Abstract
Hibernation, a natural model of tolerance to cerebral ischemia, represents a state of pronounced fluctuation in cerebral blood flow where no brain damage occurs. Numerous neuroprotective aspects may contribute in concert to such tolerance. The purpose of this study was to determine whether hibernating brain tissue is tolerant to penetrating brain injury modeled by insertion of microdialysis probes. Guide cannulae were surgically implanted in striatum of Arctic ground squirrels before any of the animals began to hibernate. Microdialysis probes were then inserted in some animals after they entered hibernation and in others while they remained euthermic. The brain tissue from hibernating and euthermic animals was examined 3 days after implantation of microdialysis probes. Tissue response, indicated by examination of hematoxylin and eosin-stained tissue sections and immunocytochemical identification of activated microglia, astrocytes, and hemeoxygenase-1 immunoreactivity, was dramatically attenuated around probe tracks in hibernating animals compared to euthermic controls. No difference in tissue response around guide cannulae was observed between groups. Further study of the mechanisms underlying neuroprotective aspects of hibernation may lead to novel therapeutic strategies for stroke and traumatic brain injury.
Clinical and experimental studies show that after central nervous system trauma, such as traumatic brain injury and acute ischemic stroke, neuronal damage continues to progress after the initial insult. 1-4 Ischemia and traumatic brain injury share several common neurodegenerative mechanisms including excitotoxicity, perturbation of Ca2+ homeostasis, inflammation, and oxidative stress. 5-10 Furthermore, the pathological events in Alzheimer’s disease and late-onset dementia may be triggered by or, at minimum, exacerbated by, impaired cerebral perfusion originating in the microvasculature and are associated with increased inflammation and oxidative stress. 11 Despite numerous therapeutic strategies found to reduce ischemia-induced damage in experimental models, only tissue plasminogen activator has proven effective in controlled clinical trials. 12-14 Although tissue plasminogen activator has improved prognosis for many stroke patients, progress in the development of effective pharmacotherapies for ischemia, traumatic brain injury, and neurodegenerative disorders has been slow.
Hibernation is a unique physiological condition known best for suppression of metabolism and body temperature, which is thought to promote survival during periods of food shortage. 15 Less well recognized are the numerous, potentially neuroprotective aspects of hibernation physiology such as leukocytopenia, immunosuppression, inhibition of protein synthesis, enhanced antioxidant defense, and metabolic suppression. 15-23 Consistent with the hypothesis that hibernation is neuroprotective, Frerichs and Hallenbeck 24 reported increased tolerance to hypoxia/aglycemia in hippocampal slices in vitro from hibernating Thirteen-lined ground squirrels. Moreover, in previous microdialysis studies in our lab, 25 hibernating and euthermic Arctic ground squirrels were readily distinguished on the basis of tissue response around the microdialysis probe. 26 In these previous studies, however, probes were in place longer in hibernating than euthermic animals and sterile microdialysis technique was not followed. The purpose of the present study was therefore to modify these experiments to systematically determine how tolerant hibernating brain tissue is to central nervous system trauma, in vivo, where multiple potentially neuroprotective mechanisms may act in concert.
Microdialysis is a well-established and accepted method for sampling neurotransmitter overflow in awake, freely moving animals. The technique has proven effective in numerous applications where correlations in neurotransmitter release and behavior are verified by pharmacological manipulations. 27,28 Nonetheless, insertion of guide cannulae and microdialysis probes into brain tissue produce stab-like wounds characteristic of traumatic brain injury with associated release of interleukin-1β, 29 gliosis, 29,30 infiltration of granulocytes, 31 and neuronal degeneration. 32 In the present study we assessed tissue pathology around microdialysis probes and guide cannulae in euthermic and hibernating Arctic ground squirrels to test the hypothesis that hibernation attenuates the posttraumatic tissue response seen with brain injury. Results show marked differences in traumatic tissue response in hibernating versus euthermic animals.
Materials and Methods
Surgery
All procedures were approved by the Institutional Animal Care and Use Committee. The animals used in this study were male and female Arctic ground squirrels weighing 650 to 840 g at the time of surgery. Surgery was performed under general anesthesia with halothane (Halocarbon Lab, Riveredge, NJ), induced at 5%, and maintained at 2.5 to 3% mixed with 100% medical grade O2 at a flow rate of 1.5 L/ml. Surgery was performed under strictly aseptic conditions. Telemetry transmitters (model VM-FH, Minimitter, OR) used to monitor core body temperature, were implanted intraperitoneally. Ground squirrels were fasted at least 4 hours before the surgery and then secured in a stereotaxic frame. Guide cannulae (CMA, Acton, MA) were stereotaxically positioned above the right and left striatum (AP, 13.5 or 14 mm; L, ±3.25 mm; D, −4.0 mm), described in detail by Osborne and colleagues, 25 and were slowly lowered 4 mm from the cortical surface. Four anchor stainless steel screws (BAS, West Lafayette, IN) were placed on the skull. Cannulae were secured to the screws with dental cement. Antibiotics (enrofloxacin; Bayer Corp., Shawnee Mission, KS, 5 mg/kg id) were given 1 day before surgery and 2 days postoperatively by subcutaneous injection.
After 10 days of postoperative recovery, the animals, housed individually under a light regime of 12:12 hours light:dark at 20 to 22°C, were transferred to the cold chamber with an ambient temperature of 2 to 4°C on a 4:20 hours light:dark cycle. Hibernation was observed using the shavings added technique. Briefly, the animal was noted to be hibernating if wood shavings placed on the back remained overnight. Body temperature was then monitored by telemetry at least 1 day before and throughout the microdialysis experiment to verify hibernating state.
Microdialysis
Microdialysis procedures and experimental protocol were the same as described in detail by Osborne and colleagues, 25 except that in the present study, all components of the microdialysis system were sterilized via heat (autoclave), ethylene oxide (Anprolene; Andersen Products, Haw River, NC) or 0.2-μm filtration (Acrodisc; Pall Corporation, Ann Arbor, MI). Briefly, the continuously perfused microdialysis probes (CMA 12/04, OD = 0.5 mm) were slowly inserted into the right and left striatum through guide cannulae while animals were hibernating or euthermic. Euthermic animals were lightly anesthetized with halothane, induced as described for surgery, maintained at 1% for ∼5 minutes. Euthermic animals recovered from anesthesia within ∼15 minutes of inserting probes. Microdialysis probes were perfused by means of a CMA/102 syringe pump (CMA, Acton, MA) with artificial cerebrospinal fluid (124 mmol/L NaCl, 2.7 mmol/L KCl, 1.2 mmol/L CaCl2, 0.85 mmol/L MgCl2, 1.4 mmol/L glucose, 24 mmol/L NaHCO3, adjusted to pH 7.4, Po2 = 70 to 80 mmHg, Pco2 = 30 to 40 mmHg by bubbling with 95% N2/5% CO2). The perfusion protocol, 0.6 μl/min for 5 hours; 0.1 μl/min for overnight, was repeated 3 days in all hibernating and euthermic animals. All microdialysis experiments were performed in the hibernaculum with an ambient temperature of 2 to 4°C. Therefore, the perfusion fluid (artificial cerebrospinal fluid) was the same temperature for both euthermic and hibernating groups.
Dialysate collected at 0.6 μl/min during the day or 0.1 μl/min overnight was analyzed for glutamate by high pressure liquid chromatography coupled with fluorescence detection. Dialysates were derivitized with o-phthaldialdehyde (OPA) reagent [1.0 ml of OPA incomplete (Sigma, St. Louis, MO), mixed with 14 μl of mercaptoethanol solution diluted in methanol in 1:10] and separated on a microbore column (Pronexus, Stockholm, Sweden) using a mobile phase of 0.1 mol/L of acetate buffer including 10% acetonitrile at pH 6.0. Derivatives of glutamate were detected using a CMA/280 fluorescence detector (CMA) and recorded on the computer via Chromatography Station for Windows software (Pronexus). Glutamate concentration was quantified by comparing peak height to an external standard.
Total white blood cell counts were performed to assess leukocytopenia during hibernation and to monitor evidence of infection. In operated animals, blood was sampled via toe nail clip during surgery. Of these animals that remained euthermic, blood was sampled on the first day of the experiment via toe nail clip and on the last day of experiment via cardiac puncture at the time of euthanasia. In operated animals that entered hibernation blood was sampled via cardiac puncture at the time of euthanasia. In unoperated control animals (hibernating and euthermic), blood was sampled via cardiac puncture at the time of euthanasia. Using a microcollection system (unopette), blood was collected into heparinized tubes. The total white blood cells were counted manually (1:100 diluted with 2.86% glacial acetic acid solution) using a hemocytometer or automatically on a Coulter T-890 (Coulter, Miami, FL) within 24 hours of sampling.
Tissue Preparation
After 3 days of microdialysis sampling, the euthermic animals were anesthetized with halothane whereas hibernators were not initially anesthetized. Rectal temperature was measured using a thermocouple in hibernating or anesthetized euthermic animals and blood was sampled via cardiac puncture. All animals were administered an intracardial injection of ketamine (2 mg/kg) and xylazine (0.6 mg/kg) before CSF sampling as described in Drew and colleagues. 22 Then under deep anesthesia, animals were perfused transcardially with at least 500 ml of 0.9% NaCl until clear. After decapitation, brains were quickly removed and placed overnight in ice-cold methacarn (60% methanol, 30% chloroform, 10% acetic acid) with gentle shaking, then transferred to 70% isopropyl alcohol and stored at 4°C until paraffin embedding.
Histology
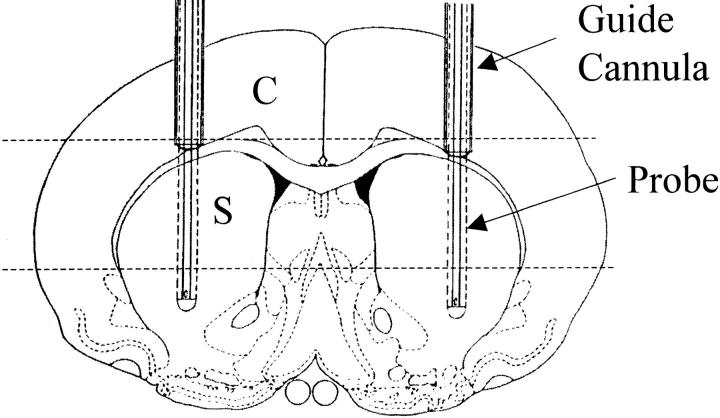
Before paraffin embedding, the whole brain was trimmed, perpendicular to the probe track, into three parts similar in thickness (Figure 1) ▶ . After conventional tissue processing procedure (Citadel 2000; Shandon, Pittsburgh, PA) each part of the brain tissue was embedded in paraffin and 6-μm-thick consecutive sections were prepared on a sliding microtome for further analysis. Hematoxylin and eosin (H&E) staining were performed on sections around probes and guide cannulae of hibernating (n = 4 striata) and euthermic (n = 6 striata) tissues to investigate pathological tissue response after traumatic brain injury.
Figure 1.
Diagram of coronal view through striatum shows position of guide cannulae (outer diameter = 0.65 mm) and microdialysis probes (outer diameter = 0.5 mm with 4-mm-long dialyzing membranes). After fixation, brains were trimmed perpendicular to the probe tracks as indicated by dashed lines. Sections for histopathological analysis (6 μm) were taken through cortex (C) and striatum (S), perpendicular to guide cannulae and microdialysis probe tracks.
Immunocytochemistry
The nonaldehyde fixed (methacarn) brain tissue sections of hibernating and euthermic squirrels were hydrated through graded ethanol after deparaffinization with xylene. Endogenous peroxidase activity in the tissue was inhibited by incubation in 3% hydrogen peroxide in methanol for 30 minutes. Sections were then incubated with 10% normal goat serum in Tris-buffered saline (50 mmol/L Tris-HCl, 150 mmol/L NaCl, pH, 7.6) for 30 minutes at room temperature to eliminate nonspecific binding. All washes and the dilutions of antibody were in 1% normal goat serum in Tris-buffered saline. After washing, the sections were incubated overnight at 4°C with either 1) immunoaffinity purified rabbit polyclonal antibody against glial fibrillary acidic protein (GFAP) (1:1000; a kind gift from Dr. Gambetti, Institute of Pathology, Case Western Reserve University), used as an established marker for reactive astrocytes; 2) biotinylated RCA-1 (1:300; Vector Laboratories, Inc., Burlingame, CA) that recognizes microglia 33 ; or 3) immunoaffinity-purified rabbit polyclonal antibody against hemeoxygenase-1 (HO-1) (Stressgen Biotechnologies Corporation, Inc., Victoria, BC, Canada) that is used as an oxidative stress marker. Sections stained by GFAP and HO-1 were then incubated in goat anti-rabbit (ICN, Costa Mesa, CA) antisera for 30 minutes followed by rabbit-specific peroxidase-antiperoxidase complex for 1 hour (Sternberger Monoclonals Inc. and ICN, Cappel): and the staining was developed using 3,3′-diaminobenzidine (DAKO Corp., Carpinteria, CA). Sections treated with RCA-1 were incubated with avidin D peroxidase (Vector Laboratories) for 1 hour and then developed by 3,3′-diaminobenzidine. The sections were then dehydrated through ascending ethanol and xylene solutions for mounting.
Statistics
Effects of surgery and hibernation state on white blood cell counts obtained on the last day of dialysis was determined using a 2 × 2 analysis of variance design followed by Tukey posthoc comparisons. Within animal comparisons across time for white blood cell counts in euthermic animals and dialysate concentrations of glutamate in euthermic and hibernating animals were made using a repeated measures analysis of variance design (SAS for Windows, Version 8; SAS Institute Inc., Cary, NC).
Results
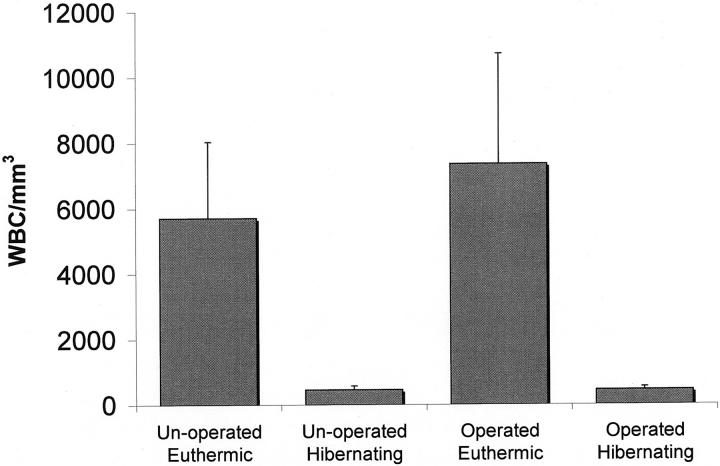
At the time probes were inserted, mean core body temperature (±SD, n = 3 to 4) of euthermic animals was (35.9 ± 1.6°C) and for hibernating animals was (4.1 ± 0.8°C). Body temperature of hibernating animals remained low (<5°C) throughout the dialysis sampling period. White blood cell counts decreased from 6357 ± 2739/mm 3 in euthermic animals to 456 ± 98/mm 3 in hibernating animals (mean ± SD, n = 8 to 10, P < 0.0001 main effect of state) (Figure 2) ▶ . White blood cell counts were similar in the operated groups compared to unoperated control animals (P = 0.31, main effect of surgery) (Figure 2) ▶ . White blood cell counts on the day of surgery and the first and last day of the experiment in euthermic animals also remained stable (mean ± SD; day of surgery, 7583/mm 3 ± 1443/mm3; day 1 of dialysis, 8812/mm 3 ± 3071/mm3; last day of dialysis 7343/mm 3 ± 3360/mm3). Similar white blood cell counts in operated and unoperated animals and stability of counts during the duration of experimental protocol were consistent with clinical observations that operated animals were free of infection.
Figure 2.
White blood cell (WBC) counts (means ± SD) for operated and unoperated euthermic and hibernating Arctic ground squirrels. Pooled (rectal) body temperature (mean ± SD, n = 7 to 9) for euthermic was (36.46 ± 0.97°C) and for hibernating was (3.32 ± 1.43°C).
Microdialysis probe and guide cannulae tracks were easily distinguished based on position (depth) of placement and a clear difference in track diameter (Figure 1) ▶ . Examination of H&E-stained tissue sections indicated an obvious response around the guide cannula in all hibernating and euthermic animals with no convincing difference among the cases (not shown). However, importantly, results from the sections with probe tracks showed a clear difference in tissue reaction between hibernating animals and controls (Figure 3) ▶ . In the hibernating animals there was simply a small space surrounded by slightly pale neural parenchyma. Routine light microscopy showed no other cellular tissue response around the probe track. By contrast, euthermic animals showed a number of histological changes, including reactive astrocytosis, microglial activation, macrophage infiltrate, and axonal swellings.
Figure 3.
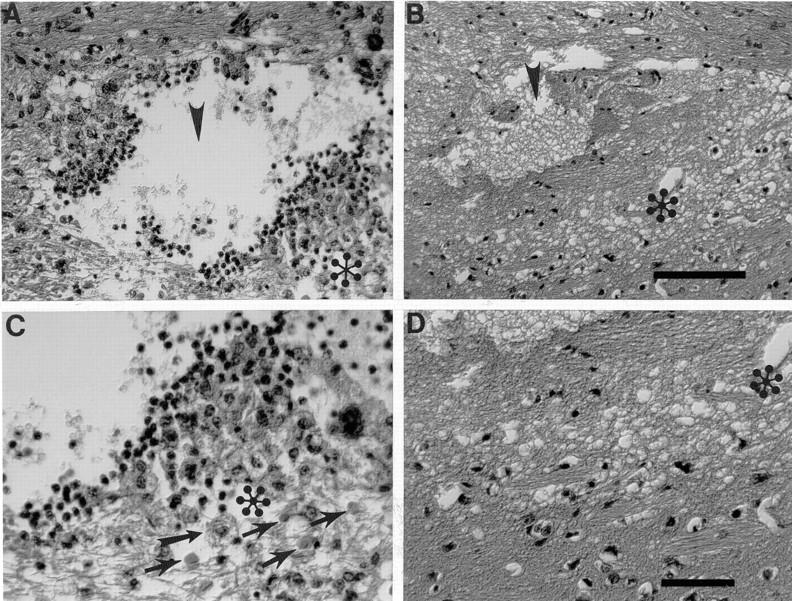
A greater tissue reaction is seen in H&E-stained sections in euthermic animals (A and C) as compared with hibernating animals (B and D). C and D are pictures with higher magnification of the area marked with (asterisk) in A and B. Arrowheads point to the probe track. Sections from euthermic animals (A and C) demonstrate the probe cavity, accompanied by mononuclear inflammatory infiltrate, accumulation of lipid-laden macrophages (one straighthead arrow), axonal swellings (four flared arrows) and fibrillary gliosis. In contrast, brain from hibernating animals (B and D) showed the probe cavity with no discernible inflammatory or reparative reaction. Specifically, no macrophages were seen, and there were no swollen axons or histological evidence of astrocytosis. There was simply a cavity surrounded by slight pallor of the surrounding neural parenchyma. Scale bars: 100 μm (A and B), 50 μm (C and D).
Activated microglia, identified by using RCA-1 binding and amoeboid shape are clearly seen around the probe tract in euthermic tissue (n = 6 striata, Figure 4A ▶ ). In contrast, the probe tract is barely discernable in hibernating tissue (n = 4 striata, Figure 4B ▶ ). While resting, ramified microglia are seen throughout the hibernating tissue section, no activated microglia are present around the probe tract. Astrocytosis, also visible in H&E-stained sections, was confirmed using antibodies against GFAP. GFAP immunoreactivity was more intense around the probe tracts in euthermic animals compared to hibernating animals (Figure 4, C and D) ▶ . HO-1 immunoreactivity, a marker of oxidative stress response induction, was localized primarily to glial cells and present around the probe tract in euthermic tissue (Figure 4E) ▶ . In contrast, HO-1 immunoreactivity was absent in hibernating tissue (Figure 4F) ▶ . The concentration of glutamate in the dialysate collected from the striatum at qualitative 0.6 μl/min and 0.1 μl/min flow rates, stabilized by day 2 and remained stable throughout 3 days of dialysis. No significant difference was observed between euthermic and hibernating animals (data not shown).
Figure 4.
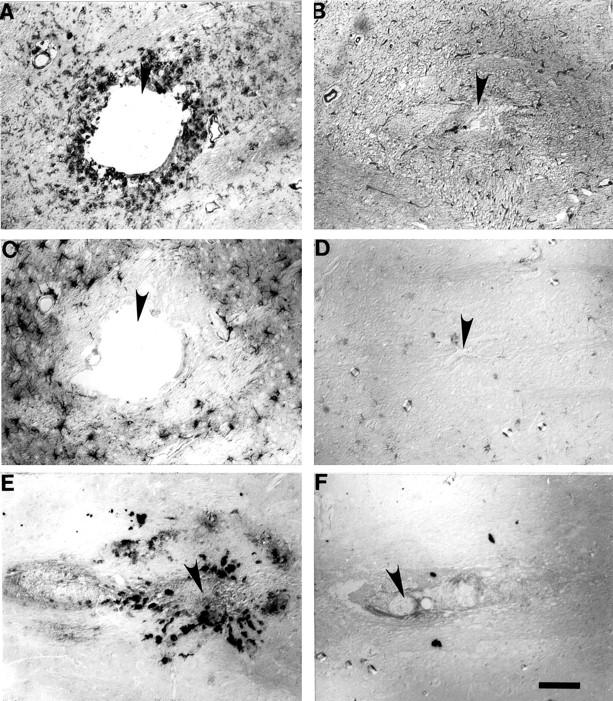
Activated microglia are visible around the probe tract (filled arrow) in euthermic tissue (A). In contrast, the probe tract (filled arrow) is barely discernable in hibernating tissue (B) where only resting (ramified) microglia (arrow) are seen. GFAP immunoreactivity around probe tract (filled arrow) is more intense in euthermic tissue (C) than hibernating tissue (D). Around probe tract (filled arrows in E and F) HO-1 immunoreactivity is seen in euthermic tissue (E) but is absent in hibernating tissue (F). Scale bars: 100 μm.
Discussion
The present results revealed by histopathological analysis suggest that hibernation is neuroprotective. In euthermic brain tissue the typical inflammatory response was evidenced by 1) the presence of activated microglia and astrocytes revealed by RCA-1 binding and GFAP immunoreactivity, and 2) an oxidative stress response identified by an increase in HO-1 immunoreactivity. Both types of responses, however, were profoundly suppressed in hibernating animals.
Traumatic brain injury, induced by insertion of microdialysis probes, induces complex cellular reactions characterized by pathological alternations of neurons and glial cells, and release of inflammatory cytokines. 29-32 The glial response is referred to as reactive gliosis. Astrocytes, the major population of glial cells within the central nervous system, become reactive and increase expression of GFAP within the cytoplasm in response to brain injury. 34,35 Microglia, resident macrophages in the normal brain, are ubiquitous and readily transformed from a ramified resting state to an amoeboid-activated appearance after trauma. 36,37 Release of neurotoxins from reactive glial cells contributes to neurodegeneration and increased neurotoxicity resulting in neuronal cell death. 38 The size of the probe tracks in euthermic striata (∼100 to 200 μm) were considerably larger than in hibernating striata yet still much smaller than the 500-μm OD of the probe. Tissue adjacent to the probe probably dies by necrosis. Subsequent macrophage infiltrate, indicated in H&E staining and activated microglia in euthermic tissue, would then be expected to remove cellular debris, and contribute to the probe track in euthermic tissue (Figure 3A ▶ and Figure 4, A and C ▶ ). In contrast, less cell death and phagocytosis in hibernating tissue likely prevented the appearance of a well-defined probe track (Figure 3B ▶ and Figure 4, B and D ▶ ). Importantly, a progressive increase in [glu]dia was not associated with or necessary for the enhanced traumatic tissue response observed in the euthermic animals.
The significant degree of protection during hibernation likely results from a combination of factors, considering the number of potentially neuroprotective aspects of hibernation physiology. For example, small decreases in intra-ischemic brain temperature decreases the extent of ischemia-induced neuronal injury, 39 and moderate hypothermia has been shown to hasten neurological recovery in patients with severe traumatic brain injury. 40 Although hypothermia has been shown to contribute to the neuroprotective state in hibernation, 24 it is unlikely that hypothermia is entirely responsible for the marked difference in brain tissue response.
Profound leukocytopenia, reported here for Arctic ground squirrels, is another potentially neuroprotective hallmark of hibernation. 17,19,23 Neutrophil adhesion and macrophage infiltration at sites of injury promote cytotoxic reactions. 41-44 Thus leukocytopenia and decreased antibody formation 18 may protect hibernating brain tissue during injury. Plasma and cerebrospinal fluid concentrations of ascorbate increase significantly during hibernation 22,23 as do other antioxidant defense systems. 45 Increased antioxidant defense systems, therefore, may further protect hibernating brain tissue. The observation that HO-1, an antioxidant protein, is induced in euthermic but not in hibernating brain supports the hypothesis that hibernation minimizes injury-induced oxidative stress.
Inhibition of protein synthesis, shown to be neuroprotective in a rodent model of focal ischemia-reperfusion, 46 also occurs during hibernation. 20,47 Thus, a number of potentially neuroprotective adaptations, in addition to profound hypothermia, leukocytopenia, and metabolic suppression, 23 characterize hibernation and likely produce additive or synergistic neuroprotective effects.
The tissue response around the guide cannulae, which were implanted before any of the animals began to hibernate, was not significantly different between animals that remained euthermic and those that entered hibernation. This strongly suggests that the neuroprotective effect was because of the hibernation state and not to the difference between animals independent of the state of hibernation. Although previous work in this laboratory 32 has shown, in rats, that perfusion of microdialysis probes has a tendency to enhance tissue damage, especially within 100 μm from the probe, the difference in tissue damage between dialyzed and nondialyzed preparations is minor at both the light and ultrastructural level. 32 This suggests that perfusion of the probe did not contribute appreciably to the degree of tissue response around the probe track. During the 6 to 8 month hibernation season, ground squirrels enter prolonged states of torpor (1 to 3 weeks) from which they periodically re-warm for brief (24 to 48 hours) periods of euthermia. Speculation still exists as to what drives periodic arousal or why it is necessary. However, it is tempting to hypothesize that a combination of adaptations has evolved in parallel with hibernation to protect vulnerable tissues during frequent periodic re-warming. This same combination of adaptations likely accounts for the profound differences in traumatic brain injury reported here. Better understanding of regulating mechanisms and neuroprotective effects of hibernation may ultimately contribute to the development of effective single and combination therapies for stroke, head trauma, and possibly neurodegenerative disease.
In summary, the current study provides evidence that hibernation plays a crucial role in protecting brain tissue against traumatic injury induced by insertion of microdialysis probes. Many neuroprotective aspects of hibernation physiology, such as hypothermia, leukocytopenia, immunosuppression, and enhanced antioxidant defense are associated with tolerance to penetrating brain injury. Further efforts should be directed at identifying the mechanisms that regulate the suppression of cellular functions in hibernation. Study of neuroprotective mechanisms in species adapted to extreme environments may guide discovery of novel therapeutic strategies.
Acknowledgments
We thank Dr. John Hallenbeck for critical review of the manuscript and Mr. Stanley Wright for secretarial assistance.
Footnotes
Address reprint requests to Kelly L. Drew, Ph.D., Institute of Arctic Biology, University of Alaska Fairbanks, P.O. Box 757000, Fairbanks, AK 99775. E-mail: ffkld@uaf.edu.
Supported by the American Heart Association (grant 98-AK-301), a University of Alaska Fairbanks President’s Special Projects Fund grant (to K. L. D.), the National Institutes of Health (grant NS38648 to M. A. S.), and NS41069 funded in part by the National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, and National Center for Research Resources.
F. Z. and X. Z. contributed equally to this article.
References
- 1.Petito CK, Feldmann E, Pulsinelli WA, Plum F: Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 1987, 37:1281-1286 [DOI] [PubMed] [Google Scholar]
- 2.Heiss WD, Huber M, Fink GR, Herholz K, Pietrzyk U, Wagner R, Winhard K: Progressive derangement of peri-infarct tissue in ischemic stroke. J Cereb Blood Flow Metab 1992, 12:193-203 [DOI] [PubMed] [Google Scholar]
- 3.Garcia JH: The evolution of brain infarcts. A review. J Neuropathol Exp Neurol 1992, 51:387-393 [DOI] [PubMed] [Google Scholar]
- 4.McIntosh TK, Juhler M, Wieloch T: Novel pharmacologic strategies in the treatment of experimental traumatic brain injury. J Neurotrauma 1998, 15:731-769 [DOI] [PubMed] [Google Scholar]
- 5.Rothman SM, Olney JW: Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol 1986, 19:105-111 [DOI] [PubMed] [Google Scholar]
- 6.Faden AI, Demediuk P, Panter SS, Vink R: The role of excitatory amino acid and NMDA receptors in traumatic brain injury. Science 1989, 244:798-800 [DOI] [PubMed] [Google Scholar]
- 7.Vila N, Castillo J, Davalos A, Chamorro A: Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000, 31:2325-2329 [DOI] [PubMed] [Google Scholar]
- 8.Gregson NA: Immune response and CNS injury. Berry M Logan A eds. Cellular Responses and Pharmacological Strategies. 1999, :pp 43-61 FL CRC Press, Boca Raton [Google Scholar]
- 9.Nagayama T, Lan J, Henshall DC, Chen D, O’Horo C, Simon RP, Chen J: Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia. J Neurochem 2000, 75:1716-1728 [DOI] [PubMed] [Google Scholar]
- 10.Shohami E, Beit-Yannai E, Horowitz M, Kohen R: Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab 1997, 17:1007-1019 [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Perry G, Smith MA, Friedland RP: Vascular abnormalities: the insidious pathogenesis of Alzheimer’s disease. Neurobiol Aging 2000, 21:357-361 [DOI] [PubMed] [Google Scholar]
- 12.: The NINDS rt-PA Study Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995, 333:1581-1587 [DOI] [PubMed] [Google Scholar]
- 13.Wardlaw JM, Warlow CP, Counsell C: Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet 1997, 350:607-614 [DOI] [PubMed] [Google Scholar]
- 14.Wahlgren NG: A review of earlier clinical studies on neuroprotective agents and current approaches. Green AR Cross AJ eds. Neuroprotective Agents and Cerebral Ischemia. 1997, :pp 337-363 Academic Press Inc., San Diego [DOI] [PubMed] [Google Scholar]
- 15.Lyman CP: The oxygen consumption and temperature regulation of hibernating hamsters. J Exp Zool 1948, 109:55-78 [DOI] [PubMed] [Google Scholar]
- 16.Snapp BD, Heller C: Suppression of metabolism during hibernation in ground squirrels (Citellus lateralis). Physiol Zool 1981, 54:297-307 [Google Scholar]
- 17.Sidky YA, Daggett LR, Auerbach R: Brown fat: its possible role in immunosuppression during hibernation. Proc Soc Exp Biol Med 1969, 132:760-763 [DOI] [PubMed] [Google Scholar]
- 18.McKenna TM, Musacchia XJ: Antibody formation in hibernating ground squirrels (Citellus tridecemlineatus). Proc Soc Exp Biol Med 1968, 129:720-724 [DOI] [PubMed] [Google Scholar]
- 19.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM: Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia.” J Cereb Blood Flow Metab 1994, 14:193-205 [DOI] [PubMed] [Google Scholar]
- 20.Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, Hallenbeck JM: Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA 1998, 95:14511-14516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gentile NT, Spatz M, Brenner M, McCarron RM, Hallenbeck JM: Decreased calcium accumulation in isolated nerve endings during hibernation in ground squirrels. Neurochem Res 1996, 21:947-954 [DOI] [PubMed] [Google Scholar]
- 22.Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME: Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res 1999, 851:1-8 [DOI] [PubMed] [Google Scholar]
- 23.Toein O, Drew KL, Chao ML, Rice ME: Dynamics of ascorbate regulation during hibernation and arousal in Arctic ground squirrels. Am J Physiology (in press) [DOI] [PubMed]
- 24.Frerichs KU, Hallenbeck JM: Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab 1998, 18:168-175 [DOI] [PubMed] [Google Scholar]
- 25.Osborne PG, Hu Y, Covey DN, Barnes BN, Katz Z, Drew KL: Determination of striatal extracellular gamma-aminobutyric acid in non-hibernating and hibernating Arctic ground squirrels using quantitative microdialysis. Brain Res 1999, 839:1-6 [DOI] [PubMed] [Google Scholar]
- 26.Hu Y, Osborne PG, Stimmelmayr R, Drew KL: Hibernation: a model of tolerance to brain trauma. Soc Neurosci 1998, 676.13
- 27.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW: Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 1997, 276:1265-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanda G, Pontieri FE, Di Chiara G: Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 1997, 276:2048-2050 [DOI] [PubMed] [Google Scholar]
- 29.Woodroofe MM, Sarna GS, Wadhwa M, Hayes GM, Louglin AJ, Tinker A, Cuzner ML: Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunology 1991, 33:227-236 [DOI] [PubMed] [Google Scholar]
- 30.Benveniste H, Diemer NH: Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol 1987, 74:234-238 [DOI] [PubMed] [Google Scholar]
- 31.de Lange EC, Danhof M, Zurcher C, de Boer AG, Breimer DD: Repeated microdialysis perfusions: periprobe tissue reactions and BBB permeability. Brain Res 1995, 702:261-265 [DOI] [PubMed] [Google Scholar]
- 32.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL: An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods 1999, 90:129-142 [DOI] [PubMed] [Google Scholar]
- 33.Mannoji H, Yeger H, Becker LE: A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol 1986, 71:341-343 [DOI] [PubMed] [Google Scholar]
- 34.Mathewson AJ, Berry M: Observations on the astrocyte response in a cerebral stab wound in adult rats. Brain Res 1985, 327:61-69 [DOI] [PubMed] [Google Scholar]
- 35.Norenberg MD: Astrocyte responses to CNS injury. J Neuropathol Exp Neurol 1994, 53:213-220 [DOI] [PubMed] [Google Scholar]
- 36.Streit WJ, Graeber MB, Kreutzberg GW: Functional plasticity of microglia: a review. Glia 1988, 1:301-307 [DOI] [PubMed] [Google Scholar]
- 37.Kreutzberg GW: Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996, 19:312-318 [DOI] [PubMed] [Google Scholar]
- 38.Hayes RL, Jenkins LW, Lyeth BG: Neurotransmitter-mediated mechanisms of traumatic brain injury. Acetylcholine and excitatory amino acids, J Neurotrauma 1992, 9(Suppl.1):S173–S187 [PubMed]
- 39.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD: Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987, 7:729-738 [DOI] [PubMed] [Google Scholar]
- 40.Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST: Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 1997, 336:540-546 [DOI] [PubMed] [Google Scholar]
- 41.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD: Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987, 7:729-738 [DOI] [PubMed] [Google Scholar]
- 42.Weiss SJ: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-375 [DOI] [PubMed] [Google Scholar]
- 43.Bowes MP, Zivin JA, Rothlein R: Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol 1993, 119:215-219 [DOI] [PubMed] [Google Scholar]
- 44.Whalen MJ, Carlos TM, Dixon CE, Robichaud P, Clark RS, Marion DW, Kochanek PM: Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J Leukoc Biol 2000, 67:160-168 [DOI] [PubMed] [Google Scholar]
- 45.Buzadzic B, Spasic M, Saicic ZS, Radojicic R, Petrovic VM, Halliwell B: Antioxidant defenses in the ground squirrel citellus citellus 2. The effect of hibernation. Free Radic Biol Med 1990, 9:407-413 [DOI] [PubMed] [Google Scholar]
- 46.Du C, Hu R, Csernansky CA, Liu XZ, Hsu CY, Choi DW: Additive neuroprotective effects of dextrorphan and cycloheximide in rats subjected to transient focal cerebral ischemia. Brain Res 1996, 718:233-236 [DOI] [PubMed] [Google Scholar]
- 47.Knight JE, Narus EN, Martin SL, Jacobsson A, Barnes BM, Boyer BB: mRNA stability and polysome loss in hibernating Arctic ground squirrels (Spermophilus parryii). Mol Cell Biol 2000, 20:6374-6379 [DOI] [PMC free article] [PubMed] [Google Scholar]