Abstract
Anaplastic large cell lymphoma (ALCL) is frequently associated with the t(2;5)(p23;q35) translocation. It creates a NPM-ALK fusion gene, fusing the anaplastic lymphoma kinase (ALK) gene (2p23) and the nucleophosmin (NPM) gene (5q35). Other rearrangements involving the ALK gene have recently been shown to be associated with ALCL, among which the ATIC-ALK rearrangement resulting from the inv(2)(p23q35) translocation is probably the most recurrent. The aims of the present study were to investigate the presence of NPM-ALK and ATIC-ALK fusion genes in ALCL, using a real-time 5′ exonuclease-based reverse-transcription polymerase chain reaction (RT-PCR). This sensitive technique was also applied to investigate whether both fusion genes might be detected in Hodgkin’s disease cases and in reactive lymphoid tissue. Results of the RT-PCR were compared to ALK immunostaining, cytogenetics, and fluorescence in situ hybridization (FISH) results. RT-PCR detected the NPM-ALK and ATIC-ALK fusions at high levels in 8 and 3 of a total of 13 ALK-positive ALCL cases. One ALK-positive ALCL case was negative for both fusion genes analyzed but revealed a new ALK-related translocation t(2;17)(p23;q25) by cytogenetic and FISH analysis. In addition, of the eight ALK-positive ALCL cases that were strongly positive for the NPM-ALK fusion, three cases also showed the presence of the ATIC-ALK fusion, although at much lower levels. Similarly, out of the three strongly positive ATIC-ALK cases, one case was positive for the NPM-ALK fusion, at low levels. Finally, the NPM-ALK and the ATIC-ALK fusions were detected, at equally low levels, respectively in 13 and 5 ALK-negative ALCL cases, in 11 and 5 Hodgkin’s disease cases and in 20 and 1 non-neoplastic lymphoid tissues. The distinction between the high- and low-level detection was confirmed by relative quantitative RT-PCR for a representative number of cases. Of interest is the fact that the high-level detection coincided with the presence of ALK gene rearrangement detected by cytogenetics and FISH and may reflect a central role of the transcript in the oncogenic mechanism of ALK-positive ALCL. Low-level detection is not supported by cytogenetics and FISH, presumably due to the presence of the transcripts in only a small minority of normal cells not detectable by these techniques. Our findings demonstrate that NPM-ALK and ATIC-ALK fusion transcripts may be detected in conditions other than ALK-positive ALCL including reactive lymphoid tissues, although at low levels, suggesting the presence of the transcripts in normal (bystander) cells. Moreover, they suggest that the ALK gene rearrangement by itself might be insufficient to induce tumor formation. They further question the validity of quantitative real-time RT-PCR for monitoring minimal residual disease in ALCL. Finally, the newly identified translocation t(2;17)(p23;q25) can be added to the list of ALK gene rearrangements occurring in ALK-positive ALCL.
Anaplastic large cell lymphoma (ALCL) was identified in 1985 1 as a lymphoid neoplasm showing anaplastic large cells, preferentially infiltrating the paracortical and intrasinusoidal lymph node regions and expressing the Ki-1 antigen (later CD30). This characteristic morphology led to the inclusion of ALCL (more specifically T/Null ALCL) in the revised European-American lymphoma (REAL) classification 2 as a distinct clinicopathological entity, showing a bimodal age distribution and presenting as a clinically aggressive disease, but curable in a high percentage of cases. Despite these characteristic features, morphological (eg, lymphohistiocytic and small cell) 3 as well as clinical variants (systemic and cutaneous) 4,5 have been described.
In 1990, 6 the association between ALCL and t(2;5)(p23;q35) was established and confirmed in a number of studies, with frequencies ranging from 15 to 75%. 7 The breakpoint involved in this translocation was cloned by Morris and colleagues, 8 demonstrating that the nucleophosmin (NPM) gene on chromosome 5q35 is fused to the previously unidentified anaplastic lymphoma kinase (ALK) gene on chromosome 2p23. The resulting chimeric NPM-ALK protein is thought to play a key role in the pathogenesis of t(2;5)-positive ALCL. Subsequently, antibodies (Abs) reacting with the ALK kinase became available, which allowed the immunohistochemical identification of ALCL cases with a 2p23/ALK- rearrangement, as the ALK protein is absent in normal lymphoid cells. 9 Importantly, expression studies with ALK Abs resulted in the description of a distinct ALK-positive ALCL subgroup, the so-called ALKomas. This particular subgroup of ALCL was shown to be associated with the presence of characteristic hallmark cells, and to have a much more favorable prognosis as compared to the ALK-negative group. 10-13
Recently, it has been demonstrated that ALK protein expression in ALCL may occasionally be the result of ALK rearrangements other than NPM-ALK. An indirect estimate of the frequency of these other rearrangements can be made based on the number of reported ALKoma cases without t(2;5) or on the number of cases without nuclear staining. The cases without nuclear staining are unlikely to be associated with the NPM-ALK rearrangement, as it results in both cytoplasmic and nuclear staining of the neoplastic cells attributable to dimerization of NPM-ALK fusion protein with wild-type NPM, which carries nuclear localization motifs. 14 Ten to twenty percent of ALKomas may thus carry ALK rearrangements other than NPM-ALK. 15 Examples already described are t(1;2)(q25;p23) resulting in a newly identified TPM3-ALK fusion gene, 16 t(2;3)(p23;q21) fusing the TFG and ALK genes, 17 inv(2)(p23q35) creating the ATIC-ALK fusion gene, 18-21 the CLTCL-ALK fusion gene 22 and the not further characterized t(1;2)(q21;p23). 23
Interestingly, NPM-ALK transcripts have been detected in Hodgkin’s disease (HD) cases 24-26 suggesting it to be not as specific as previously assumed. Alternatively, these findings may indicate a common pathogenesis of ALCL and HD. 24 However, other investigators failed to detect t(2;5) in HD and considered the studies demonstrating t(2;5) in HD as controversial. 24,27-29 Finally, the detection of t(2;5) in peripheral blood of healthy individuals has also been reported. 30
The present study was aimed at investigating the presence of ALK gene rearrangements in various lymphoid tissues including ALCL cases, HD cases and reactive lymphoid tissue. Results obtained by a real-time 5′ exonuclease-based reverse transcription-polymerase chain reaction (RT-PCR) to detect NPM-ALK and ATIC-ALK fusion genes were compared to those obtained by ALK protein immunostaining, cytogenetics and fluorescence in situ hybridization (FISH).
Materials and Methods
Cases
Thirty-three ALCL cases (cases 1 to 33), 22 HD cases (cases 34 to 55) (Table 1 ▶ ), and 31 cases of reactive lymphoid tissue without evidence of lymphoma (cases 56 to 86, not listed in Table 1 ▶ ), were selected from the database of the Department of Pathology of the University Hospitals of the Catholic University of Leuven, Belgium. All cases were documented by a freshly frozen tissue block. The reactive lymphoid tissue samples included lymph nodes (n = 29) and spleens (n = 2).
Table 1.
Summary of the Results of the Anaplastic Large Cell Lymphoma and Hodgkin’s Disease Cases
| Case | Diagnosis | ALK IHC | RT-PCR* | FISH | Karyotype | |||
|---|---|---|---|---|---|---|---|---|
| GAPDH | NPM-ALK | ATIC-ALK | Status | Chromosome 2 abnormalities | ||||
| 1 | ALCL | pos | 21 | 17 | 37 | ND | A-M | t(2;5)(p23;q35) |
| 2† | ALCL | pos | 15 | 19 | neg | ND | A-C | t(2;5)(p23;q35) |
| 3† | ALCL | pos | 21 | 19 | neg | NPM-ALK | ND | |
| 4 | ALCL | pos | 18 | 19 | neg | NPM-ALK | ND | |
| 5† | ALCL | pos | 19 | 20 | neg | NPM-ALK | A-C | t(2;5)(p23;q35) |
| 6† | ALCL | pos | 17 | 20 | neg | NPM-ALK | ND | |
| 7† | ALCL | pos | 24 | 21 | neg | NPM-ALK | ND | |
| 8 | ALCL | pos | 19 | 23 | neg | NPM-ALK | ND | |
| 9 | ALCL | pos | 22 | 37 | neg | NPM-ALK | A-M | t(2;5)(p23;q35) |
| 10† | ALCL | pos | 15 | 33 | 21 | ALK-R | A-M | inv(2)(p23q35) |
| 11† | ALCL | pos | 24 | 36 | 25 | ALK-R | A-C | inv(2)(p23q35) |
| 12† | ALCL | pos | 21 | 37 | 22 | ALK-R | A-C | inv(2)(p23q35) |
| 13 | ALCL | pos | 15 | neg | neg | ALK-R | A-M | t(2;17)(p23;q25) |
| 14 | ALCL | neg | 16 | 37 | neg | ND | A-C | N |
| 15 | ALCL | neg | 17 | 30 | neg | No ALK-R | A-C | +2;+2 |
| 16 | ALCL | neg | 17 | 32 | 30 | ND | A-C | N |
| 17 | ALCL | neg | 17 | 32 | 33 | ND | ND | |
| 18 | ALCL | neg | 20 | 33 | neg | ND | ND | |
| 19 | ALCL | neg | 17 | 34 | 36 | ND | ND | |
| 20 | ALCL | neg | 21 | 35 | neg | ND | ND | |
| 21 | ALCL | neg | 16 | 35 | neg | ND | N | N |
| 22 | ALCL | neg | 23 | 35 | 36 | ND | ND | |
| 23 | ALCL | neg | 15 | 35 | 37 | ND | ND | N |
| 24 | ALCL | neg | 17 | 36 | neg | No ALK-R | A-C | N |
| 25 | ALCL | neg | 16 | 37 | neg | ND | ND | |
| 26 | ALCL | neg | 16 | 38 | neg | ND | ND | |
| 27 | ALCL | neg | 19 | neg | neg | ND | ND | |
| 28 | ALCL | neg | 23 | neg | neg | ND | ND | N |
| 29 | ALCL | neg | 20 | neg | neg | ND | ND | |
| 30 | ALCL | neg | 22 | neg | neg | ND | ND | |
| 31 | ALCL | neg | 18 | neg | neg | ND | A-C | add(2)(q37) |
| 32 | ALCL | neg | 16 | neg | neg | No ALK-R | ND | |
| 33 | ALCL | neg | 15 | neg | neg | ND | A-C | N |
| 34 | HD | neg | 18 | 33 | 34 | ND | A-S | N |
| 35 | HD | neg | 19 | 33 | 34 | ND | A-C | N |
| 36 | HD | neg | 18 | 34 | neg | ND | ND | |
| 37 | HD | neg | 20 | 36 | 36 | ND | ND | |
| 38 | HD | neg | 18 | 36 | neg | ND | A-M | N |
| 39 | HD | neg | 17 | 36 | neg | ND | A-C | N |
| 40 | HD | neg | 16 | 36 | neg | ND | ND | |
| 41 | HD | neg | 20 | 37 | neg | ND | ND | |
| 42 | HD | neg | 19 | 37 | 38 | ND | N | |
| 43 | HD | neg | 16 | 38 | neg | ND | N | |
| 44 | HD | neg | 17 | 39 | neg | ND | N | |
| 45 | HD | neg | 16 | neg | neg | ND | A-C | N |
| 46 | HD | neg | 16 | neg | neg | ND | ND | |
| 47 | HD | neg | 20 | neg | 38 | ND | A-C | N |
| 48 | HD | neg | 18 | neg | neg | ND | ND | |
| 49 | HD | neg | 21 | neg | neg | ND | ND | |
| 50 | HD | neg | 17 | neg | neg | ND | ND | |
| 51 | HD | neg | 17 | neg | neg | ND | A-C | N |
| 52 | HD | neg | 21 | neg | neg | ND | ND | |
| 53 | HD | neg | 23 | neg | neg | ND | ND | |
| 54 | HD | neg | 15 | neg | neg | ND | ND | |
| 55 | HD | neg | 21 | neg | neg | ND | ND | |
*Positive results are expressed by CT values.
†Cases included in previous studies. 12,21
IHC, immunohistochemistry; neg, negative; ND, not done; N, normal; A, abnormal; -S, simple (1 chromosomal aberration); -M, moderate (1 to 4 chromosomal aberrations); -C, complex (>4 chromobsomal aberrations); ALK-R; ALK rearrangement.
Morphology and Immunohistochemistry
All cases were reviewed on hematoxylin and eosin-stained paraffin-embedded tissue sections. Immunophenotyping was performed on fixed paraffin-embedded sections or on frozen tissue sections. Monoclonal Abs reacting to CD30 (BerH2), CD15 (LeuM1), CD20 (L26) and CD3 (Leu4) were applied, using a streptavidin-biotin-peroxidase three-stage technique. The peroxidase reaction was developed using 3,3′-diaminobenzidine tetrahydrochloride (Dako, Glostrup, Denmark) and hydrogen peroxide, 0.01% v/v. Antibodies were purchased from Becton Dickinson (San Jose, CA) and from Dako. The tetrahydrochloride immunohistochemical labeling with the monoclonal ALK1 antibody was performed as previously described. 12
The diagnosis of ALCL and HD was made according to the REAL classification 2 and according to the recommendations of the World Health Organization advisory committee. 31
Real-Time Detection of the NPM-ALK and the ATIC-ALK Fusion Transcripts
All cases were analyzed using a newly-developed real-time PCR assay. In short, total RNA was extracted from 5 to 8 sections of 25 μm thickness from freshly frozen tissue blocks using Trizol reagent (Life Technologies, Merelbeke, Belgium). One microgram of total RNA was converted into cDNA using Superscript reverse transcriptase according to the manufacturer’s recommendations (Life Technologies). Five microliters of the RT reaction (20 μl) were then used as template for a 50-μl reaction for the real-time detection of the NPM-ALK and ATIC-ALK fusion transcripts using the TaqMan Universal Master Mix and an ABI Prism 7700 Sequence Detection System (PE Corporation, Foster City, CA). Two sets of primers (15 pmol each) combined with a TaqMan probe (20 pmol) were used for the respective transcripts. The primer directed to the ALK portion of the transcripts was the same for both transcripts (primer ALKr: 5′-TGTACTCAGGGCTCTGCAGCT). Forward primers used were NPMf: 5′-GGGCCAGTGCATATTAGTGGA and ATICf: 5′-CTGTACACACTGCAGCCCAAG. The TaqMan probes were 6-carboxy-fluorescein (FAM)-labeled and bridged the breakpoints (N/A: 5′-AGCACTTAGT AGTGTACCGCCGGAAGCACC and A/A: 5′-CCATCACAGTGTACCGCCGGAAGC).
The quality of the synthesized cDNA was verified during the same run by amplification of a 101-bp fragment of the GAPDH gene, which is constitutively expressed in all cells (primers 5′-AGCCTCAAGATCATCAGCAATG and 5′-ATGGACTGTGGTCATGAGTCCTT. TaqMan probe: 5′-JOE-CCAACTGCTTAGCACCCCTGGCC). Amplification conditions were 2 minutes at 50°C (allowing AmpEraseUNG treatment) and 10 minutes at 95°C, followed by 43 cycles of denaturation (95°C, 15 seconds), extension (60°C, 1 minute). Results of this real-time PCR method are expressed as the CT value, that represents the cycle at which fluorescence raises above a threshold value. CT values below 40 are considered positive. The lower the CT value, the higher the positivity of the sample, suggesting the higher concentration of the target sequence in the starting material.
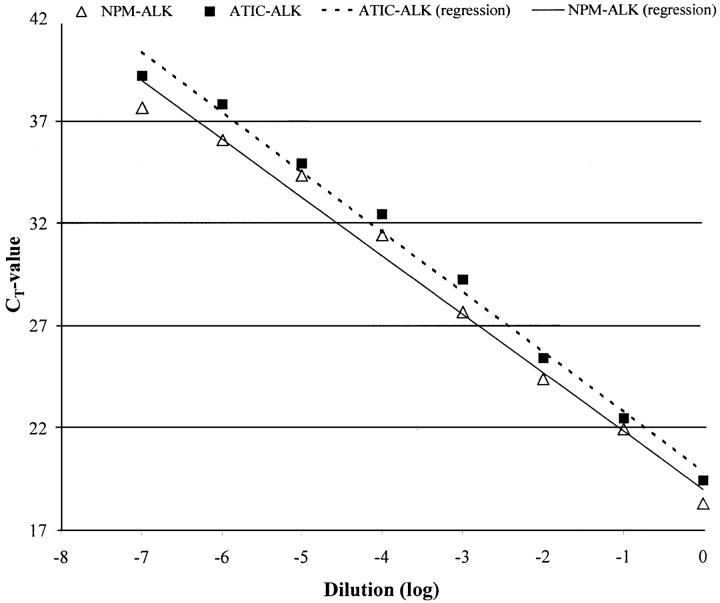
The sensitivity of the assay was determined by amplification of a NPM-ALK-positive and an ATIC-ALK-positive control sample, diluted 10 to 10 8 times in diethyl pyrocarbonate water, and was 10−7 for both. The sensitivity study is illustrated in Figure 1 ▶ .
Figure 1.
Sensitivity study of the real-time RT-PCR method for the detection of the NPM-ALK and the ATIC-ALK fusion genes. Ten-fold dilutions (in diethyl pyrocarbonate water) were made from the cDNA of two samples, positive for NPM-ALK and ATIC-ALK. The RT-PCR was performed with triplicates and the resulting CT values were averaged and plotted on the chart. A strong linear relationship between the CT and the degree of dilution of the starting material was obtained for both NPM-ALK and ATIC-ALK detection.
Cloning and Sequencing of the Products
A limited number (n = 3) (cases 59, 61, and 65) of NPM-ALK PCR products were sequenced to verify the specificity of the PCR. Cloning was performed using the pGEM-T easy vector system (Promega, Leiden, the Netherlands). In short, the PCR products were gel-purified and inserted in the pGEM-T vector. Blue-white screening with X-gal and IPTG (Life Technologies, Merelbeke, Belgium) was used to pick the appropriate colonies for sequencing. Six white colonies were checked for the presence of the insert and were sequenced over the full length in both directions.
Quantitative Real-Time RT-PCR for NPM-ALK
Quantification was performed in a representative part of the cases namely four ALK-positive ALCL cases (cases 1, 2, 3, and 6), four ALK negative ALCL cases (cases 17–19 and 21), eight HD cases (cases 34–37, 39, 40, 45, and 48) and eight reactive tissues (cases 56, 59–62, 66, 77, and 79). The NPM-ALK fusion gene in the samples was quantified by measuring CT and by using a standard curve to determine the starting target quantity. As the precise amount of total cDNA added to each reaction mix and its quality are both difficult to assess, the GAPDH transcripts were also quantified as an endogenous reference and each sample was normalized by dividing the NPM-ALK target amount by the GAPDH target amount. Each of the normalized target values was divided by a designated calibrator-normalized target value. Final results are expressed as N-fold differences in NPM-ALK level relative to the GAPDH transcript amount and the calibrator.
The standard curves for both NPM-ALK and GAPDH were constructed with 10-fold serial dilutions in diethyl pyrocarbonate water of cDNA prepared from total RNA extracted from case 1. The series of diluted cDNA’s were aliquoted and stored at −20°C until use. Each PCR run was performed with triplicates, of which results were averaged, and included no template controls, 7 points of the standard curves and 8 unknowns. Amplification conditions were similar to those used for the qualitative RT-PCR (see above).
Prevention of Carryover Contamination
Carryover of PCR product was avoided by using the real-time RT-PCR method as it is a closed tube assay that requires no post-PCR handling. Three tubes had to be opened for sequencing, but this was performed in a separate room, preventing contact of products with the PCR setup area. Moreover, the master mix that was used contains dUTP instead of dTTP, and the enzyme AmpEraseUNG (uracil-N-glycosylase). UNG treatment removes dUTP containing carryover PCR products 32 and is followed by thermal inactivation of UNG before the actual PCR. In our laboratory neither NPM-ALK nor ATIC-ALK detection, was performed with a conventional RT-PCR lacking the dUTP-AmpEraseUNG system.
Apart from these specific characteristics of the real-time RT-PCR, general recommendations were taken in account to prevent carryover of RNA/cDNA during handling before the real-time PCR, such as regularly changing gloves, maintaining separate areas, and using positive-displacement pipets and sterile filter tips. In addition, cutting and collecting of the frozen tissue sections was performed with specific care to prevent carryover of small fragments of tissue. The knife of the cryostat was thoroughly cleaned after the cutting of each sample.
Numerous no-template controls (dH2O) were included at the beginning of the extraction procedures and remained consistently negative.
Cytogenetics and FISH
Chromosome analysis was performed according to standard protocols. Cells from lymph nodes were cultured for one day without stimulation. Three to 10 G-banded metaphases were analyzed. Chromosome abnormalities are presented in accordance with the International System for Human Cytogenetic Nomenclature (1995). 33
FISH was performed as previously described. 34 The ALK and NPM loci were investigated using an ALK P1 clone (designated ALK-DMPC-HFF#1–1111H1) and three cosmid clones (13, 15–2, and 47C12) from the 5q35 region located immediately centromeric to the NPM locus. 35 In t(2;5)-negative but ALK+ cases, rearrangement of ALK was analyzed with the Vysis LSI ALK probe assay (Vysis, Inc., Downer’s Grove, IL) that contains two differently labeled probes located either 3′ telomeric (spanning 250 kb and labeled with SpectrumOrange) or 5′ centromeric (spanning 300 kb and labeled with SpectrumGreen) of the t(2;5) breakpoint of the ALK gene at 2p23. The FISH data were collected on a Leitz DMRB fluorescence microscope equipped with a cooled black and white CCD camera (Photometrics, Tuscon, AZ) run by SmartCapture software (Vysis, Stuttgart, Germany).
Results
Results from the ALCL and the HD cases are summarized in Table 1 ▶ .
ALK Immunohistochemical Staining
ALK staining was positive in the tumor cells of 13 ALCL cases. In 9 of the latter cases (cases 1–9), cytoplasmic as well as nuclear staining was found, whereas the remaining 4 cases (cases 10–13) showed only cytoplasmic staining. None of the HD cases and none of the reactive tissue samples showed obvious ALK protein expression.
RT-PCR Results
Control of RNA Quality
All RNA samples were of sufficient quality as verified by the RT-PCR detection of a fragment of the GAPDH gene. The CT values resulting from this control RT-PCR ranged from 15 to 24 for the ALCL and the HD cases and from 14 to 26 for the reactive lymphoid tissues.
ALK Fusion Transcripts in the 33 ALCL Cases
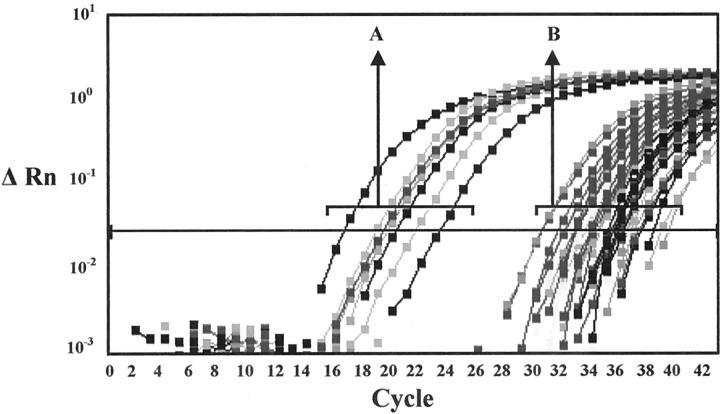
Of the 13 ALK-positive ALCL cases, 12 cases showed positive signals for NPM-ALK. Of these 12 cases, 8 cases were strongly positive (CT range, 17 to 23) and 4 were only weakly positive (CT range, 33–37) (Figure 2) ▶ . Of the latter 4 cases, 3 were also strongly positive for the ATIC-ALK fusion (cases 10–12) (CT range, 20 to 25). These three cases showed only cytoplasmic ALK staining, suggesting that the ATIC-ALK rearrangement was responsible for the ALK overexpression. Case 9, which showed massive involvement with cytoplasmic and nuclear ALK staining on the paraffin section, was only weakly positive for NPM-ALK. This finding might be explained by the fact that the frozen material only contained very small areas of tumor, which might have largely disappeared in subsequent sections cut for RNA extraction. One ALK-positive case with cytoplasmic and nuclear ALK staining that was strongly positive for NPM-ALK was also positive for ATIC-ALK, although weakly (case 1) (CT 37). Another ALK-positive ALCL case was negative for both ALK fusions (case 13).
Figure 2.
Results of real-time detection of the NPM-ALK fusion transcripts. x-axis: cycle number. y-axis: ΔRn = normalized signal generated from the sample minus the baseline signal established in the first few cycles of the PCR. The horizontal line indicates the threshold ΔRn value. The CT value is the cycle number at which the increase of the signal above this threshold can first be detected. Curves indicated by A represent the strongly positive signals generated from all ALK-positive ALCL cases with t(2;5)(p23;q35) (CT range, 17–23), excluding one case (case 9). Curves indicated by B represent the weakly positive signals generated from part of the ALK-negative ALCL, HD, and reactive tissue cases, from part of the ALK-positive ALCL cases without t(2;5)(p23;q35) (cases 10–12) and from 1 ALK-positive ALCL with t(2;5)(p23;q35) (case 9) (CT range, 30–39).
Of the 20 ALK-negative ALCL cases, 13 cases were positive for NPM-ALK (Figure 2) ▶ , of which 5 cases were also positive for ATIC-ALK. However, the signals resulting from all of these cases were weak (CT range, 30–38).
ALK Fusion Transcripts in the 22 Hodgkin’s Disease Cases
Eleven of 22 Hodgkin’s disease cases showed positive signals for NPM-ALK. CT values for these cases ranged from 31 to 39 (Figure 2) ▶ . Four of these cases (cases 34, 35, 37, and 42) also showed weak positive signals (range, 34–38) for the ATIC-ALK fusion. One additional case showed a positive signal for the ATIC-ALK fusion, but was negative for the NPM-ALK fusion.
ALK Fusion Transcripts in Reactive Tissues
Twenty of 31 reactive tissues (cases 56 to 75) showed positive results for the presence of NPM-ALK fusion transcripts with CT values ranging from 33 to 38 (Figure 2) ▶ . Sequencing of the products of three cases (cases 59, 61 and 65) (CT range, 34–36) confirmed that these weak fluorescent signals represented true amplification of the NPM-ALK fusion. Another case (case 76) showed a weak positivity (CT = 38) for the presence of the ATIC-ALK fusion.
Relative Quantification of NPM-ALK Fusion Transcripts
Results of real-time relative quantitative RT-PCR are summarized in Table 2 ▶ , showing the NPM-ALK target amounts detected in 24 samples, normalized to the GAPDH target amount (NPM-ALKN) and relative to the result of case 35 (resulting in the NPM-ALKN/C value). The latter result was designated as the calibrator as it was the strongest positive of all low-level samples. Based on the NPM-ALKN/C values, there was an obvious distinction between high-level and low-level detection in respectively ALK-positive ALCL cases (cases 1 to 3 and 6) on the one hand and ALK-negative ALCL cases, HD cases and reactive tissue cases (all other cases) on the other hand. More specifically, the presence of NPM-ALK in the high-level samples was 165-fold to 124,571-fold higher than that in the low-level samples.
Table 2.
Relative Quantification of the NPM-ALK Fusion Genes in 24 Cases by Real-Time RT-PCR
| Case | Diagnosis | NPM-ALKN* | NPM-ALKN/C† |
|---|---|---|---|
| 1 | ALK pos ALCL | 1.078E-01 | 2109 |
| 2 | ALK pos ALCL | 2.464E-02 | 482.1 |
| 3 | ALK pos ALCL | 2.732E-02 | 534.5 |
| 6 | ALK pos ALCL | 8.447E-03 | 165.3 |
| 17 | ALK neg ALCL | 7.326E-06 | 0.1433 |
| 18 | ALK neg ALCL | 1.328E-06 | 0.02600 |
| 19 | ALK neg ALCL | 2.120E-06 | 0.04148 |
| 21 | ALK neg ALCL | 8.653E-07 | 0.01693 |
| 34 | HD | 6.287E-06 | 0.1230 |
| 35 | HD | 5.111E-05‡ | 1.0000 |
| 36 | HD | 1.08E-05 | 0.2115 |
| 37 | HD | 1.042E-05 | 0.2039 |
| 39 | HD | 6.494E-06 | 0.1271 |
| 40 | HD | 2.838E-06 | 0.5553 |
| 45 | HD | 0 | 0 |
| 48 | HD | 0 | 0 |
| 56 | Non-neoplastic | 2.381E-06 | 0.04659 |
| 59 | Non-neoplastic | 3.090E-06 | 0.06046 |
| 60 | Non-neoplastic | 4.672E-06 | 0.09141 |
| 61 | Non-neoplastic | 1.712E-05 | 0.3346 |
| 62 | Non-neoplastic | 9.231E-16 | 0.1806 |
| 66 | Non-neoplastic | 2.096E-06 | 0.04101 |
| 77 | Non-neoplastic | 0 | 0 |
| 79 | Non-neoplastic | 0 | 0 |
*Normalized amount of NPM-ALK, determined by dividing the average NPM-ALK value by the average GAPDH value.
†Normalized amount of NPM-ALK divided by a designated calibrator value (‡).
Karyotyping and FISH
Cytogenetic studies and/or FISH analysis detected 2p23 abnormalities in all ALK-positive ALCL cases but in none in any of the ALK-negative ALCL cases or HD cases analyzed.
Cytogenetic studies were performed in 15 ALCL and in 10 HD cases. In the former group, 2p23 abnormalities were identified in 8 cases (cases 1, 2, 5, and 9–13) including four with a t(2;5)(p23;q35), three with the inv(2)(p23q35) and one with a t(2;17)(p23;q25). The latter case was the one ALK-positive ALCL case in which both analyzed ALK fusion genes were not detected (case 13). Six ALCL cases revealed an abnormal karyotype, but without structural aberrations of the short arm of chromosome 2. A normal karyotype was found in one ALCL case. In the HD group, seven cases showed clonal chromosomal abnormalities without 2p aberrations, and normal karyotype was observed in three cases (Table 1) ▶ .
FISH detection of the ALK/2p23 rearrangement was performed in 14 cases. In 7 of cases, FISH assay for a t(2;5) was used and a fusion signal indicating the NPM-ALK rearrangement was found either in available abnormal metaphases (cases 5 and 9) or in interphase cells (cases 3, 4, 6–8). Three ALK-positive ALCL cases (cases 10–12) lacking the NPM-ALK fusion in abnormal metaphases, showed a variant ALK rearrangement namely the inv(2)(p23q35) using the 5′/3′ end ALK FISH assay (Vysis). 21 The same set of probes was used to demonstrate the ALK rearrangement in t(2;17)(p23;q25) found in case 13. FISH performed in cases 15, 24, and 32 did not reveal the 2p23 abnormalities.
Discussion
In the present study we applied a real-time RT-PCR technique to detect NPM-ALK and ATIC-ALK fusion transcripts in ALCL cases, in HD cases, and in non-neoplastic lymphoid tissue samples. RT-PCR results were correlated to those obtained by ALK staining, cytogenetic studies and FISH analysis. Our results demonstrate a difference between ALK protein expression as detected by immunohistochemistry and the presence of the ALK fusion transcripts: ALK staining was positive in only 12 of 25 ALCL cases showing one or both fusion transcripts, however exclusively in those cases showing ALK fusion detection at high levels. In addition, we found that both fusion transcripts can be detected in HD, as well as in reactive conditions, although at low levels. All data obtained by karyotyping and FISH were concordant with the ALK immunohistochemistry results. Interestingly, one ALK-positive ALCL case (case 13) showed none of both analyzed ALK fusion genes but revealed the presence of a new translocation t(2;17)(p23;q25) by cytogenetic and FISH analysis. In our study, ALK rearrangements other than NPM-ALK occurred in 31% of ALK-positive ALCL cases, much more frequently than previously thought. 15
For the ALCL cases, the difference between ALK staining and RT-PCR results might be explained by the higher sensitivity of the real-time RT-PCR method, which was 10−7, compared to conventional RT-PCR methods. The TaqMan technology also accounts for the high specificity through the obligate binding of the fluorescent probe spanning the breakpoints. The reliability of the method for NPM-ALK detection was confirmed by sequencing of three amplification products showing weak positivity (cases 59, 61, and 65). Carryover contamination should be considered as another possible explanation for our RT-PCR results. However, in our opinion, contamination is very unlikely due to the specific characteristics of the real-time method (closed tube assay and dUTP/UNG technique) and the general PCR recommendations that were conscientiously followed during all procedures.
Discrepancies among immunohistochemical staining results, cytogenetical analysis, and RT-PCR results were also observed by Ott and colleagues, 36 who found two ALCL cases showing the presence of the NPM-ALK fusion transcript by RT-PCR without cytogenetic positivity and without positive immunostaining with anti-p80. The authors suggested false positivity to be a possible explanation. Alternatively, two other explanations were proposed, the presence of only a minor fraction of tumor cells carrying the translocation and the existence of rearrangements in a non-neoplastic bystander cell.
The latter explanation may now be supported by our results showing the presence at a low level of the NPM-ALK fusion transcripts in almost two-thirds of the non-neoplastic tissues analyzed. The level at which the fusion transcript is detected in these non-neoplastic tissues is comparable to the level observed in the ALCL cases lacking ALK protein expression suggesting indeed the presence of NPM-ALK fusion transcripts in the background reactive cells and not in the tumor cells. In addition, a weak positivity was also observed for the ATIC-ALK fusion transcript, although in only one reactive tissue sample. This much lower frequency of ATIC-ALK fusion transcript detection in reactive tissue biopsies might reflect its lower frequency in ALCL cases, thus supporting our findings.
The possible presence of NPM-ALK in non-neoplastic cells may also explain its detection in HD cases, as shown by us in both the present study and previously by others. 24-26 Orscheschek and colleagues 24 found 11 of 13 cases to be positive by RT-PCR, concluding that HD and ALCL might be pathogenetically related and that detection of t(2;5) may be used as an indicator for clonality in HD. However, Trümper and colleagues 25 also found NPM-ALK in HD but acknowledged that the t(2;5) should not be considered as important in terms of pathogenesis of HD, based on its very low detection rate in RS single cells (<5% of RS cells in 2 of 9 HD cases). Moreover, in their subsequent study they demonstrated the existence of NPM-ALK in normal peripheral blood samples of 14 of 29 healthy individuals, 30 suggesting that the detection of NPM-ALK in HD is coincidental.
Our study may also support the findings of Beylot-Barry and colleagues, 37 who found that CD30+ cutaneous lymphoproliferative diseases may contain NPM-ALK transcripts in the absence of ALK expression.
The presence of tumor-specific fusion genes in non-neoplastic cells is not new. Earlier, the fusion genes resulting from t(14;18) and t(9;22) were also shown not to be restricted to malignant cells. 38-39 Such genes may constitute abnormalities that, by themselves, are not able to generate a neoplastic proliferation without the interaction with as yet unidentified tumorogenic events. Experiments with a retroviral NPM-ALK gene transfer mouse model have indeed suggested that NPM-ALK expression alone may not be sufficient to produce lymphoma, as the latency period was relatively long and no cell lines could be established from the mouse tumors. 40 The c-myc proto-oncogene appears to be frequently altered in ALCL 41 and might thus represent a candidate gene required for co-operating with NPM-ALK in malignant transformation. Alternatively, tumor specific fusion genes may be expressed in hematopoietic cells that have entered an apoptotic pathway before acquiring a characteristic leukemic karyotype. 42
Not only the presence of so-called tumor-specific fusion genes in normal tissue but also the high frequency of this finding in our study is remarkable. This indicates that an ALK rearrangement might be very easily acquired, which may be supported by the numerous partner genes hitherto identified 16-23 or remaining to be identified such as the one on chromosome 17q25 in case 13 of the present study. Moreover, recently, Lawrence and colleagues 43,44 demonstrated the presence of TPM3-ALK and TPM4-ALK fusion genes in inflammatory myofibroblastic tumors, indicating that ALK rearrangement is not restricted to lymphocytes but may affect different cell lineages.
Based on our findings, one may question the reliability of real-time RT-PCR as a tool to be used in the diagnosis and follow up of lymphomas, in particular of ALCL cases. Firstly, the application of high sensitivity real-time RT-PCR may overestimate the presence of the t(2;5) in the ALCL cases investigated, thereby preventing the exact evaluation of prognosis at the time of diagnosis. Immunohistochemical demonstration of ALK expression within the tumor cells should always be accomplished to make the diagnosis of ALK-positive ALCL. Alternatively, for diagnostic purposes, it may be considered either to score as positive only those samples with CT values below a certain threshold value (eg, 30) or to rely only on results obtained by routine low to moderate sensitivity RT-PCR. Secondly, our findings demonstrate that quantitative followup of minimal residual disease using the TaqMan technology may be problematic due to the presence of the target sequence in apparently normal bystander cells. When used for monitoring minimal residual disease in ALCL, the high sensitivity of this technique may be considered not only a big advantage but also a major drawback. Quantitative real-time RT-PCR has already been used to monitor minimal residual disease in chronic myeloid leukemia, showing, however, confusing results; for instance, a positive PCR reaction does not always equal relapse. This might be explained by the “too high sensitivity” of the technique, as it may detect BCR-ABL transcripts in normal cells. 39,42 Extensive studies using real- time PCR should be performed to evaluate the significance of low levels of fusion transcripts and their relevance for the prognosis of patients.
In summary, our findings demonstrate that NPM-ALK and ATIC-ALK fusion transcripts, which result in ALCL from t(2;5) and inv(2), respectively, may also be detected in nonmalignant cells. These results question whether both fusion transcripts are sufficient to induce tumor formation and whether real-time RT-PCR monitoring of minimal residual disease is of prognostic significance.
Acknowledgments
The authors thank Miet Vanherck for excellent technical assistance.
Footnotes
Address reprint requests to Dr. B. Maes, Department of Pathology, University Hospitals, Catholic University of Leuven, Minderbroedersstraat 12, B-3000 Leuven, Belgium. E-mail: brigitte.maes@uz.kuleuven.ac.be.
Supported by a grant from the Belgian Cancer Association to C. D. W.-P.
References
- 1.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H: The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66:848-858 [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 3.Kadin ME: Anaplastic large cell lymphoma and its morphological variants. Cancer Surv 1997, 30:77-86 [PubMed] [Google Scholar]
- 4.Paulli M, Berti E, Rosso R, Boveri E, Kindl S, Klersy C, Lazzarino M, Borroni G, Menestrina F, Santucci M, Gambini C, Vassallo G, Magrini U, Sterry W, Burg G, Geerts ML, Meijer CJLM, Willemze R, Feller AC, Müller-Hermelink HK, Kadin ME: CD30/Ki-1-positive lymphoproliferative disorders of the skin —clinicopathologic correlation and statistical analysis of 86 cases: a multicentric study from the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Project Group. J Clin Oncol 1995, 13:1343-1354 [DOI] [PubMed] [Google Scholar]
- 5.Vergier B, Beylot-Barry M, Pulford K, Michel P, Bosq J, de Muret A, Beylot C, Delaunay MM, Avril MF, Dalac S, Bodemer C, Joly P, Groppi A, de Mascarel A, Bagot M, Mason DY, Wechsler J, Merlio JP: Statistical evaluation of diagnostic and prognostic features of CD30+ cutaneous lymphoproliferative disorders: a clinicopathologic study of 65 cases. Am J Surg Pathol 1998, 22:1192-1202 [DOI] [PubMed] [Google Scholar]
- 6.Mason DY, Bastard C, Rimokh R, Dastugue N, Huret JL, Kristoffersson U, Magaud JP, Nezelof C, Tilly H, Vannier JP, Hemet J, Warnke R: CD30-positive large cell lymphomas ( a “Ki-1 lymphoma”) are associated with a chromosomal translocation involving 5q35. Br J Haematol 1990, 74:161-168 [DOI] [PubMed] [Google Scholar]
- 7.Chan WC: The t(2;5) or NPM-ALK translocation in lymphomas: diagnostic considerations. Adv Anat Pathol 1996, 3:396-399 [Google Scholar]
- 8.Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP: ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase. Oncogene 1997, 14:2175-2188 [DOI] [PubMed] [Google Scholar]
- 9.Pulford K, Lamant L, Morris SW, Butler LH, Wood KM, Stroud D, Delsol G, Mason DY: Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997, 89:1394-1404 [PubMed] [Google Scholar]
- 10.Falini B, Bigerna B, Fizzotti M, Pulford K, Pileri SA, Delsol G, Carbone A, Paulli M, Magrini U, Menestrina F, Giardini R, Pilotti S, Mezzelani A, Ugolini B, Billi M, Pucciarini A, Pacini R, Pelicci PG, Flenghi L: ALK expression defines a distinct group of T/null lymphomas (“ALK lymphomas”) with a wide morphological spectrum. Am J Pathol 1998, 153:875-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugieres L, Terrier-Lacombe MJ, Haralambieva E, Pulford K, Pileri S, Morris SW, Mason DY, Delsol G: ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998, 91:2076-2084 [PubMed] [Google Scholar]
- 12.Pittaluga S, Wlodarska I, Pulford K, Campo E, Morris SW, Van den Berghe H, De Wolf-Peeters C: The monoclonal antibody ALK1 identifies a distinct morphological subtype of anaplastic large cell lymphoma associated with 2p23/ALK rearrangements. Am J Pathol 1997, 151:343-351 [PMC free article] [PubMed] [Google Scholar]
- 13.Shiota M, Mori S: Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Leukemia 1997, 11 (Suppl 3):538-540 [PubMed] [Google Scholar]
- 14.Mason DY, Pulford KA, Bischof D, Kuefer MU, Butler LH, Lamant L, Delsol G, Morris SW: Nucleolar localization of the nucleophosmin-anaplastic lymphoma kinase is not required for malignant transformation. Cancer Res 1998, 58:1057-1062 [PubMed] [Google Scholar]
- 15.Pulford K, Falini B, Cordell J, Rosenwald A, Ott G, Muller-Hermelink HK, MacLennan KA, Lamant L, Carbone A, Campo E, Mason DY: Biochemical detection of novel anaplastic lymphoma kinase proteins in tissue sections of anaplastic large cell lymphoma. Am J Pathol 1999, 154:1657-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamant L, Dastugue N, Pulford K, Delsol G, Mariame B: A new fusion gene TPM3-ALK in anaplastic large cell lymphoma created by a (1;2)(q25;p23) translocation. Blood 1999, 93:3088-3095 [PubMed] [Google Scholar]
- 17.Hernandez L, Pinyol M, Hernandez S, Bea S, Pulford K, Rosenwald A, Lamant L, Falini B, Ott G, Mason DY, Delsol G, Campo E: TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 1999, 94:3265-3268 [PubMed] [Google Scholar]
- 18.Trinei M, Lanfrancone L, Campo E, Pulford K, Mason DY, Pelicci PG, Falini B: A new variant anaplastic lymphoma kinase (ALK)-fusion protein (ATIC-ALK) in a case of ALK-positive anaplastic large cell lymphoma. Cancer Res 2000, 60:793-798 [PubMed] [Google Scholar]
- 19.Colleoni GW, Bridge JA, Garicochea B, Liu J, Filippa DA, Ladanyi M: ATIC-ALK: A novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35). Am J Pathol 2000, 156:781-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z, Cools J, Marynen P, Cui X, Siebert R, Gesk S, Schlegelberger B, Peeters B, De Wolf-Peeters C, Wlodarska I, Morris SW: Inv(2)(p23q35) in anaplastic large-cell lymphoma induces constitutive anaplastic lymphoma kinase (ALK) tyrosine kinase activation by fusion to ATIC, an enzyme involved in purine nucleotide biosynthesis. Blood 2000, 95:2144-2149 [PubMed] [Google Scholar]
- 21.Wlodarska I, De Wolf-Peeters C, Falini B, Verhoef G, Morris SW, Hagemeijer A, Van den Berghe H: The cryptic inv(2)(p23q35) defines a new molecular genetic subtype of ALK-positive anaplastic large-cell lymphoma. Blood 1998, 92:2688-2695 [PubMed] [Google Scholar]
- 22.Touriol C, Greenland C, Lamant L, Pulford K, Bernard F, Rousset T, Mason DY, Delsol G: Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 2000, 95:3204-3207 [PubMed] [Google Scholar]
- 23.Rosenwald A, Ott G, Pulford K, Katzenberger T, Kuhl J, Kalla J, Ott MM, Mason DY, Muller-Hermelink HK: t(1;2)(q21;p23) and t(2;3)(p23;q21): two novel variant translocations of the t(2;5)(p23;q35) in anaplastic large cell lymphoma. Blood 1999, 94:362-364 [PubMed] [Google Scholar]
- 24.Orscheschek K, Merz H, Hell J, Binder T, Bartels H, Feller AC: Large-cell anaplastic lymphoma-specific translocation (t[2;5] [p23;q35]) in Hodgkin’s disease: indication of a common pathogenesis? Lancet 1995, 345:87-90 [DOI] [PubMed] [Google Scholar]
- 25.Trumper L, Daus H, Merz H, von Bonin F, Loftin U, Cochlovius C, Moller P, Feller AC, Pfreundschuh M: NPM/ALK fusion mRNA expression in Hodgkin and Reed-Sternberg cells is rare but does occur: results from single-cell cDNA analysis. Ann Oncol 1997, 8-(Suppl 2):83-87 [PubMed] [Google Scholar]
- 26.Yee HT, Ponzoni M, Merson A, Goldstein M, Scarpa A, Chilosi M, Menestrina F, Pittaluga S, De Wolf-Peeters C, Shiota M, Mori S, Frizzera G, Inghirami G: Molecular characterization of the t(2;5) (p23; q35) translocation in anaplastic large cell lymphoma (Ki-1) and Hodgkin’s disease. Blood 1996, 87:1081-1088 [PubMed] [Google Scholar]
- 27.Sarris AH, Luthra R, Papadimitracopoulou V, Waasdorp M, Dimopoulos MA, McBride JA, Cabanillas F, Duvic M, Deisseroth A, Morris SW, Pugh WC: Amplification of genomic DNA demonstrates the presence of the t(2;5) (p23;q35) in anaplastic large cell lymphoma, but not in other non-Hodgkin’s lymphomas, Hodgkin’s disease, or lymphomatoid papulosis. Blood 1996, 88:1771-1779 [PubMed] [Google Scholar]
- 28.Lamant L, Meggetto F, al Saati T, Brugieres L, de Paillerets BB, Dastugue N, Bernheim A, Rubie H, Terrier-Lacombe MJ, Robert A, Rigal F, Schlaifer D, Shiuta M, Mori S, Delsol G: High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood 1996, 87:284-291 [PubMed] [Google Scholar]
- 29.Wellmann A, Otsuki T, Vogelbruch M, Clark HM, Jaffe ES, Raffeld M: Analysis of the t(2;5)(p23;q35) translocation by reverse transcription-polymerase chain reaction in CD30+ anaplastic large-cell lymphomas, in other non-Hodgkin’s lymphomas of T-cell phenotype, and in Hodgkin’s disease. Blood 1995, 86:2321-2328 [PubMed] [Google Scholar]
- 30.Trumper L, Pfreundschuh M, Bonin FV, Daus H: Detection of the t(2;5)-associated NPM/ALK fusion cDNA in peripheral blood cells of healthy individuals. Br J Haematol 1998, 103:1138-1144 [DOI] [PubMed] [Google Scholar]
- 31.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD: The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November. Ann Oncol 1997, 1999:10:1419–1432 [DOI] [PubMed]
- 32.Longo MC, Berninger MS, Hartley JL: Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 1990, 93:125-128 [DOI] [PubMed] [Google Scholar]
- 33.ISCN: An International System for Human Genetic Nomenclature. Edited by F Mitelman. Basel: Karger. 1995
- 34.Dierlamm J, Wlodarska I, Michaux L, La Starza R, Zeller W, Mecucci C, Van den Berghe H: Successful use of the same slide for consecutive fluorescence in situ hybridization (FISH) experiments. Genes Chromosomes Cancer 1996, 16:261-264 [DOI] [PubMed] [Google Scholar]
- 35.Mathew P, Sanger WG, Weisenburger DD, Valentine M, Valentine V, Pickering D, Higgins C, Hess M, Cui X, Srivastava DK, Morris SW: Detection of the t(2;5)(p23;q35) and NPM-ALK fusion in non-Hodgkin’s lymphoma by two-color fluorescence in situ hybridization. Blood 1997, 89:1678-1685 [PubMed] [Google Scholar]
- 36.Ott G, Katzenberger T, Siebert R, DeCoteau JF, Fletcher JA, Knoll JH, Kalla J, Rosenwald A, Ott MM, Weber-Matthiesen K, Kadin ME, Muller-Hermelink HK: Chromosomal abnormalities in nodal and extranodal CD30+ anaplastic large cell lymphomas: infrequent detection of the t(2;5) in extranodal lymphomas. Genes Chromosomes Cancer 1998, 22:114-121 [DOI] [PubMed] [Google Scholar]
- 37.Beylot-Barry M, Groppi A, Vergier B, Pulford K, Merlio JP: Characterization of t(2;5) reciprocal transcripts and genomic breakpoints in CD30+ cutaneous lymphoproliferations. Blood 1998, 91:4668-4676 [PubMed] [Google Scholar]
- 38.Limpens J, Stad R, Vos C, de Vlaam C, de Jong D, van Ommen GJ, Schuuring E, Kluin PM: Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood 1995, 85:2528-2536 [PubMed] [Google Scholar]
- 39.Biernaux C, Loos M, Sels A, Huez G, Stryckmans P: Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood 1995, 86:3118-3122 [PubMed] [Google Scholar]
- 40.Kuefer MU, Look AT, Pulford K, Behm FG, Pattengale PK, Mason DY, Morris SW: Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 1997, 90:2901-2910 [PubMed] [Google Scholar]
- 41.Inghirami G, Macri L, Cesarman E, Chadburn A, Zhong J, Knowles DM: Molecular characterization of CD30+ anaplastic large-cell lymphoma: high frequency of c-myc proto-oncogene activation. Blood 1994, 83:3581-3590 [PubMed] [Google Scholar]
- 42.Faderl S, Talpaz M, Kantarjian HM, Estrov Z: Should polymerase chain reaction analysis to detect minimal residual disease in patients with chronic myelogenous leukemia be used in clinical decision making? Blood 1999, 93:2755-275910216068 [Google Scholar]
- 43.Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CDM, Fletcher JA: TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000, 157:377-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladanyi M: Aberrant ALK tyrosine kinase signaling: different cellular lineages, common oncogenic mechanisms? Am J Pathol 2000, 157:341-345 [DOI] [PMC free article] [PubMed] [Google Scholar]