, Klug MG, Field LJ: Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 1994, 264:98-101 [DOI] [PubMed] [Google Scholar]
- 31.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE: Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med 1998, 4:929-933 [DOI] [PubMed] [Google Scholar]
- 32.Robinson SW, Cho PW, Levitsky HI, Olson JL, Hruban RH, Acker MA, Kessler PD: Arterial delivery of genetically labelled skeletal myoblasts to the murine heart: long-term survival and phenotypic modification of implanted myoblasts. Cell Transplantation 1996, 5:77-91 [DOI] [PubMed] [Google Scholar]
- 33.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD: Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest 1996, 98:2512-2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu RC-J, Zibaitis A, Kao RL: Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg 1995, 60:12-18 [PubMed] [Google Scholar]
- 35.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM: Embryonic stem cell lines derived from human blastocysts. Science 1998, 282:1145-1147 [DOI] [PubMed] [Google Scholar]
- 36.Koh GY, Klug MG, Soonpaa MH, Field LJ: Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest 1993, 92:1548-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiMorio JX, Stockdale FE: Myoblast transfer into skeletal muscle. Unresolved questions of new muscle formation from injected myogenic cells. 1992, :pp 329-340 HM Blau. New York, Raven Press Ltd., Neuromuscular Development and Disease. Edited by AM Kelly [Google Scholar]
- 38.Delcarpio JB, Claycomb WC: Cardiomyocyte transfer into the mammalian heart. Cell-to-cell interactions in vivo and in vitro. Ann NY Acad Sci 1995, 752:267-285 [DOI] [PubMed] [Google Scholar]
- 39.Klug MG, Soonpaa MH, Koh GY, Field LJ: Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest 1996, 98:216-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J-I, Umezawa A, Ogawa S: Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999, 103:697-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpion JB, Bahinski A, Isso NJ, Jr: Hl-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998, 95:2979-2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiden JM: Beating the odds: a cardiomyocyte cell line at last. J Clin Invest 1999, 103:591-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison SJ, Shah NM, Anderson DJ: Regulatory mechanisms in stem cell biology. Cell 1997, 88:287-298 [DOI] [PubMed] [Google Scholar]