Abstract
Chemoattraction of macrophages and T cells into the normal endometrium and inflammatory sites within endometriotic foci is mediated by chemokine gene expression. mRNA transcripts encoding regulated on activation, normal T-cell-expressed and -secreted (RANTES), a monocyte and T-cell chemokine, were demonstrated in the stroma of normal endometrium and endometriotic implants using in situ mRNA hybridization. Epithelial glands failed to express RANTES mRNA. In histological serial sections, we observed CD68-positive macrophages in the stroma of endometriotic implants adjacent to regions with prominent RANTES mRNA hybridization. In adjacent sections, monoclonal antibodies against tumor necrosis factor (TNF)-α showed this cytokine to be localized to stromal and epithelial compartments of the endometriotic implant with weak staining in unaffected ovarian tissue. Subconfluent monolayers of endometriotic stromal cells were tested for RANTES gene expression in situ, but we could only detect RANTES mRNA in isolated stromal cells after treatment with TNF-α. No RANTES mRNA was observed in unstimulated stromal cells or TNF-α stimulated or unstimulated epithelial cells. The data are consistent with a model in which proinflammatory cytokines (eg, TNF-α) induce RANTES gene expression limited to specific cells within endometrial and endometriotic stroma. Production of this chemokine, in turn, stimulates recruitment of CD68-positive macrophages into these tissues.
Endometriosis is a chronic gynecological disorder that affects millions of reproductive-aged women world-wide. The clinical hallmarks of this disorder are ectopic endometrial implants, commonly associated with evidence of intraperitoneal inflammation. Haney and colleagues 1 were the first to identify macrophages as the most abundant leukocytes in the pelvic fluid of women with endometriosis. Subsequent studies indicated that the total concentration 2 and activation status 3 of macrophages were specifically increased in endometriosis. Activated T cells also appear to accumulate preferentially in the peritoneal fluid of endometriosis cases. 2,4
Resident leukocytes within the stroma of endometriotic lesions have been characterized histologically. 5,6 As noted in the surrounding pelvic fluid, endometriotic implants predominantly contain macrophages and T cells, and eosinophils have been reported recently. 7 We and others have proposed that recruitment of leukocytes to the vicinity of endometriotic lesions is mediated by specific chemokines synthesized by cells within the implants. Leukocyte chemoattractants, such as monocyte chemotactic protein-1 (MCP-1), 8,9 vascular endothelial growth factor, 10,11 interleukin-8, 12-14 and eotaxin 15 are expressed in endometriotic implants. Because of the phenotypic inflammatory exudate of endometriosis, we studied the expression of regulated on activation, normal T cell expressed and secreted (RANTES), a β-chemokine selective for macrophage and T-cell recruitment. RANTES protein was identified immunohistochemically in stromal cells of normal endometrium and ectopic implants 16 and could be detected in high concentrations in peritoneal fluid of women with endometriosis. 4 The current experiments were designed to verify RANTES synthesis within the endometrium and endometriotic implants and to identify which cells were responsible for RANTES gene expression. In situ mRNA hybridization, combined with immunohistochemistry to localize specific cell types within the implants, was used to achieve this objective. The findings support our hypothesis that local cytokine networks regulate RANTES gene expression and the attraction of macrophages and T cells into eutopic and ectopic endometrium.
Materials and Methods
Patient Recruitment and Characterization
Healthy ovulatory women, who had not received hormones or GnRH agonist therapy for at least 6 months before surgery, were recruited after they had provided written informed consent under a study protocol approved by the Committee on Human Research at the University of California, San Francisco. Women with ovarian endometriomata (n = 8) were identified laparoscopically and staged according to a modification of the revised American Fertility Society system. 12 Controls also were evaluated by laparoscopy and were women with subserosal leiomyomata or without pelvic pathology (n = 11).
Sources of Tissues
Tissue specimens were obtained at the time of laparoscopy, which was scheduled during the mid-proliferative phase of the cycle. Endometrial and endometriosis biopsies were collected under sterile conditions and prepared for histochemistry or cell culture, as described previously. 16
Preparation of Hybridization Probes
For in situ hybridization, 202-bp RANTES cDNA templates were synthesized by polymerase chain reaction, having engineered T7 RNA polymerase-binding site sequences 5′ to the antisense oligomers for the gene products (Table 1) ▶ . Run-off transcripts were synthesized by incubation of T7 RNA polymerase with 35S-UTP and unlabeled rNTPs as described previously. 17
Table 1.
RANTES Primers Used in Generating Antisense and Sense Control Riboprobe Templates
| Antisense primers |
| 5′ Primer |
| CAC TGC CCC GTG CCC ACA TCA A |
| 3′ Primer |
| CGT AGG CTA ATA CGA CTC ACT ATA GGG AGG CTG TGA GAG TCT CCA TCC TAG CTC ATC TCC AAA |
| Sense control primers |
| 5′ Primer |
| CGT AGG CTA ATA CGA CTC ACT ATA GGG AGG CTG TGA CAC TGC CCC GTG CCC ACA TCA A |
| 3′ Primer |
| GAC TCT CCA TCC TAG CTC ATC TCC AAA |
Underlined letters represent T7 polymerase site sequence.
Immunohistochemistry
Endometrial and endometrioma tissues were fixed for 24 hours in 2% paraformaldehyde and 0.5% glutaraldehyde, paraffin-embedded, cut in serial sections of 5 μm, and stained using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Immunoperoxidase staining was performed overnight at 4°C, using mouse monoclonal IgG antibodies against human cytokeratin-18 (1:2000 dilution; Sigma, St. Louis, MO), human CD-68 (dilution 1:100; DAKO Corp., Carpinteria, CA), human RANTES (dilution 1:100; R&D Systems, Minneapolis, MN), or TNF-α (dilution 1:100, R&D Systems). Controls for the immunostaining specificity of TNF-α included sections stained with anti-TNF-α antibodies immunoabsorbed with 10 μg/ml of recombinant human TNF-α (R&D Systems). Diaminobenzidine (Zymed, South San Francisco, CA) was used as the chromagen. All sections also were lightly counterstained with hematoxylin.
In Situ Hybridization of Tissue Sections
For detection of RANTES mRNA, paraffin-embedded endometriotic and endometrial specimens were dewaxed and hybridized as described previously. 17,18 After pretreatment with proteinase K (1 μg/ml), serial tissue sections were incubated in a hybridization mixture containing the 35S-labeled RANTES antisense RNA probe (500 ng/ml) or RANTES sense RNA control probe (500 ng/ml) in 10 mmol/L Tris-HCl, pH 7.4, 50% (v/v) deionized formamide, 600 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 0.02% polyvinylpyrrolidone, 0.02% Ficoll, 0.05% bovine serum albumin, 10% dextran sulfate, 10 mmol/L dithiothreitol, 200 μg/ml denatured sonicated salmon sperm DNA, and 100 μg/ml rabbit liver tRNA. Hybridization was performed overnight at 42°C. After hybridization, tissue sections were washed as described previously. 17 Nonhybridized single-stranded RNA probes were digested by RNase A (20 μg/ml) in 10 mmol/L of Tris-HCl (pH 8.0)/0.5 mol/L NaCl for 30 minutes at 37°C. For autoradiography, slides were dipped in Kodak NTB-2 nuclear track emulsion (Integra Biosciences, Inc., Woburn, MA). After exposure for 4 weeks at 4°C, slides were developed and counterstained with hematoxylin and eosin.
Human Endometrial and Endometriosis Cell Cultures
Primary endometrial and endometriotic cell cultures were prepared from biopsies as described previously. 16 Glandular epithelial cells were separated from stromal cells and debris by filtration through narrow gauge sieves. Stromal cells were subcultured twice to eliminate contamination by macrophages or other leukocytes. Extensive characterization of cell cultures prepared using this protocol confirmed that they were >95% pure and retained functional markers of their endometrial and endometriotic origin in vivo. 12
Preparation of Cells for in Situ Hybridization
Endometriotic stromal and epithelial cells were plated onto Lab-Tek four-chamber slides (NUNC, Rochester, NY). The complete medium was removed and replaced with fresh minimal essential medium-α containing 2.5% fetal calf serum and antibiotics and the cells were cultured for 48 hours in the absence or presence of TNF-α (100 ng/ml, Sigma Chemical Co.), and fixed in 95% ethanol. In situ hybridization was performed as described above.
Results
In Situ Hybridization and Immunohistochemistry of Tissue Sections
In situ hybridization was used to localize RANTES mRNA in fixed tissues. A section of normal mid-proliferative endometrium is shown in Figure 1A ▶ . RANTES gene expression could be detected in the stromal compartment adjacent to epithelial glands, but the glands themselves did not contain any RANTES message. No specific autoradiographic signals were observed when serial endometrial tissue sections were hybridized with the RANTES sense RNA-control probe (Figure 1B) ▶ . Similar results were observed in 8 of 11 normal endometrial biopsies. Endometriotic implants of the ovary also showed RANTES mRNA in the stromal compartment indicated by black arrows and white arrows. The epithelial cell lining subjacent to the stroma and the unaffected ovarian stroma (top) did not contain RANTES mRNA (Figure 1C) ▶ . No specific autoradiographic signals were observed when the endometriotic tissue sections were hybridized with the RANTES sense RNA-control probe (Figure 1D) ▶ . Similar results were observed in seven of eight endometriotic sections.
Figure 1.
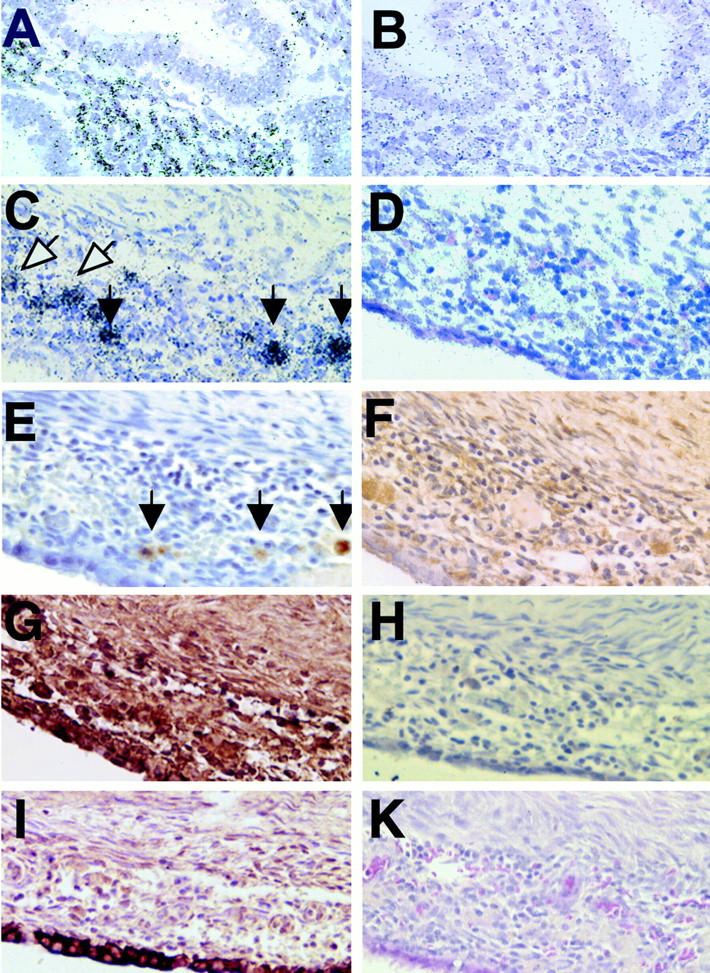
In situ hybridization of RANTES mRNA in normal mid-proliferative human endometrium (A). RANTES gene expression could be detected in the stromal compartment adjacent to epithelial glands, but the glands themselves did not contain RANTES message. No specific autoradiographic signal was observed when serial tissue sections were hybridized with the RANTES sense RNA-control probe (B). Endometriotic implants also showed RANTES message in the stromal compartment with several intense spots (black arrows) and other more diffuse hybridization (white arrows). The epithelial cell lining of the endometrioma and the unaffected ovarian stroma (top) did not contain RANTES message (C). No specific autoradiographic signal was observed when endometriotic tissue sections were hybridized with the RANTES sense RNA-control probe (D). Immunohistochemical localization of CD68-positive macrophages (E, black arrows) in an adjacent endometriotic tissue section. Monoclonal antibodies against human RANTES demonstrated this protein in the endometriotic stroma, with sparing of the cyst epithelium (F). Monoclonal antibodies against TNF-α showed the protein in the epithelial and stromal compartments of the endometriotic implant. Portions of the ovary uninvolved with endometriosis showed less TNF-α immunostaining (G). Control experiments were performed on serial endometriotic sections stained with TNF-α antibodies immunoabsorbed with excess TNF-α protein (H). Epithelial cells lining the endometriotic cyst were cytokeratin-positive (I). All sections were also counterstained with hematoxylin. H&E staining shows the morphology of the endometriotic implant (K). Original magnifications, ×200.
Immunohistochemistry of serial sections demonstrated CD-68-positive macrophages within the implant (Figure 1E) ▶ . These regions correspond to RANTES mRNA-positive spots in Figure 1C ▶ (black arrows), whereas other RANTES mRNA-positive areas had no apparent macrophages (Figure 1C ▶ , white arrows). Monoclonal antibodies against human RANTES demonstrated this protein in the endometriotic stroma, with sparing of the cyst epithelium (F). Monoclonal antibodies against TNF-α showed that this cytokine was diffusely present in both stromal and epithelial compartments of the endometriotic implant. Regions of the ovary uninvolved with endometriosis showed less intense TNF-α immunostaining (Figure 1G) ▶ . Control experiments were performed on serial endometriotic sections stained with TNF-α antibodies immunoabsorbed with excess TNF-α protein (Figure 1H) ▶ . The latter treatment eliminated the staining pattern and verified the specificity of the immunolocalization. An adjacent section, stained with monoclonal antibodies against cytokeratin, specifically decorated the epithelial lining of the endometriotic cyst (Figure 1I) ▶ . Figure 1K ▶ is a control section stained with hematoxylin and eosin to show the morphology of the endometriotic implant.
RANTES in Situ Hybridization in Purified Endometrial Cell Populations
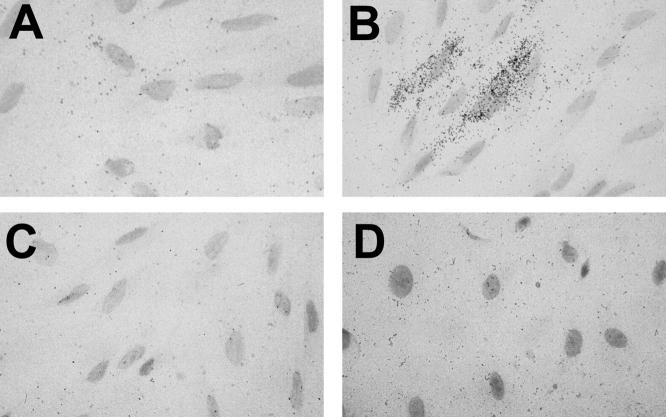
Isolated endometriotic stromal and epithelial cells were examined subsequently for RANTES mRNA expression by in situ hybridization. Subconfluent monolayers of endometriotic stromal cells were cultured for 48 hours. No RANTES mRNA was detectable under conditions in which the medium contained no added cytokine (Figure 2A) ▶ . After culturing the cells in the presence of TNF-α (100 ng/ml) for 48 hours (Figure 2B) ▶ , RANTES mRNA was identified in 10 to 20% of the stromal cells. No specific autoradiographic signals were observed when TNF-α-stimulated endometriotic stromal cells were hybridized with the RANTES sense RNA-control probe (Figure 2C) ▶ . Subconfluent monolayers of endometriotic epithelial cells also did not reveal evidence of RANTES mRNA expression, even after 48 hours of cytokine stimulation (Figure 2D) ▶ .
Figure 2.
Isolated primary endometriotic stromal and epithelial cells were examined for RANTES mRNA expression by in situ hybridization. Subconfluent monolayers of endometriotic stromal cells were cultured for 48 hours. No RANTES mRNA was detectable under conditions in which the medium contained no added cytokine (A). After culturing the cells in the presence of TNF-α (100 ng/ml) for 48 hours (B), RANTES mRNA was expressed in 10 to 20% of the stromal cells. No specific autoradiographic signal was observed when TNF-α stimulated endometriotic stromal cells were hybridized with the RANTES sense RNA-control probe (C). Subconfluent monolayers of epithelial cells did not reveal evidence of RANTES expression, even after TNF-α stimulation (D). Original magnifications, ×600.
Discussion
Twenty years ago, gynecologists first observed the accumulation of specific leukocytes within the peritoneal cavities of women with endometriosis 1 and during the past decade the same leukocytes were noted to infiltrate both eutopic and ectopic endometrium. 5 We now have come to understand that leukocyte trafficking from the circulation into tissues is highly regulated via concentration gradients of selective chemokines. Rather than each leukocyte responding to a single cognate chemoattractant, considerable biological redundancy and promiscuity of chemokine specificity exist. Among the proteins with predominant monocyte affinity are the β-chemokines, RANTES, MCPs 1 to 4 and macrophage inflammatory proteins 1α and β. These chemokines are ligands with variable affinities for the receptors CCR1 to CCR5, which are expressed on the surface of responsive immune cells. 19
As the predominant leukocytes within peritoneal fluid 1 and ectopic implants 5 of women with endometriosis include macrophages and T cells, our laboratory has focused on the expression of one chemokine, RANTES, with activity for both cell types. RANTES originally was isolated as a cDNA from CD8+ T cells 20 that encodes an 8-kd secreted chemokine protein. Expression of RANTES can be induced within hours of stimulation by proinflammatory stimuli, such as TNF-α. 21
In the current study we found that RANTES mRNA was localized to stroma, consistent with protein localization and in vitro expression in isolated endometrial cells. 16 CD68-positive macrophages were co-localized with RANTES mRNA-expressing stromal cells (black arrows, Figure 1C ▶ ), however, some RANTES mRNA-positive stromal regions (white arrows, Figure 1C ▶ ) were devoid of macrophages. A plausible interpretation of this observation is that CD68-positive macrophages are recruited to areas of stromal RANTES expression. The distribution of TNF-α protein in endometriosis implants is very similar to that described in the functionalis region of normal eutopic endometrium. 22
Our in situ hybridization data indicated that RANTES mRNA-positive stromal cells were in proximity to TNF-α-positive epithelial and stromal cells. This observation suggested that the latter cells are potential paracrine or autocrine sources, respectively, of cytokines that stimulate RANTES mRNA production in endometrial and endometriotic stroma. Endometrial expression of TNF-α mRNA is increased in the late secretory phase and during endometrial bleeding. 23 This is consistent with our observation that normal endometrial RANTES mRNA concentrations, as determined by solution hybridization experiments, were highest in the secretory phase. 16 However, our immunohistochemistry analyses were not sensitive or quantitative enough to verify this finding. 16 To optimize reproducibility we limited the current study to proliferative phase sampling of normal and endometriosis biopsies, which precluded further analysis of the effects of ovulatory cycle stage and endocrine milieu on RANTES expression. Our group previously investigated the effects of 10 nmol/L of 17β-estradiol on RANTES secretion by endometrial and endometriosis stromal cells in vitro, but we observed no stimulatory action. 24 Recently, however, Akoum and colleagues 25 studying the related MCP-1 chemokine in mixed endometriotic cell cultures, observed that 10 nmol/L of estradiol increased interleukin-1β-induced MCP-1 mRNA production by 200% and protein secretion by 65%. They suggested that the effects of estradiol on MCP-1 expression are indirectly mediated via enhanced cytokine sensitivity.
Our in vitro findings (Figure 2B) ▶ suggest that a subpopulation of endometriotic stromal cells is responsible for RANTES mRNA expression. This result was unanticipated based on total RNA from solubilized stromal cell cultures 16 and differs from the apparently more generalized distribution of RANTES protein. This observation is of interest given the recent appreciation of heterogeneity among mesenchymal cells in certain tissues. The emergence of a discrete subpopulation of stromal cells may occur during endometriotic differentiation as has been described for presumed stem cells within bone marrow stroma. 26 Current studies in the laboratory are directed to characterize the subset of endometriotic stromal cells responsible for robust synthesis of RANTES gene transcripts.
In summary, this study confirmed that cells within the eutopic and ectopic endometrial stroma are the sites of RANTES mRNA transcription. A combination of in situ hybridization and immunohistochemistry in tissue specimens, coupled with primary endometriotic cell cultures, was used. The findings support the hypothesis that TNF-α, produced by endometriotic implant epithelial and/or stromal cells, can induce expression of RANTES mRNA in a subset of endometriotic stromal cells. These cells, in turn, synthesize RANTES protein, which serves to recruit the accumulation of CD68-expressing macrophages into the endometriotic lesion. We postulate that the inflammatory environment created by this cascade contributes to the clinical symptoms that typify endometriosis.
Acknowledgments
We thank Victor Chao for preparing the RNA probe templates and Evelyn Garrett for her assistance with histology and in situ hybridization.
Footnotes
Address reprint requests to Robert N. Taylor, M.D., Ph.D., Center for Reproductive Sciences, University of California, 505 Parnassus Ave., San Francisco, CA 94143. E-mail: rtaylor@socrates.ucsf.edu.
Supported by Fortune-Project F1241133, the Deutsche Forschungs-gemeinschaft (to D. H.), and a grant from the National Institutes of Health/National Institute of Child Health and Human Development, through cooperative agreement U54-HD37321, as part of the Specialized Cooperative Centers Program in Reproduction Research (to D. H. and R. N. T.).
References
- 1.Haney AF, Muscato JJ, Weinberg JB: Peritoneal fluid cell populations in infertility patients. Fertil Steril 1981, 35:696-698 [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Faris HM, Schiff I, Anderson DJ: Characterization of leukocyte subpopulations in the peritoneal fluid of women with endometriosis. Fertil Steril 1988, 50:216-222 [PubMed] [Google Scholar]
- 3.Olive DL, Weinberg JB, Haney AF: Peritoneal macrophages and infertility: the association between cell number and pelvic pathology. Fertil Steril 1985, 44:772-777 [DOI] [PubMed] [Google Scholar]
- 4.Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV: Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol 1993, 169:1545-1549 [DOI] [PubMed] [Google Scholar]
- 5.Klein NA, Pergola GM, Tekmal RR, Montoya IA, Dey TD, Schenken RS: Cytokine regulation of cellular proliferation in endometriosis. Ann NY Acad Sci 1994, 734:322-332 [DOI] [PubMed] [Google Scholar]
- 6.Cirkel U, Ochs H, Mues B, Zwadlo G, Sorg C, Schneider HP: Inflammatory reaction in endometriotic tissue: an immunohistochemical study. Eur J Obstet Gynecol Reprod Biol 1993, 48:43-50 [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal RD, Samoszuk M, Taylor AP, Brown G, Alisauskas R, Goldenberg DM: Degranulating eosinophils in human endometriosis. Am J Pathol 2000, 156:1581-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL: Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod 1996, 2:40-45 [DOI] [PubMed] [Google Scholar]
- 9.Akoum A, Lemay A, McColl S, Paradis I, Maheux R: Elevated concentration and biologic activity of monocyte chemotactic protein-1 in the peritoneal fluid of patients with endometriosis. Fertil Steril 1996, 66:17-23 [PubMed] [Google Scholar]
- 10.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK: Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest 1996, 98:482-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN: Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 1996, 81:3112-3118 [DOI] [PubMed] [Google Scholar]
- 12.Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN: Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril 1995, 63:929-932 [PubMed] [Google Scholar]
- 13.Bergqvist A, Nejaty H, Froysa B, Bruse C, Carlberg M, Sjoblom P, Soder O: Production of interleukins 1beta, 6 and 8 and tumor necrosis factor alpha in separated and cultured endometrial and endometriotic stromal and epithelial cells. Gynecol Obstet Invest 2000, 50:1-6 [DOI] [PubMed] [Google Scholar]
- 14.Fasciani A, D’Ambrogio G, Bocci G, Monti M, Genazzani AR, Artini PG: High concentrations of the vascular endothelial growth factor and interleukin-8 in ovarian endometriomata. Mol Hum Reprod 2000, 6:50-54 [DOI] [PubMed] [Google Scholar]
- 15.Hornung D, Dohrn K, Sotlar K, Greb RR, Wallwiener D, Kiesel L, Taylor RN: Localization in tissues and secretion of eotaxin by cells from normal endometrium and endometriosis. J Clin Endocrinol Metab 2000, 85:2604-2608 [DOI] [PubMed] [Google Scholar]
- 16.Hornung D, Ryan IP, Chao VA, Vigne J-L, Schriock ED, Taylor RN: Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab 1997, 82:1621-1628 [DOI] [PubMed] [Google Scholar]
- 17.Klingel K, Rieger P, Mall G, Selinka H-C, Huber M, Kandolf R: Visualization of enteroviral replication in myocardial tissue by ultrastructural in situ hybridization: identification of target cells and cytopathic effects. Lab Invest 1998, 78:1227-1237 [PubMed] [Google Scholar]
- 18.Kandolf R, Ameis D, Kirschner P, Canu A, Hofschneider PH: In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci USA 1987, 84:6272-6276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi D, Zlotnik A: The biology of chemokines and their receptors. Ann Rev Immunol 2000, 18:217-242 [DOI] [PubMed] [Google Scholar]
- 20.Schall TJ, Jongstra J, Dyer BJ, Jorgensen J, Clayberger C, Davis MM, Krensky AM: A human T cell-specific molecule is a member of a new gene family. J Immunol 1988, 141:1018-1025 [PubMed] [Google Scholar]
- 21.Ortiz BD, Krensky AM, Nelson PJ: Kinetics of transcription factors regulating the RANTES chemokine gene reveal a developmental switch in nuclear events during T-lymphocyte maturation. Mol Cell Biol 1996, 16:202-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt JS, Chen H-L, Hu X-L, Tabibzadeh S: Tumor necrosis factor-α messenger ribonucleic acid and protein in human endometrium. Biol Reprod 1992, 47:141-147 [DOI] [PubMed] [Google Scholar]
- 23.Tabibzadeh S, Satyaswaroop PG, von Wolff M, Strowitzki T: Regulation of TNF-α mRNA expression in endometrial cells by TNF-α and by oestrogen withdrawal. Mol Hum Reprod 1999, 5:1141-1149 [DOI] [PubMed] [Google Scholar]
- 24.Taylor RN, Ryan IP, Moore ES, Hornung D, Shifren JL, Tseng JF: Angiogenesis and macrophage activation in endometriosis. Ann NY Acad Sci 1997, 828:194-207 [DOI] [PubMed] [Google Scholar]
- 25.Akoum A, Jolicoeur C, Boucher A: Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J Clin Endocrinol Metab 2000, 85:896-904 [DOI] [PubMed] [Google Scholar]
- 26.Sitnicka E, Wang QR, Tsai S, Wolf NS: Support versus inhibition of hematopoiesis by two characterized stromal cell types. Stem Cells 1995, 13:655-665 [DOI] [PubMed] [Google Scholar]