Abstract
Dimerization co-factor of hepatocyte nuclear factor 1 (HNF1)/pterin-4α-carbinolamine dehydratase (DCoH/PCD) is both a positive co-factor of the HNF1 homeobox transcription factors and thus involved in gene regulation as well as an enzyme catalyzing the regeneration of tetrahydrobiopterin. Dysfunction of DCoH/PCD is associated with the human disorders hyperphenylalaninemia and vitiligo. In Xenopus, overexpression of the protein during development induces ectopic pigmentation. In this study loss of function experiments using DCoH/PCD-specific antibodies demonstrated that the protein is also absolutely necessary for pigment cell formation in Xenopus. In normal human skin DCoH/PCD protein is weakly expressed in the basal layer of the epidermis that consists of keratinocytes and melanocytes. Whereas only 4 of 25 benign nevi reacted with DCoH/PCD-specific antibodies, high protein levels were detectable in melanoma cell lines and 13 of 15 primary malignant melanoma lesions. The comparison with the commonly used melanoma markers S100 and HMB45 demonstrated that DCoH/PCD has an overlapping but distinct expression pattern in melanoma lesions. In addition to human colon cancer, this is the second report about the overexpression of DCoH/PCD in human tumor cells indicating that the protein might be involved in cancerogenesis.
Dimerization co-factor of hepatocyte nuclear factor 1 (HNF1)/pterin-4α-carbinolamine dehydratase (DCoH/PCD) is one of the rare examples of a bifunctional protein combining a role in gene regulation on the transcriptional level and having an apparently unrelated enzymatic function. The protein was originally purified from liver cell nuclei because of its stable association with the transcription factor HNF1α. 1 The POU/homeobox factor HNF1α and the closely related HNF1β are involved in the regulation of many differentially expressed genes especially in liver, kidney, the intestinal tract, and pancreas via binding as homo- or heterodimers to a well-characterized DNA recognition element in the promoters of these genes. 2 It has been shown that two DCoH/PCD molecules bind to a HNF1 dimer forming a transcriptionally active heterotetrameric complex. 1,3-5 Thus, DCoH/PCD might act as a positive co-factor for HNF1 in a cell type-specific manner extending its regulatory properties in a given cellular context. In fact, DCoH/PCD and HNF1 proteins are co-expressed in liver and kidney whereas for example HNF1 but not DCoH/PCD is detectable in colon, 6,7 probably reflecting the fact that HNF1 regulates different target genes in these tissues. In this context it is noteworthy that DCoH/PCD, although absent in normal adult colon, is expressed in the developing vertebrate gut during embryogenesis and also in human colon cancer. 4,7
In the absence of HNF1 DCoH/PCD forms homotetramers that have 4-α-carbinolamine dehydratase activity. This enzyme is involved in the regeneration of tetrahydrobiopterin (BH4), the obligatory electron donator for the phenylalanine hydroxylase. 8 BH4 is also essential for tyrosine hydroxylase and tryptophan hydroxylase function catalyzing the first step of dopamine and serotonin neurotransmitter synthesis, and it stimulates the nitric oxide synthase forming citrullin and nitric oxide. 9 The importance of DCoH/PCD in the regeneration of tetrahydrobiopterin is shown by several mutations in the DCoH/PCD gene that cause hyperphenylalaninemia. 10-13 This harmful metabolite acts as a competitive inhibitor of the phenylalanine hydroxylase thus repressing the formation of tyrosine, the precursor of catecholamines and melanin. 12,13 A similar molecular mechanism is also discussed for the role of DCoH/PCD in vitiligo, a skin depigmentation disorder, as the PCD activity is nearly absent in the depigmented skin areas, 14,15 whereas enzymatic activity is detectable in normal human skin. 16 Confirming a crucial role for DCoH/PCD in pigmentation, we recently demonstrated that overexpression of DCoH/PCD in Xenopus induces the formation of ectopic pigmentation and an increase of tyrosinase activity, which is the rate-limiting enzyme in melanin synthesis. 17 Because a DCoH/PCD mutant with impaired dehydratase function retains the ability to induce pigmentation, we concluded that DCoH/PCD is not only an essential enzyme for melanin biosynthesis, but might also regulate the differentiation of pigment-producing cells. 17 In fact, a function for DCoH/PCD independent of the phenylalanine hydroxylase system and of HNF1 is proposed by several authors as the protein is expressed in cells devoid of BH4 and HNF1 including neural crest derived cell types, 18 rat brain, 19 the vertebrate egg, and early embryos. 4,6 This function might include the interaction of DCoH/PCD with yet unknown partners as the crystal structure of DCoH/PCD constitutes a tetramer containing two saddle-shaped grooves similar to TBP (TATA box binding protein) that bears the potential to bind other macromolecules. 20-22
To investigate the role of DCoH/PCD in pigment cell formation we analyzed the effect of DCoH/PCD protein inhibition during Xenopus development. As DCoH/PCD is structurally and functionally conserved among the vertebrates, 1,4,8 we assume that the results are also relevant for mammals. To directly address a possible role for DCoH/PCD in human melanocytes, we determined its expression pattern in human skin, benign and dysplastic nevi, primary melanoma lesions, and melanoma cell lines.
Materials and Methods
Production and Purification of the DCoH/PCD-Specific Antibodies
Rabbit polyclonal antibodies were obtained after standard immunization by (Eurogentec, Herstal, Belgium) using recombinant histidine-tagged Xenopus DCoH/PCD. 4 The recombinant protein was isolated from Escherichia coli following the manufacturer’s instructions (Qiagen, Hilden, Germany). The polyclonal antibodies were purified for microinjection into Xenopus eggs using the his-tagged fusion protein covalently coupled to MoBiTec-DVS agarose (2 mg/ml). Antibodies were eluted with 100 mmol/L of glycine, pH 2.5, and neutralized with 0.1 volume of 1 mol/L of Tris buffer, pH 8, after extensive washing with phosphate-buffered saline.
Microinjection into Fertilized Xenopus Eggs
In vitro fertilization and culture of Xenopus eggs and embryos was performed as described by Peng. 23 A volume of 25 to 50 nl of purified DCoH/PCD-specific antibodies (100 μg/ml in 15 mmol/L Tris, 88 mmol/L NaCl, 1 mmol/L KCl, pH 7.4) was injected into fertilized eggs that were allowed to develop until stage 42 (3 days at room temperature). For control experiments affinity-purified goat α-rabbit polyclonal antibodies (Roche, Mannheim, Germany) were used. Successful injection was monitored using co-injection of green fluorescence protein mRNA as described elsewhere. 24 The Xenopus DCoH/Rc/CMV expression vector 4 was cut with NaeI to perform in vitro transcription of capped mRNA using T7 polymerase. 17 Approximately 100-pg GFP and 250-pg DCoH/PCD synthetic mRNA were used for each microinjection into the two-cell stage.
Cell Culture and Transfection
NIH3T3 fibroblasts and BLM34 melanoma cells (kindly provided by Hans-Christoph Kirch, Dept. of Molecular Biology, University of Essen) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, and 100 U/ml penicillin and streptomycin each. NIH3T3 cells were transfected with the Xenopus DCoH/PCD expression vector as described previously 4 using lipofectamine (Gibco, Karlsruhe, Germany).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was kindly provided by Stephan Wagner, Dept. of Dermatology, University of Essen. The RT-reaction was performed as described 25 using 2 μg of RNA. For the amplification of specific transcripts in the presence of α-32P-CTP the following primers, annealing temperatures, and cycle numbers were used. DCoH/PCD (human and mouse): upstream: 5′-CGGAAT TCATATGGCTGGCAAAGCACACAG-3′; downstream: 5′-CGGGATCCTATGTCATGG ACACTGCTAC-3′, 55°C, 28 cycles. HNF1α: upstream: 5′-GTGTCTACAACTGGTTTG CC-3′; downstream: 5′-TGTAGACACTGTCACTAAGG-3′, 52°C, 40 cycles. GAPDH: upstream: 5′-ACCACAGTCCATGCCATCAC-3′; downstream: 5′-TCCACCACCCTGTTG CTGTA-3′, 62°C, 28 cycles. Twenty μl of the 50 μl reactions were separated on 6% polyacrylamide gels and products were visualized by autoradiography.
Western Blotting
Twenty μg of protein of whole BLM34 cell extract and Xenopus liver were separated on 15% sodium dodecyl sulfate gel and transferred to nitrocellulose. After blocking with 0.5% blocking reagent (Roche) the blots were incubated overnight with DCoH/PCD-specific rabbit antibodies diluted 1:20,000. Peroxidase-conjugated α-rabbit antibodies were used to visualize DCoH/PCD using the ECL system (Amersham, Freiburg, Germany). For control experiments the antibodies were incubated with the recombinant DCoH/PCD-histidine fusion protein (10 μg/ml).
Immunofluorescence and Immunohistochemistry
Cells were cultured on glass slides and fixed using methanol at −20°C for 20 minutes before staining with rabbit polyclonal DCoH/PCD antibodies (1:5000 diluted) followed by a Cy3-coupled α-rabbit secondary antibody (Roche) and counterstained with Hoechst 33342 (Sigma, Taufkirchen, Germany).
Tissues were fixed overnight in neutral-buffered formalin and embedded in paraffin. For immunohistochemistry, dewaxed paraffin sections were placed in a microwave at 350 W, containing 0.1 mol/L buffered sodium citrate (pH 6), boiled for 3 × 5 minutes, and chilled to room temperature. The sections were incubated with rabbit polyclonal DCoH/PCD antibodies (paraffin, 1:400 dilution) or with mouse monoclonal antibodies against S100 and HMB45 as recommended (DAKO, Hamburg, Germany). Visualization of primary antibodies was performed using the alkaline-phosphatase anti-alkaline phosphatase technique and New Fuchsin (DAKO) as chromogen. 26 The sections were counterstained with hematoxylin.
Results
To address the significance of DCoH/PCD in pigment cell formation we injected epitope-purified polyclonal antibodies into fertilized Xenopus eggs to knock out the maternal and zygotic protein. The injected antibodies were stable for 3 days in the developing embryos as they were detectable in swimming larvae using a secondary labeled antibody (data not shown). The survival rate of injected eggs was low (5 to 10%) and most of the developing larvae that reached gastrula and neurula stages showed multiple malformations frequently affecting the axis formation and head development. However, we found very similar results using control antibodies raised against rabbit IgG demonstrating that these phenotypes reflect nonspecific effects because of the introduction of immunoglobulins.
Nevertheless, 20 α-DCoH-injected embryos survived and developed properly with respect to gastrulation, axis formation, and size (Figure 1) ▶ ; all of these larvae had a characteristic phenotype that was not observed using control antibodies. Figure 1a ▶ shows an untreated 3-day-old swimming larva (stage 42) with pigmented dermal melanocytes distributed all over the head and tail skin and pigmentation of the eye (Figure 1c) ▶ . In contrast, the α-DCoH-injected embryos (Figure 1b) ▶ failed to develop any pigmented melanocytes in the epidermis and the pigmentation of the eye is somewhat weaker compared to the control (Figure 1, d and c) ▶ . Figure 1e ▶ shows the phenotype of a functional DCoH/PCD protein translated from in vitro-synthesized DCoH/PCD mRNA that was injected into one blastomere of the Xenopus two-cell stage resulting in the expression of DCoH/PCD on the left side of the body. The formation of ectopic pigment cells in the skin of developing embryos was restricted to the DCoH/PCD overexpressing area (Figure 1e) ▶ . Taken together, DCoH/PCD is essential for melanin synthesis and is capable to induce ectopic differentiation of melanocytes during development.
Figure 1.
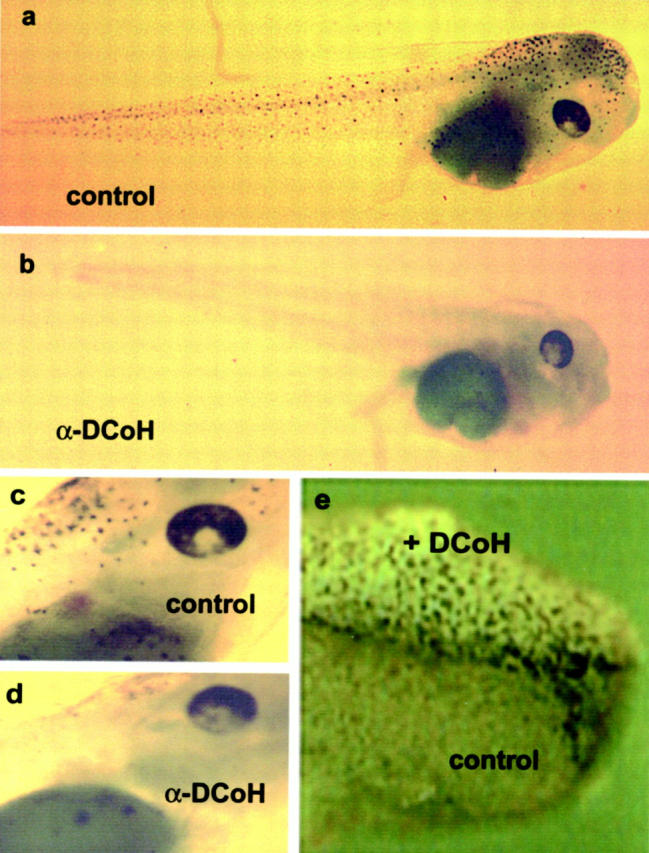
Inhibition of DCoH/PCD during Xenopus embryogenesis suppresses pigmentation. a: Three-day old embryo (swimming larva, stage 42) with normal skin pigmentation. b: Larva of the corresponding stage derived from a fertilized egg that received DCoH/PCD-specific antibodies failed to develop dermal pigment cells. c and d: Eye regions of both larvae demonstrating that the ocular pigmentation is also disturbed (original magnification, ×4). Note some diffuse pigmentation below the eye in both larvae. e: Anterior region of a stage 25 embryo (2-day-old embryo) derived from a two-cell stage that was injected with DCoH/PCD mRNA into one blastomere (original magnification, ×8). Ectopic pigment cells were evenly distributed on the left side of the body, which expressed the introduced DCoH/PCD mRNA, whereas no dermal pigmentation is normally seen at this stage of development (right side).
To investigate, whether the crucial role of DCoH/PCD in melanocyte development found in Xenopus is conserved in mammals, we analyzed the expression of DCoH/PCD in the melanoma cell line BLM34. 42 The specificity of DCoH/PCD antibodies was proven because DCoH/PCD was readily detectable in NIH3T3 fibroblasts after transfection with a DCoH/PCD expression vector (Figure 2a ▶ ; left, red staining), whereas endogenous DCoH/PCD was hardly detectable in these cells using immunofluorescence (data not shown). The DCoH/PCD staining revealed that transfected DCoH/PCD is expressed in the nucleus and the cytoplasm to a similar extent (Figure 2a ▶ , left). Staining of BLM34 melanoma cells that were not transfected, gave intense staining indicating that DCoH/PCD is expressed in this cell line (Figure 2b) ▶ . The protein is sequestered predominantly in the nucleus, but is also detectable in the perinuclear zone and the cytoplasm. The distribution of DCoH/PCD probably reflects its bifunctional character acting as a regulator in the nucleus as well as an enzyme in the cytoplasm. 23-25 Immunoblotting using DCoH/PCD-specific polyclonal antibodies (Figure 2c) ▶ revealed a single protein of ∼12 kd in whole-cell extracts of BLM34 cells, which corresponds to the expected size of DCoH/PCD. As previously described, the human protein (lane 1) migrates somewhat slower than the Xenopus homologue detected in liver extract (lane 2), although both proteins have the same molecular weight and consist of 104 amino acids with 85% identity. 6 The staining is specific for DCoH/PCD as preincubation of the antibodies with recombinant DCoH/PCD abolished staining (lanes 3 and 4).
Figure 2.
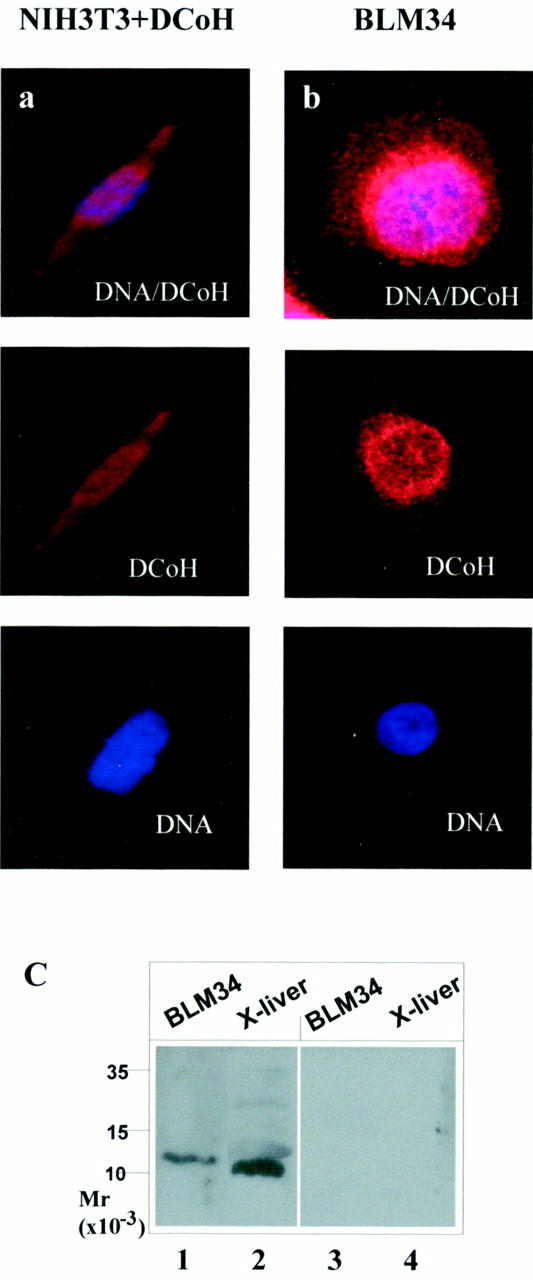
DCoH/PCD-protein is expressed in the melanoma cell line BLM34. a: Detection of DCoH/PCD in a NIH3T3 cell after transfection with a DCoH/PCD expression vector and in a BLM3445 cell (b) using rabbit polyclonal antibodies raised against DCoH/PCD and a secondary Cy3-labeled antibody. Top: Co-staining of DCoH/PCD and DNA (Hoechst 33342). Middle and bottom: The separate signals for DCoH/PCD and DNA, respectively. DCoH/PCD staining was observed in the nucleus and the cytoplasma. c: Western blot using DCoH/PCD-specific antibodies as the primary antibody before (lanes 1 and 2) and after (lanes 3 and 4) preincubation with recombinant DCoH/PCD protein to detect DCoH/PCD in whole-cell extracts of BLM34 cells and Xenopus liver.
To analyze the expression of DCoH/PCD in normal melanocytes and melanoma cells we used RT-PCR to detect its mRNA in normal skin, tumor samples (mel1 and mel2), and the melanoma cell lines SKmel and HT144. In all of these specimens the DCoH/PCD mRNA was present (Figure 3A ▶ , top) under conditions that failed to detect the transcripts in NIH3T3 fibroblasts. Transcripts of HNF1α, the homeobox transcription factor interacting with DCoH/PCD in liver and kidney, were not detectable in these samples, whereas the HNF1α RNA was clearly amplified using RNA of the hepatoma cell line HepG2 (Figure 3A ▶ , middle). The absence of HNF1α in skin and melanoma samples excludes the possible contribution of this transcription factor to the DCoH/PCD-mediated pigmentation in normal or malignant melanocytes. Because DCoH/PCD mRNA was detectable in normal human skin, we determined the expression pattern of DCoH/PCD using immunohistochemistry. Figure 3B ▶ shows faint staining of the epidermal basal layer within keratinocytes and few melanocytes (arrows). The protein was sometimes sequestered in the cytoplasm of keratinocytes, whereas in a few cells it was also detectable in the nucleus (asterisks). There are also individual cells without staining, demonstrating that the intensity of the staining and thus the expression level of DCoH/PCD varies among the cells of the basal layer.
Figure 3.
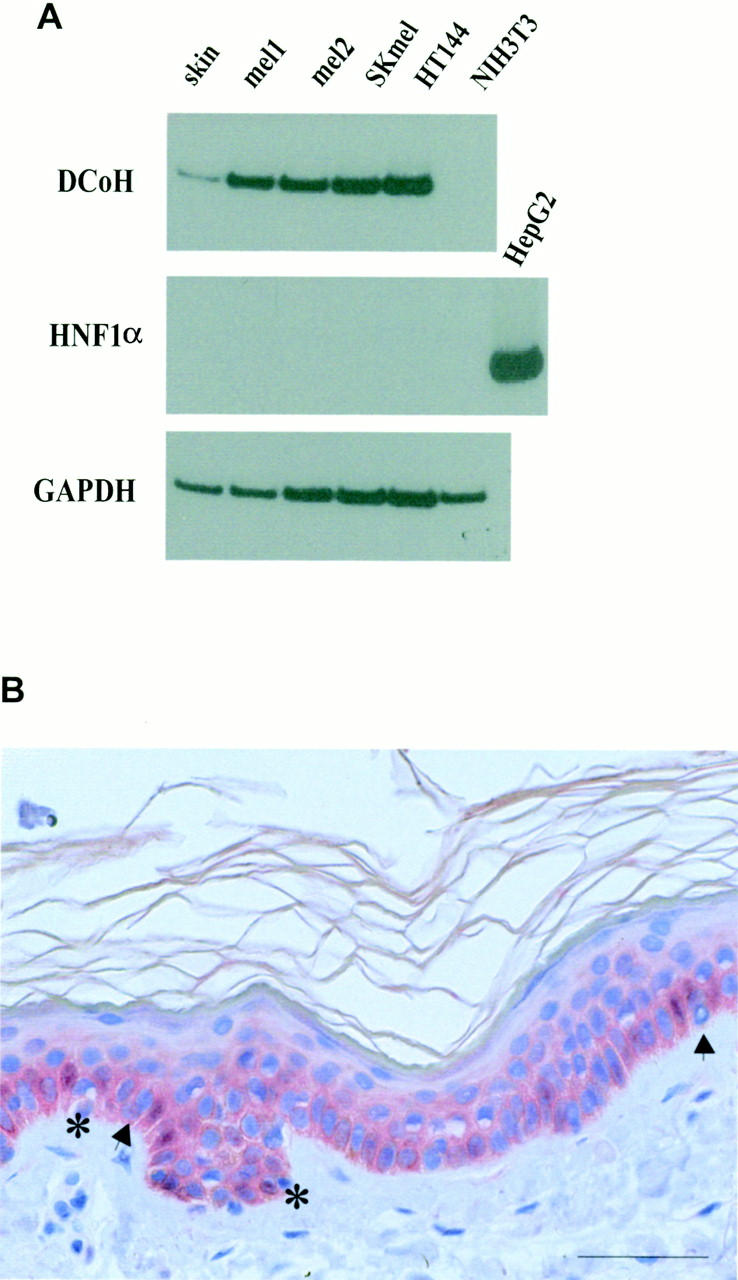
DCoH/PCD is detectable in primary human malignant melanoma lesions, melanoma cell lines, and normal human skin. A: RNA of different sources (lane 1, normal skin; lanes 2 and 3, two primary nodular melanoma lesions; lane 4, SK melanoma cell line; lane 5, HT144 melanoma cell line; and lane 6, NIH3T3 fibroblasts) was isolated and analyzed by RT-PCR for the presence of transcripts for DCoH/PCD (top), HNF1α (middle), and the housekeeping gene GAPDH (bottom). Control reactions performed without reverse transcriptase revealed no amplification (data not shown). B: Normal human skin immunostained with DCoH/PCD antibodies revealed faint staining of basal keratinocytes and melanocytes (arrows) (original magnification, ×200). Note the staining of the cytoplasm and the nucleus (asterisks) of individual cells. The immunoreactivity disappeared after preincubation of the antibodies with recombinant DCoH/PCD-protein (data not shown). Scale bar, 50 μm.
Given the importance of DCoH/PCD for pigmentation during embryogenesis and the weak expression in normal skin, we studied its expression in several types of pigmented lesions (Table 1 ▶ and Figure 4 ▶ ). Interestingly, DCoH/PCD staining was detectable in only 4 of 25 (16%) benign nevi and 5 of 11 (45%) dysplastic nevi (Table 1) ▶ . In contrast, DCoH/PCD was positive in 13 of 15 (87%) primary melanoma lesions (Figure 4 ▶ ; g, h, and i, and Table 1 ▶ ). Staining can be present in nearly 100% of the tumor cells but is absent in the surrounding normal healthy dermis (Figure 4h) ▶ . Higher power microscopy revealed that the DCoH/PCD staining in the tumor cells is not uniform with regard to its intensity and its subcellular distribution (Figure 4i) ▶ . There are single cells and patches within the tumor that are differentially stained although they are histologically indistinguishable from the neighboring cells (Figure 4h) ▶ . Furthermore, in most of the cells DCoH/PCD staining is in the cytoplasm as well as in the nucleus, but there are single cells predominantly stained in the cytoplasm (Figure 4h) ▶ and vice versa (Figure 4i) ▶ . The strong DCoH/PCD staining in melanoma cells is in striking contrast to the weak expression in normal epidermis, benign nevi (P < 0.003, compared to HMB45 and S100, chi-square test) and dysplastic nevi (Table 1) ▶ . Therefore, DCoH/PCD showed a higher specificity for malignant melanoma cells than the commonly used antibodies against S100 and HMB45.
Table 1.
Staining Characteristics in 51 Melanocytic Lesions
| DCoH/PCD+ (% positive) | HMB45+ (% positive) | S100+ (% positive) | |
|---|---|---|---|
| Benign nevi | 4/25* | 10/19 | 12/15 |
| (16%) | (53%) | (80%) | |
| Dysplastic nevi | 5/11 | 8/8 | 7/9 |
| (45%) | (100%) | (78%) | |
| Primary melanoma lesion | 13/15 | 10/13 | 12/14 |
| (87%) | (77%) | (86%) |
*P < 0.003, compared to HMB45 and S100, chi-square test.
Figure 4.
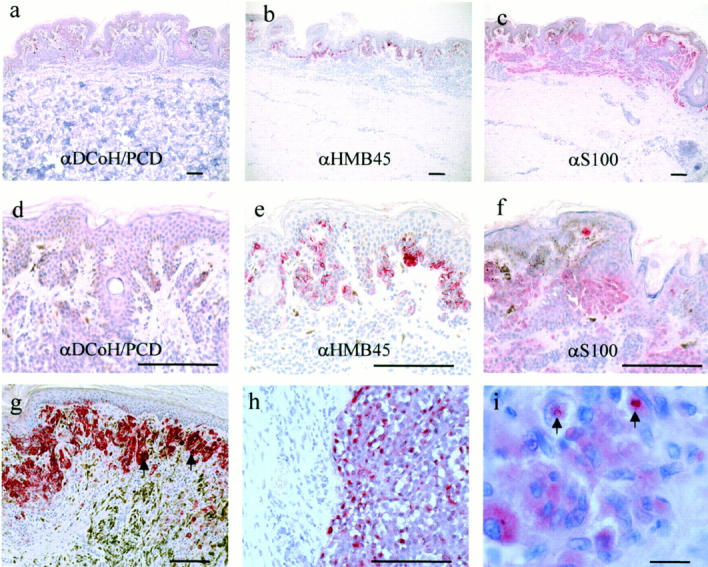
DCoH/PCD is frequently overexpressed in primary melanoma lesions, but not in benign nevi. a: Benign nevi showed absent to weak DCoH/PCD staining in clusters of nevus cells (original magnification, ×50). For comparison, the staining of serial sections with anti-HMB45 (b and e) and anti-S100 (c and f) is also shown. d: Higher power view revealed the negativity of nevus cells for DCoH/PCD, whereas anti-HMB45 (e) and anti-S100 (f) stained nevus cells (original magnification, × 200). g: Primary superficially spreading melanoma lesions staining positive with DCoH/PCD polyclonal antibodies (original magnification, ×100). Note also some DCoH/PCD-positive melanophages (arrows). h: Higher power view of a different area of a primary nodular melanoma lesion demonstrates the characteristics of the DCoH/PCD staining with individual cells staining primarily in the cytoplasm (original magnification, ×200, respectively). Scale bars, 50 μm (a–h). i: Several melanoma cells show nuclear DCoH/PCD staining (arrows) (original magnification, ×1000). Scale bar, 50 μm (i).
To evaluate whether the expression of DCoH/PCD in melanoma cells represents a potential new melanoma marker, we stained serial sections of primary human melanoma lesions with the DCoH/PCD-specific polyclonal antibodies and commonly used antibodies detecting the melanoma markers S100 and HMB45 (Figure 5, a–i) ▶ . Anti-S100 detects a group of calcium-binding proteins expressed in normal melanocytes of the skin as well as in malignant melanoma cells. 27 In contrast the HMB45 antigen is associated with melanosomes of stages 1 and 2, thus predominantly staining early melanosomes with active melanin synthesis. 28 Two selected primary malignant melanoma lesions (Figure 5, a–c ▶ , represents the superficially-spreading type; and Figure 5, d–i ▶ , the nodular growth pattern, respectively) show different levels and patterns of DCoH/PCD-staining (Figure 5 ▶ ; a, d, and g) compared to a serial section stained with anti-HMB45 (Figure 5 ▶ ; b, e, and h) and anti-S100 (Figure 5 ▶ ; c, f, and i). In Figure 5f ▶ , S100 is being expressed in ∼90% of the melanoma cells. Among the positive cells the staining is not uniform with lower expression in the center. The anti-HMB45-staining is restricted to one-third of the tumor with a complete lack of staining in clusters of tumor cells (Figure 5, e and h) ▶ . In contrast to S100 and HMB45, the DCoH/PCD-staining is more evenly distributed throughout the tumor, staining nearly 100% of the melanoma cells including cells that are negative for S100 and HMB45 (Figure 5, d and g) ▶ .
Figure 5.
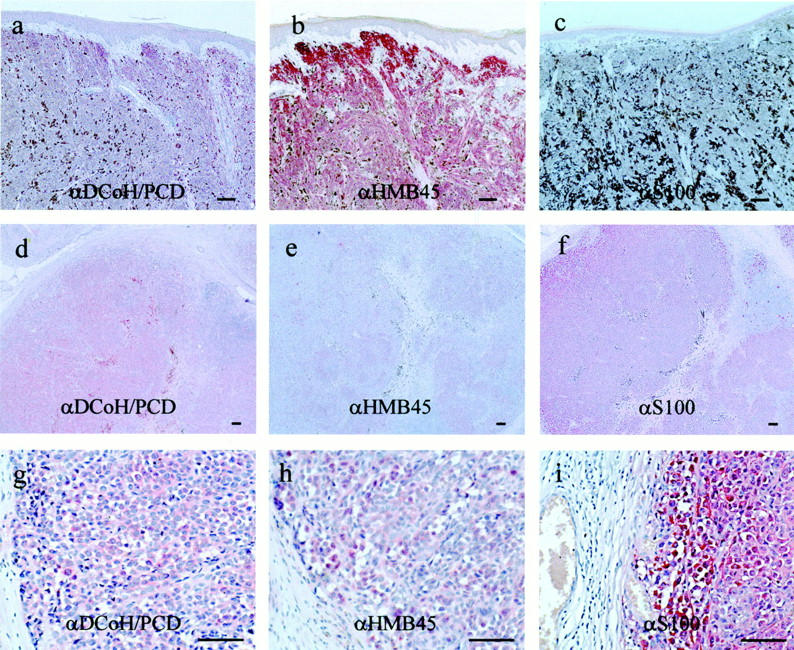
DCoH/PCD expression in melanoma lesions is distinct from the melanoma markers S100 and HMB45. Serial sections of a primary superficially spreading melanoma lesion (a–c) and a primary nodular melanoma lesion (d–i) were either stained with the polyclonal antibodies specific for DCoH/PCD (a, d, and g), or commercially available monoclonal antibodies against HMB45 (b, e, and h), and S100 (c, f, and i). Original magnifications: ×100 (a–c); ×50 (d–f); and ×200 (g–i). Note the distinct staining pattern including cells that are negative for S100 and HMB45. Scale bars, 50 μm.
Taken together, DCoH/PCD represents a melanoma-associated antigen that is significantly higher expressed in melanoma cells than their normal counterparts and has an expression pattern distinct from that of the markers anti-S100 and anti-HMB45.
Discussion
Based on our recent data that overexpression of DCoH/PCD results in ectopic pigmentation in Xenopus, 17 we asked whether this protein is also necessary for the normal pigmentation. To address this question we inhibited the endogenous factor by injection of DCoH/PCD-specific antibodies into fertilized Xenopus eggs and monitored the effects on pigmentation in the developing larvae. This loss of function approach has been successfully used in several studies investigating the function of maternal proteins, 29,30 because the activity of these factors cannot be inhibited by RNA-antisense approaches. DCoH/PCD is a maternal factor stored in the egg, detectable in early blastula stages, and also expressed zygotically. 4 We assume that the maternal as well as the zygotic protein are blocked by the injected antibodies in the embryo, because the antibodies are detectable for up to 3 days in swimming larvae (data not shown). The characteristic phenotype of the manipulated embryos is the almost complete lack of pigment cells in the epidermis. The epidermal melanocytes originate from neural crest cells, a multipotent progenitor line that gives rise to several cell types (eg, cartilage, glia, and neurons), 31 which raises the question of the role of DCoH/PCD for neural crest-cell differentiation and proliferation during development. We addressed this question by detecting the neural crest marker slug 32 in DCoH/PCD-overexpressing and thus hyperpigmented embryos and found the amount and distribution of slug-expressing cells unchanged (E Pogge v. Strandmann, unpublished data). Therefore, DCoH/PCD is most likely not involved in early differentiation and proliferation of the neural crest cells, but is rather critical for pigment-cell lineage restriction within the neural crest-cell population.
The hypo- and hyperpigmentation in Xenopus because of DCoH/PCD loss and gain of function might depend on its enzymatic activity and/or on a putative regulatory function in pigment-producing cells. In humans it has been reported that the lack of the DCoH/PCD dehydratase activity in the skin of vitiligo patients and the observed accumulation of harmful 7-biopterin might cause depigmentation. 14,15 However, the fact that patients with hyperphenylalaninemia in combination with elevated 7-biopterin levels because of mutations in the DCoH/PCD gene do not develop vitiligo is not consistent with such a model. 16 Therefore, a contribution of the regulatory activity of DCoH/PCD to the development of vitiligo is an attractive alternative explanation. In this context it is noteworthy that the phenylalanine hydroxylase gene is a downstream target of HNF1/DCoH as DCoH/PCD potentiates the HNF1-dependent transcription of the PAH gene in transfections. 32 Furthermore we have shown that a DCoH/PCD mutant without the dehydratase function retains the potential to induce ectopic pigmentation in Xenopus. 17 Regardless of the potential mechanism, our finding that DCoH/PCD inhibition blocks pigment cell formation in Xenopus favors the model that the dysfunction of DCoH/PCD is not simply a consequence but rather the reason for certain skin depigmentation diseases in vertebrates.
If DCoH/PCD plays a role in the human melanocytes it should likely be also expressed in melanoma cell lines. We proved the distribution of DCoH/PCD in BLM34 cells in the nucleus as well as in the cytoplasm that is consistent with a dual function in nuclear gene regulation and dehydratase activity in the cytoplasm. A similar distribution has been observed in several other cell types, whereas a predominant nuclear localization is detectable in early embryogenesis and in cells derived from the neural crest of the rat. 6,18,33,34 In human skin weak protein levels are found in the basal and suprabasal layers of the epidermis. This cell layer contains keratinocytes and melanocytes forming a functional unit responsible for pigment synthesis and melanin distribution in the skin. 35 The expression pattern is consistent with the detection of DCoH/PCD in cultures of human epidermal keratinocytes. 16 Although DCoH/PCD expression in cultured dermal fibroblasts was reported, 34 the fibroblasts of the dermis are free of DCoH/PCD staining. In conclusion, the expression pattern in skin supports a potential role of DCoH/PCD in the regulation of pigmentation in humans.
Because the transcription factor HNF1 is not expressed in human skin, it is tempting to speculate about other transcription factors as partners of DCoH/PCD in melanocytes. One such candidate is mitf (microphthalmia-associated transcription factor) a basic helix-loop-helix zipper factor that is critically involved in melanocyte differentiation. 36,37 In fact, mutations in this gene cause a lack of skin pigmentation and defects in eye development and are associated with the human disorder Waardenburg syndrome II. 38 Moreover, misexpression of mitf in mouse fibroblasts converts their fate to cells with melanocyte characteristics. 38 More strikingly, in zebrafish loss of function with the mitf homologue nacre leads to the lack of melanocytes, whereas gain of function induces the formation of ectopic pigment cells, 39 a phenotype reminiscent to the DCoH/PCD phenotype in Xenopus. Like DCoH/PCD the overexpression of mitf in most, if not all, melanocytic lesions has recently been reported. 36
Consistent with the strong expression of DCoH/PCD in the melanoma cell line BLM34, DCoH/PCD is overexpressed in primary malignant melanoma lesions, but not routinely in benign pigmented nevi. The DCoH/PCD staining is intense and limited to melanoma cells, but it is not uniformly distributed within the tumor. This reflects the fact that melanoma cells, although clonally expanded, constitute a heterogeneous cell population with respect to protein expression, which is even more evident using anti-S100 or anti-HMB45 antibodies. Further experiments will elucidate whether tumor progression and malignancy correlate with the cellular expression level of DCoH/PCD in dysplastic nevi and melanoma lesions.
The overexpression of DCoH/PCD might be a prerequisite or even a reason for the growth advantage of the malignant cell or represent a secondary effect resulting from another selective growth advantage. An enhanced carbinolamine dehydratase activity might confer a growth advantage for certain cells as a mitogenic effect of tetrahydrobiopterin on PC12 rat pheochromocytoma, thymocytes, and on C6 glioma cells has been reported. 40,41 DCoH/PCD overexpression has also been reported in human colon carcinoma in contrast to the lack of expression in normal colon 7 raising the question whether DCoH/PCD might be generally associated with dedifferentiation and/or proliferation in certain cell types.
With regard to DCoH/PCD acting as an enzyme contributing to the supply of the melanin precursor tyrosine, its overexpression may reflect an increased melanin synthesis that is frequently observed in malignant melanoma lesions. However, this seems unlikely because the strong expression of DCoH/PCD in melanoma cells may occur independently of the melanin synthesis, and because the overexpression is also found in BLM34 cells that are amelanotic. Furthermore, we show that DCoH/PCD expression is high in amelanotic areas of a given melanoma lesion that are not stained with anti-HMB45 antibodies, and low in benign pigmented nevi. The comparison of DCoH/PCD staining with anti-S100 and anti-HMB45 reveals that DCoH/PCD expression is distinct from these current melanoma markers, thus potentially representing a new diagnostic tool.
Acknowledgments
We thank Kerstin Heise (University of Essen) for support in immunohistochemistry techniques; Fabian Esser (University of Essen) for laser scan analysis; and Stephan Wagner and Hans-Christoph Kirch for kindly donating RNAs. We are thankful for the sponsorship of Fumedica GmbH, Germany (http://www.fumedica.de).
Footnotes
Address reprint requests to Dr. Elke Pogge v. Strandmann, Institute of Cell Biology (Cancer Research), Universitätsklinikum Essen, Hufelandstrasse 55, D-45122 Essen Germany. E-mail: pogge.v.strandmann@uni-essen.de.
Supported by Deutsche Forschungsgemeinschaft STR530/1-2, Lise-Meitner fellowship (to E. P. v. S).
References
- 1.Mendel DB, Khavari PA, Conley PB, Graves MK, Hansen LP, Admon A, Crabtree GR: Characterisation of a cofactor that regulates dimerization of a mammalian homeodomain protein. Science 1991, 254:1762-1767 [DOI] [PubMed] [Google Scholar]
- 2.Cereghini S: Liver-enriched transcription factors and hepatocyte differentiation. FASEB J 1996, 10:267-282 [PubMed] [Google Scholar]
- 3.Rhee KH, Stier G, Becker PB, Suck D, Sandaltzopoulos R: The bifunctional protein DCoH modulates interactions of the homeodomain transcription factor HNF1 with nucleic acids. J Mol Biol 1997, 265:20-29 [DOI] [PubMed] [Google Scholar]
- 4.Pogge v. Strandmann E, Ryffel GU: Developmental expression of the maternal protein XDCoH, the dimerization cofactor of the homeoprotein LFB1 (HNF1). Development 1995, 121:1216–1217 [DOI] [PubMed]
- 5.Johnen G, Kowlessur D, Citron BA, Kaufman S: Studies on the enzymatic and transcriptional activity of the dimerization cofactor for hepatocyte nuclear factor1. Proc Natl Acad Sci USA 1995, 94:13469-13474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogge v. Strandmann E, Senkel S, Ryffel GU: The bifunctional protein DCoH/PCD, a transcription factor with a cytoplasmic enzymatic activity, is a maternal factor in the rat egg and expressed tissue specifically during embryogenesis. Int J Dev Biol 1998, 42:53–59 [PubMed]
- 7.Eskanazi R, Thöny B, Svoboda M, Robberecht P, Dassesse D, Heizmann CW, Van Laethem JL, Resibois A: Overexpression of pterin-4α-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 in human colon cancer. Am J Pathol 1999, 155:1105-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citron BA, Davis MD, Milstien S, Gutierrez J, Mendel DB, Crabtree GR, Kaufman S: Identity of 4α-carbinolamin dehydratase, a component of the phenylalanine hydroxylation system, and DCoH, a transregulator of homeoproteins. Proc Natl Acad Sci USA 1992, 89:11891-11894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thöny B, Auerbach G, Blau N: Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 2000, 347:1-16 [PMC free article] [PubMed] [Google Scholar]
- 10.Adler C, Ghisla S, Rebrin I, Haavik J, Heizmann CW, Blau N, Kuster T, Curtius HC: 7-substituted pterins in humans with suspected pterin-4α-carbinolamine dehydratase deficiency. Eur J Biochem 1992, 208:139-144 [DOI] [PubMed] [Google Scholar]
- 11.Citron BA, Kaufman S, Milstien S, Naylor EW, Greene CL, Davis MD: Mutations in the 4α-carbinolamine dehydratase gene leads to mild hyperphenylalaninemia with defective cofactor metabolism. Ann J Hum Genet 1993, 53:768-774 [PMC free article] [PubMed] [Google Scholar]
- 12.Thöny B, Neuheiser F, Kierat L, Blaskovics M, Arn PH, Ferreira P, Rebrin I, Ayling J, Blau N: Hyperphenylalaninemia with high levels of 7-biopterin is associated with mutations in the PCBD gene encoding the bifunctional protein pterin-4α-carbinolamine dehydratase and transcriptional coactivator (DCoH). Am J Hum Genet 1998, 62:1302-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thöny B, Neuheiser F, Kierat L, Rolland MO, Guibaud P, Schluter T, Germann R, Heidenreich RA, Duran M, de Klerk JB, Ayling JE, Blau N: Mutations in the pterin-4α-carbinolamine dehydratase (PCBD) gene leads to a benign form of hyperphenylalaninemia. Hum Genet 1998, 103:162-167 [DOI] [PubMed] [Google Scholar]
- 14.Schallreuter KU, Wood JM, Pittelkow MR, Gutlich M, Lemke KR, Rodl W, Swanson NN, Hitzemann K, Ziegler I: Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 1994, 263:1444-1446 [DOI] [PubMed] [Google Scholar]
- 15.Schallreuter KU, Wood JM, Ziegler I, Lemke KR, Pittelkow MR, Lindsey NJ, Gutlich M: Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. Biochem Biophys Res Commun 1994, 1226:181-192 [DOI] [PubMed] [Google Scholar]
- 16.Lei XD, Woodworth CD, Johnen G, Kaufman S: Expression of 4α-carbinolamine dehydratase in human epidermal keratinocytes. Biochem Biophys Res Commun 1997, 238:556-559 [DOI] [PubMed] [Google Scholar]
- 17.Pogge v. Strandmann E, Senkel S, Ryffel GU: Ectopic pigmentation in Xenopus in response to DCoH/PCD, the cofactor of HNF1 transcription factors/pterin-4α-carbinolamine dehydratase. Mech Dev 2000, 91:53–60 [DOI] [PubMed]
- 18.Resibois A, Cuvelier L, Svoboda M, Heizman CW, Thöny B: Immunohistochemical localisation of pterin-4α-carbinolamine dehydratase in rat peripheral organs. Histochem Cell Biol 1999, 111:381-390 [DOI] [PubMed] [Google Scholar]
- 19.Depaepe V, Cuvelier L, Thöny B, Resibois A: Pterin-4α-carbinolamine dehydratase in rat brain. I. Patterns of colocalisation with tyrosine hydroxylase. Brain Res Mol Brain Res 2000, 75:76-88 [DOI] [PubMed] [Google Scholar]
- 20.Endrizzi JA, Cronk JD, Wang W, Crabtree GR, Alber T: Crystal structure of DCoH, a bifunctional protein-binding transcriptional coactivator. Science 1995, 268:556-559 [DOI] [PubMed] [Google Scholar]
- 21.Ficner R, Sauer UH, Stier G, Suck D: Three-dimensional structure of the bifunctional protein PCD/DCoH, a cytoplasmic enzyme interacting with transcription factor HNF1. EMBO J 1995, 14:2034-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ficner R, Sauer U, Ceska T, Stier G, Suck D: Crystallization and preliminary crystallographic studies of recombinant dimerization cofactor of transcription factor HNF1/pterin-4α-carbinolamine dehydratase from liver. FEBS Lett 1995, 357:62-64 [DOI] [PubMed] [Google Scholar]
- 23.Peng HB: Appendix A: solutions and protocols. Methods Cell Biol 1991, 36:657-662 [PubMed] [Google Scholar]
- 24.Nastos A, Pogge v. Strandmann E, Weber H, Ryffel GU: The embryonic expression of the tissue-specific transcription factor HNF1α in Xenopus: rapid activation by HNF4 and delayed induction by mesoderm inducers. Nucleic Acids Res 1998, 26:5602–5608 [DOI] [PMC free article] [PubMed]
- 25.Holewa B, Zapp D, Drewes T, Senkel S, Ryffel GU: HNF4β, a new gene of the HNF4 family with distinct activation and expression profiles in oogenesis and embryogenesis of Xenopus laevis. Mol Cell Biol 1997, 17:687-694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY: Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP-complexes). J Histochem Cytochem 1984, 32:219-229 [DOI] [PubMed] [Google Scholar]
- 27.McNutt NS: The S100 family of multipurpose calcium-binding proteins. J Cutan Pathol 1998, 25:521-529 [DOI] [PubMed] [Google Scholar]
- 28.Kapur RP, Bigler SA, Skelly M, Gown AM: Anti-melanoma monoclonal antibody HMB45 identifies an oncofetal glycoconjugate associated with immature melanosomes. Histochem Cytochem 1992, 40:202-212 [DOI] [PubMed] [Google Scholar]
- 29.Stebbins-Boaz B, Hake LE, Richter JD: CPEB controls the cytoplasmatic polyadenylation of cyclin, CDK2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J 1996, 15:2582-2592 [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Whitman M: FAST-1 is a key maternal effector of mesoderm inducers in the early Xenopus embryo. Development 1999, 126:5621-5634 [DOI] [PubMed] [Google Scholar]
- 31.Mayer R, Morgan R, Sargent MG: Induction of the prospective neural crest of Xenopus. Development 1995, 121:767-777 [DOI] [PubMed] [Google Scholar]
- 32.Lei XD, Kaufman S: Identification of hepatic nuclear factor 1 binding sites in the 5′flanking region of the human phenylalanine hydroxylase gene: implication of a dual function of phenylalanine hydroxylase stimulator in the phenylalanine hydroxylation system. Proc Natl Acad Sci USA 1998, 95:1500-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sourdive D, Transy C, Garbay S, Yaniv M: The bifunctional DCoH protein binds to HNF1 independently of its 4-α-carbinolamine dehydratase activity. Nucleic Acids Res 1997, 25:1476-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei XD, Kaufman S: Characterization of expression of the gene for human pterin carbinolamine dehydratase/dimerization cofactor of HNF1. DNA Cell Biol 1999, 18:243-252 [DOI] [PubMed] [Google Scholar]
- 35.Bagnara JT: The Pigmentary System. 1998:pp 9-35 Oxford University Press, Oxford
- 36.King R, Weilbaecher KN, McGill G, Cooley E, Mihm M, Fisher DE: Microphthalmia transcription factor: a sensitive and specific melanocyte marker for melanoma diagnosis. Am J Pathol 1999, 155:731-738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goding CR: Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev 2000, 14:1712-1728 [PubMed] [Google Scholar]
- 38.Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long JE, Meyers KA, Aaronson SA, Miki T: Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet 1996, 14:50-54 [DOI] [PubMed] [Google Scholar]
- 39.Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DEW: Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 1999, 126:3757-3767 [DOI] [PubMed] [Google Scholar]
- 40.Anastasiadis PZ, Kuhn DM, Levine RA: Tetrahydrobiopterin uptake into rat brain synaptosomes, cultured PC12 cells, and rat striatum. Brain Res 1994, 665:77-84 [DOI] [PubMed] [Google Scholar]
- 41.Schott K, Brand K, Hatakeyama K, Kagamiyama H, Maier J, Werner T, Ziegler I: Control of cell-cycle-associated tetrahydrobiopterin synthesis in rat thymocytes. Exp Cell Res 1992, 200:105-109 [DOI] [PubMed] [Google Scholar]
- 42.Zuckerman JE, Raffin TA, Brown JM, Newman RA, Etiz BB, Sikic B: In vitro selection and characterization of a bleomycin-resistant subline of B16 melanoma. Cancer Res 1986, 46:1748-1753 [PubMed] [Google Scholar]


