Abstract
Cutaneous melanocytic neoplasms are known to acquire variable characteristics of neural crest differentiation. Melanocytic nevus cells in the dermis and desmoplastic melanomas often display characteristics of nerve sheath differentiation. The extent and nature of neuronal differentiation characteristics displayed by primary and metastatic melanoma cells are not well understood. Here, we describe induction of a juvenile isoform of microtubule-associated protein 2 (MAP-2c) in cultured metastatic melanoma cells by the differentiation inducer hexamethylene bisacetamide. Up-regulation of this MAP-2 isoform, a marker for immature neurons, is accompanied by extended dendritic morphology and down-regulation of tyrosinase-related protein 1 (TYRP1/gp75), a melanocyte differentiation marker. In a panel of cell lines that represent melanoma tumor progression, MAP-2c mRNA and the corresponding ∼70-kd protein could be detected predominantly in primary melanomas. Immunohistochemical analysis of 61 benign and malignant melanocytic lesions showed abundant expression of MAP-2 protein in melanocytic nevi and in the in situ and invasive components of primary melanoma, but only focal heterogeneous expression in a few metastatic melanomas. In contrast, MAP-2-positive dermal nevus cells and the invasive cells of primary melanomas were TYRP1-negative. This reciprocal staining pattern in vivo is similar to the in vitro observation that induction of the neuronal marker MAP-2 in metastatic melanoma cells is accompanied by selective extinction of the melanocytic marker TYRP1. Our data show that neoplastic melanocytes, particularly at early stages, retain the plasticity to express the neuron-specific marker MAP-2. These observations are consistent with the premise that both benign and malignant melanocytes in the dermis can express markers of neuronal differentiation.
Melanocytes arise from the neural crest, which also gives rise to peripheral neurons, glial cells, and neuroendocrine cell types. 1 Neoplastic melanocytes are known to exhibit certain differentiation characteristics of other neural crest derivatives. For example, some benign nevus cells that migrate into the dermis morphologically resemble Schwann cells of the peripheral nervous system. 2 Similarly, desmoplastic (neurotropic) melanomas, which arise most often in sun-damaged skin, share many characteristics of peripheral nerve sheath tumors, including nerve involvement and expression of neural protein markers. 3 Other studies have shown expression of neuron-associated markers such as intermediate filament protein peripherin, neuropeptide substance P, muscarinic acetylcholine receptors, and neuron-specific enolase in primary and metastatic melanomas. 4-7 These observations suggest that human cutaneous melanocytes maintain plasticity of differentiation. Neoplastic transformation presumably allows them to exhibit characteristics of other neural crest derivatives. Although the dermal environment is thought to facilitate alternative pathways of differentiation in neoplastic melanocytes, signaling mechanisms involved in such trans-differentiation are not well understood. 2
In this study, we describe expression of a neuron-selective marker microtubule-associated protein 2 (MAP-2) in melanoma in vivo and its induction in melanoma cells in vitro. MAPs are a family of proteins expressed predominantly in neuronal cells and are associated with the dendritic morphology of neurons. 8 MAP-2, a neuron-specific MAP primarily localized to dendrites, stabilizes microtubule bundles and allows outgrowth of cellular processes. 9 Multiple isoforms of MAP-2, which are regulated during development, have been described. Thus, whereas the high molecular weight (∼280 kd) mature forms, MAP-2a and MAP-2b, persist throughout the life of the neuron, the juvenile isoform (∼70 kd) MAP-2c, derived by alternative splicing of MAP-2 mRNA, appears during development and diminishes in adult neurons. 10 Expression of this neuron-selective MAP-2 in melanocytes and melanocytic lesions has not been investigated. Our data show that MAP-2 is expressed abundantly in a majority of melanocytic nevi and primary melanomas, but weakly and heterogeneously in a few metastatic melanomas in vivo. In metastatic melanoma cell lines in vitro, MAP-2 can be induced by treatment with hexamethylene bisacetamide (HMBA), a pharmacological compound known to induce terminal differentiation of mouse erythroleukemia cells and a variety of human tumor cells. 11 Induction of MAP-2 by HMBA is accompanied by polydendritic morphology and down-regulation of the melanocytic differentiation marker TYRP1/gp75. Treatment with HMBA does not repress other melanocytic markers tested including tyrosinase, DCT/TYRP2, SILV/Pmel17, and microphthalmia-associated transcription factor (MITF). 12,13 This reciprocal relationship between the induction of MAP-2 and extinction of TYRP1 is also observed in the expression pattern of these two proteins in melanocytic neoplasms in vivo. The significance of this reciprocal expression of the melanocytic marker TYRP1 and the neuronal marker MAP-2 in differentiation of melanocytic lesions and the possible consequences of MAP-2 expression on melanoma tumor progression will be discussed.
Materials and Methods
Cell Culture
Primary culture of human melanocytes was initiated from neonatal foreskins. Fresh skin specimens were washed three times with Hanks’ balanced salt solution and excess fat was removed. The samples were cut into small pieces and incubated in 0.25% trypsin solution at 4°C overnight. Epidermis was separated from the dermis and epidermal cells were suspended and cultured in Ham’s F10 nutrient medium with 10% fetal bovine serum, 85 nmol/L 12-O-tetradecanoylphorbol-13-acetate (TPA), 0.1 mmol/L 3-isobutyl-1-methylxanthine (IBMX), 2.5 nmol/L cholera toxin (CT), and 100 μg/ml geneticin.
Primary (WM35, WM75, WM98-1, WM115, and WM793) and metastatic (WM451Lu) human melanoma cell lines were kindly provided by Dr. Meenhard Herlyn (The Wistar Institute, Philadelphia, PA). WM35 is derived from an early-stage radial growth phase primary lesion (Breslow thickness 0.69 mm, Clark level II) and the patient was cured after surgical removal of the lesion. WM35 cells do not metastasize in nude mice. 14 WM75 is derived from vertical growth phase (VGP) primary melanoma (Breslow thickness 6.25 mm, Clark level IV) from a patient who also had a subsequent metastatic lesion. WM98-1 is derived from a VGP primary (Breslow thickness 5.4 mm, Clark level IV) and the patient had a recurrence of melanoma during 5-year clinical follow-up. WM98-1 is tumorigenic in nude mice. 15,16 WM115 is derived from a VGP primary melanoma (Breslow thickness 2.24 mm, Clark level III) in a patient who had a recurrence 9 months later. 16 WM793 is derived from a VGP primary melanoma (Breslow thickness 0.55 mm, Clark level II) in a patient who did not have a recurrence during 10-year clinical follow-up. 16 These WM lines were grown in Ham’s F10 medium containing 10% fetal bovine serum and 1% antibiotic-antimycotic mixture. Metastatic melanoma cell lines SK-MEL-19 and SK-MEL-23 clone 22 (cl.22) cells described earlier, 17,18 were grown in minimal essential medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1% glutamine, and 1% antibiotic-antimycotic mixture. Cells were seeded at a density of 5 × 10 5 cells/10 ml of culture medium in 100-mm dishes. Culture medium, fetal bovine serum, Hanks’ balanced salt solution, antibiotic-antimycotic mixture, geneticin, nonessential amino acids, and glutamine were purchased from Life Technologies, Inc., Bethesda, MD. TPA, 3-isobutyl-1-methylxanthine, and CT were from Sigma Chemical Co., St. Louis, MO. HMBA was obtained from Aldrich Chemical Co., Milwaukee, WI.
RNA Isolation
Cells grown as monolayers were washed twice with Hanks’ balanced salt solution, harvested by trypsinization, and washed once with ice-cold PBS. PolyA+ RNA and total RNA were isolated from cell pellets using MicroFastTrack mRNA (Invitrogen Corp., Carlsbad, CA) and Ultraspec-II RNA isolation system (Biotecx Laboratories, Inc., Houston, TX), respectively. Total RNAs were treated with DNase I (Clontech Laboratories Inc., Palo Alto, CA) for differential display reverse transcriptase-polymerase chain reaction (PCR) to remove the remaining genomic DNA.
Differential Display
Reverse transcriptase-PCR was performed using a GeneAmp System 2400 (Perkin-Elmer Corp., Foster City, CA). Differential display was performed using a Delta differential display kit (Clontech Laboratories Inc.) following the manufacturer’s instructions. First-strand cDNA was synthesized from 2 μg of total RNA isolated from 70 to 85% confluent control or 5 mmol/L of HMBA-treated (for 48 hours) SK-MEL-19 cells using an oligo (dT) primer. Diluted cDNA (1:12.5 and 1:50) was used to amplify the differential display-PCR product in the presence of [α-33P] dATP (Dupont NEN, Boston, MA) using a random combination of arbitrary primers and oligo (dT) primers. PCR product was resolved by electrophoresis in a 5% polyacrylamide, 8-mol/L urea sequencing gel. The gel was dried and exposed to Biomax MS film (Kodak, Rochester, NY). Bands expressed differentially between untreated and treated samples were cut, eluted, re-amplified, and sequenced by the ABI 377 DNA sequencer (Perkin-Elmer Corp., Foster City, CA).
Northern Analysis
Northern analysis was performed as described previously using a Northern Max kit and a Strip-EZ DNA probe synthesis and removal kit (Ambion, Inc., Austin, TX). 12 The blots were washed at room temperature for 20 minutes with 2× SSC, 0.5% sodium dodecyl sulfate (SDS), followed by washes at 55 to 60°C for 20 minutes with 0.5%× SSC, 0.5% SDS, and then 0.1%× SSC, 0.5% SDS. The 410-bp cDNA template for the MAP-2 probe was amplified by PCR using a set of primers flanking the region of MAP-2 cDNA identical to differential display-PCR fragment (sense: 5′ ATCAAATGGTCCACTAGGCG 3′; antisense: 5′ GCACTTCAAGGGAAGCTGAT 3′). The cDNA templates for tyrosinase, TYRP1, DCT/TYRP2, MITF probes were generated as described before. 12 Human GAPDH probe was from Ambion. Human β-actin probe template (838 bp) was amplified using primers from Clontech Laboratories, Inc. (sense: 5′ ATCTGGCACCACACCTTCTACAATGAGCTGCG 3′; antisense: 5′ CGTCATA CTCCTGCTTGCTGATCCACATCTGC 3′). MAP-2, tyrosinase, TYRP1, DCT/TYRP2, MITF, GAPDH, and β-actin probes detected a single mRNA band at ∼6.0 kb, 1.9 kb, 2.8 kb, 4.5 kb, 5.5 kb, 1.4 kb, and 1.8 kb, respectively. 12 Band intensity was quantitatively analyzed with an ImageQuaNT software (Molecular Dynamics, Sunnyvale, CA). Relative intensities of MAP-2 signals were obtained by normalizing to GAPDH.
Western Blot Analysis
Western blot analysis was performed as described earlier. 12 Briefly, cells were solubilized in lysis buffer containing 1% SDS, 10 mmol/L Tris, pH 7.4, and proteinase inhibitors (Boehringer Mannheim, Indianapolis, IN). Protein content was estimated using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Total cellular protein was subjected to 9% SDS-polyacrylamide gel electrophoresis, and transferred electrophoretically to a polyvinylidene difluoride membrane (NEN Life Science, Boston, MA). The blots were incubated in blocking buffer [1% bovine serum albumin in Tris-buffered saline (TBS) containing 10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl] at room temperature for 3 hours, and then at 4°C overnight with addition of the primary antibodies diluted in TBS. Anti-MAP-2 mAbs HM-2 (Sigma) and M13 (Zymed Laboratories, San Francisco, CA) were used at 1:1000; anti-γ-tubulin polyclonal antibody (Sigma) was used at 1:5000. Blots were washed with TBST (TBS containing 0.1% Tween 20) with frequent changes of wash buffer. They were then incubated with donkey anti-mouse (for HM-2 and M13) or anti-rabbit (for γ-tubulin) horseradish peroxidase antibody (Amersham Pharmacia Biotech Inc., Piscataway, NJ) or alkaline phosphatase-conjugated goat anti-mouse IgG (BioRad Laboratories, Hercules, CA) diluted in TBST at 1:2000 to 1:2500 for 1 to 3 hours, and washed again with TBST with frequent changes of wash buffer. Protein bands were detected either colorimetrically or by chemiluminescence using an ECL kit (Amersham Pharmacia Biotech Inc.) and exposed to Kodak X-ray film for 5 seconds to 15 minutes.
Immunohistochemistry Analysis
Tissue specimens were fixed in 10% neutral-buffered formalin, processed by routine histological method, and embedded in paraffin. Standard sections were cut and collected on positively charged slides and immunohistochemical studies for TYRP1 (1:80, mel-5; Signet Laboratories; Dedham, MA), gp100 (1:100, HMB45; DAKO Corporation; Carpinteria, CA), Melan A/MART-1 (1:5; Novocastra Laboratories; Burlingame, CA), neuron-specific enolase (1:50; DAKO; Glostrup, Denmark), neurofilament protein p68 (1:5; Accurate Chemical and Scientific Co.; Westbury, NY), low-affinity nerve growth factor receptor (1:40, p75NGFR; Boehringer Mannheim; Indianapolis, IN), and neural adhesion molecule (1:40, CD56/N-CAM; Becton-Dickinson; San Jose, CA) were performed using standard immunoperoxidase techniques on a Ventana autostainer (Ventana Medical Systems, Tucson, AZ). Immunohistochemical studies for MAP-2 (M13, prediluted; Zymed) were performed manually using the manufacturer’s Histostain-Plus kit, which uses a standard streptavidin-biotin amplification method and a 3-amino-9-ethylcarbazole chromogen.
Results
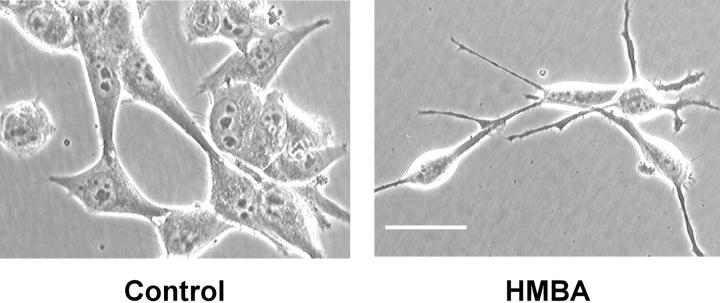
We reported earlier that the treatment of human pigmented melanocytic cells in culture with the differentiation-inducing agent HMBA inhibits cell growth and causes selective down-regulation of the melanocyte differentiation marker TYRP1. 12,13,19 Flow cytometric analysis showed that treatment with HMBA results in accumulation of cells in G0/G1 phase and a significant decrease in population of cells in G2/M phase (data not shown). This change in growth kinetics upon treatment with HMBA is accompanied by formation of long dendrites in all melanoma cells tested. 12,13,19 In Figure 1 ▶ , the effect of HMBA on the morphology of a representative melanoma cell line SK-MEL-19 is shown.
Figure 1.
Induction of dendritic morphology by HMBA. Morphology of human metastatic melanoma cell line SK-MEL-19 treated with 5 mmol/L of HMBA for 5 days. Scale bar, 100 μm.
Identification of MAP-2 Expression in Melanoma Cells by Differential Display Analysis
To characterize changes in gene expression associated with growth inhibition and dendritic morphology of melanoma cells, we performed differential display analysis using RNA obtained from control and HMBA-treated SK-MEL-19 melanoma cells. Arbitrary primer P4 (ATTAACCCTCACTAAATGCTGGTAG) and oligo dT primer T7 (CATTATGCTGAGTGATATCTTTTTTTTTGA) amplified a cDNA that is overexpressed in treated cells. Nucleotide sequence analysis of the ∼450-bp cDNA band showed 98% identity to 3′-untranslated region within exon 19 of 10.2-kb human MAP-2 cDNA (GenBank Accession No. U32996; between nucleotides 1931 to 2384). cDNA probes derived from this region detect 6-kb and 9-kb alternative splice variants of MAP-2 mRNAs that produce, respectively, a juvenile polypeptide form (MAP-2c) of ∼70 kd and two mature forms (MAP-2a and MAP-2b) of ∼280 kd. 10
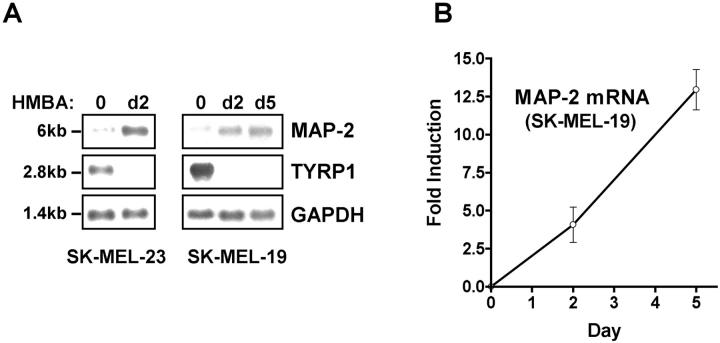
A 410-bp PCR-amplified cDNA fragment nested within the 450-bp differential display fragment was used to probe polyA+ RNA isolated from control and HMBA-treated melanoma cells. In control metastatic melanoma SK-MEL-19 and SK-MEL-23 cl.22 cells, a weak 6-kb band representing the alternatively spliced MAP-2 mRNA could be seen. Treatment of melanoma cells with HMBA for 2 to 5 days resulted in a significant up-regulation of MAP-2 expression (Figure 2A) ▶ . In SK-MEL-19 cells treated with HMBA for 48 hours, a fourfold increase in MAP-2 mRNA was noted. Prolonged presence of the inducer resulted in continued accumulation (up to 12-fold) of MAP-2 in SK-MEL-19 cells (Figure 2B) ▶ . Thus, Northern analysis confirmed the identification of MAP-2 as a differentially expressed gene in melanoma cells treated with the differentiation inducer. In the middle panel of Figure 2A, a ▶ concomitant down-regulation of the melanocyte differentiation marker TYRP1 mRNA by the inducer in SK-MEL-19 and SK-MEL-23 cl.22 is shown.
Figure 2.
Up-regulation of MAP-2 mRNA in melanoma cells by HMBA. A: Northern blot analysis. Three μg of polyA+ RNA isolated from untreated SK-MEL-19 and SK-MEL-23 cl.22 melanoma cells and cells treated with HMBA for 2 to 5 days was electrophoresed, blotted, and hybridized with 32P-labeled probes as described in Materials and Methods. B: Quantitative analysis of MAP-2 mRNA induction in SK-MEL-19 melanoma cells. Band intensities were measured, normalized to GAPDH, and plotted as relative intensities. Data shown are means ± SEM from three independent experiments.
Expression of MAP-2 in Melanoma Cell Lines
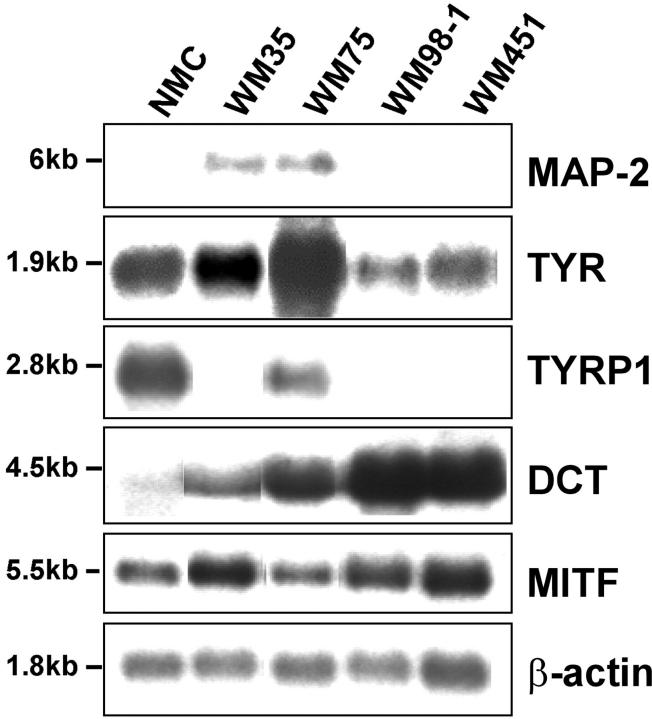
Expression of MAP-2 was studied in a panel of well-characterized cell lines that represent melanoma progression. 14-16 PolyA+ RNA isolated from neonatal foreskin melanocytes, primary radial growth phase melanoma cell line WM35, primary VGP melanoma cell lines WM75 and WM98–1, and metastatic melanoma WM451 was analyzed by Northern blot hybridization (Figure 3) ▶ . In primary melanoma cell lines WM35 and WM75, the 6-kb MAP-2 mRNA was readily detected. MAP-2 mRNA was not detectable in normal melanocytes, primary melanoma WM98–1, or metastatic melanoma WM451. The variable expression of melanocyte differentiation markers tyrosinase, TYRP1, DCT, and MITF in these cell lines is shown (Figure 3) ▶ . These data show that melanocytes at early stages of tumor progression activate transcription of the neuronal differentiation marker MAP-2 and produce an alternatively processed MAP-2c transcript normally found in immature neurons.
Figure 3.
Expression of MAP-2 mRNA in human melanocytes and melanoma cells. Northern blot analysis of 3 μg of polyA+ RNA isolated from cultured neonatal melanocytes (NMC), primary radial growth phase melanoma cell line WM35, primary VGP melanoma cell lines WM75 and WM98–1, and metastatic melanoma cell line WM451. Expression of MAP-2 and the melanocytic markers tyrosinase, TYRP1, DCT, and MITF were analyzed using 32P-labeled cDNA probes as described in Materials and Methods. Human β-actin probe was used to determine variation in RNA loading.
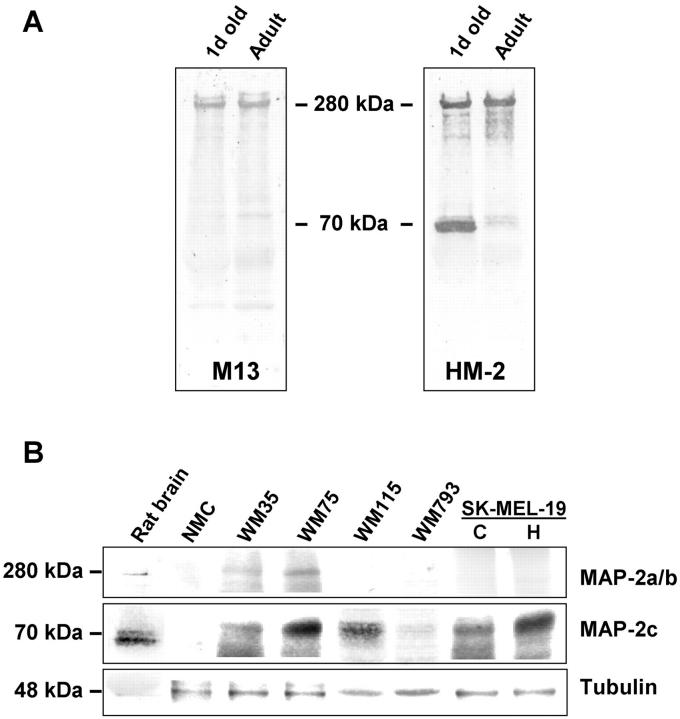
Western blot analysis was also used to detect the expression of MAP-2 protein and the specificity of available anti-MAP-2 antibodies. As shown in Figure 4A ▶ , mAb HM-2 detected both the immature juvenile ∼70-kd and mature ∼280-kd isoforms found in 1-day-old rat brain extracts and the 280-kd mature form in the adult brain extracts. On the other hand, mAb M13 detected only the mature 280-kd form in newborn and adult rat brain extracts. Western blot analysis of detergent extracts of melanoma cell lines with mAb HM-2 is shown in Figure 4B ▶ . A protein band of ∼70 kd corresponding to the MAP-2c isoform, consistent with the presence of 6-kb MAP-2 mRNA, could be detected in WM35, WM75, and all primary melanoma cell lines tested and the metastatic cell line SK-MEL-19, but not in normal melanocytes. For comparison, amounts of MAP-2 isoforms detectable in newborn rat brain extracts are also shown (first lane in Figure 4B ▶ ). Human melanoma MAP-2c appears to migrate slightly slower than the ∼70-kd doublet of rat brain isoform. In SK-MEL-19 cells treated with HMBA, an increase in the amount of MAP-2c protein was apparent. Although the 9-kb mRNA that produces a mature 280-kd protein was not detectable by Northern blotting, a faint protein band corresponding to the MAP-2a and MAP-2b isoforms could be detected in WM35 and WM75 melanoma cell lines.
Figure 4.
Immunoblotting analysis of MAP-2 expression in melanocytes and melanoma cells. A: Specificity of anti-MAP-2 mAbs M13 and HM-2 was tested using 1-day-old and adult rat brain extracts. Protein bands corresponding to 70-kd MAP-2c and ∼280-kd MAP-2a and MAP-2b isoforms are indicated. B: Forty μg of detergent extracts of melanocytes and melanoma cells were analyzed by Western blotting using mAb HM-2. NMC, melanocytes; WM35, radial growth phase primary melanoma cell line; WM75, WM115, and WM793, VGP primary melanoma cell lines. C and H, control and HMBA-treated (for 5 days) SK-MEL-19 cells. One-day-old rat brain extract was used as positive control. Immunoblotting with a polyclonal anti-γ-tubulin antibody is shown to estimate variation in amount of protein loaded in each lane.
Pathways of Regulation of MAP-2 Expression in Melanoma in Vitro
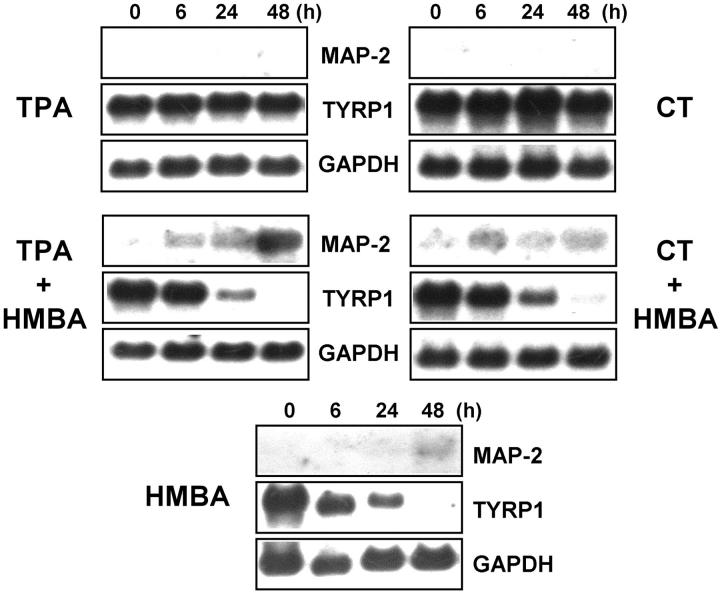
To understand the possible pathways involved in up-regulation of MAP-2 by HMBA, we tested the effects of phorbol ester TPA (a modifier of protein kinase C activity) and CT (a cAMP inducer) on MAP-2 expression in melanoma cells. SK-MEL-19 cells were treated with TPA or CT for 6, 24, and 48 hours and MAP-2 expression was studied by Northern blot analysis of total RNA. As shown in Figure 5 ▶ , treatment of cells with TPA or CT alone did not induce MAP-2 expression. Up-regulation of MAP-2 expression in cells treated with HMBA could be detected by 48 hours. When HMBA was added together with TPA or CT, a significant increase in MAP-2c mRNA could be detected as early as 6 hours after treatment. Similarly, whereas treatment with TPA or CT alone did not cause down-regulation of TYRP1 mRNA, treatment with HMBA alone or in combination with TPA or CT resulted in extinction of TYRP1 expression. These data suggest that although agents that affect protein kinase C and cAMP pathways themselves have no effect on MAP-2 expression in melanoma cells, these agents can facilitate HMBA-mediated induction of MAP-2. These data also suggest that there is a reciprocal relationship between pathways that regulate the expression of the melanocytic marker TYRP1 and the neuronal marker MAP-2 in melanoma cells.
Figure 5.
Role of cAMP and protein kinase C signaling pathways in up-regulation of MAP-2. SK-MEL-19 melanoma cells were treated with 5 mmol/L of HMBA, 2.5 nmol/L of CT, 10 ng/ml TPA, or a combination of HMBA and CT or HMBA and TPA for 6, 24, and 48 hours. Ten μg of total RNA/lane was electrophoresed and blotted for Northern analysis. Blots were probed sequentially with 32P-labeled MAP-2, TYRP1, and GAPDH probes.
MAP-2 Expression in Melanocytic Lesions in Vivo
To test whether MAP-2 is also expressed in human melanocytic lesions in vivo, immunohistochemistry was performed using anti-MAP-2 mAb M13. A total of 61 individual paraffin-embedded specimens were tested. These included 10 congenital and acquired melanocytic nevi, 9 primary malignant melanomas, and 42 metastatic melanomas. Whereas the majority of nevi (60%) and many primary melanomas (44%) were strongly MAP-2-positive (+++ to ++), only a small percentage of metastatic melanomas (24%) had foci of MAP-2-stained cells (Table 1) ▶ . Fisher’s exact test showed a strong association between the number of lesions showing strong MAP-2 reactivity and the characteristics of the melanocytic lesions (P = 0.0039). As shown in Figure 6A ▶ , in a malignant melanoma arising in a nevus, both the dermal nevus cells and the cells within the early primary melanoma showed strong cytoplasmic staining for MAP-2. TYRP1-specific mAb MEL-5 stained melanocytes and melanoma cells within epidermis and at the dermal-epidermal junction, whereas the early invasive disease and the intradermal nevus cells were less intensely stained.
Table 1.
Immunohistochemical Staining of Melanocytic Lesions with Anti-MAP-2 Antibody
| Lesion | MAP-2 staining intensity | ||||
|---|---|---|---|---|---|
| n | +++ | ++ | + | − | |
| Nevi | 10 | 6 (60%) | 0 (0%) | 1 (10%) | 3 (30%) |
| Primary melanoma | 9 | 3 (33.3%) | 1 (11.1%) | 2 (22.2%) | 3 (33.3%) |
| Metastatic melanoma | 42 | 2 (5%) | 8 (19%) | 14 (33%) | 18 (43%) |
The relative intensity of staining of paraffin embedded tissue section with mAb M13 is shown as: no detectable immunoreactivity (−), or increasing intensity of reactivity (+, ++, and +++). n, number of specimens tested. Data are shown as the number of specimens positive or negative for MAP-2 staining (numbers in parentheses are percent total specimens). Nevi include both congenital and acquired types; primary melanomas include 2 desmoplastic specimens and 7 conventional melanomas, and metastatic melanomas include 26 lesions from lymph nodes, 13 from brains and 3 other anatomical sites (one lesion each from lung, bone, and parotid gland). Analysis of the data using Fisher’s exact test (r × c contingency table) showed strong association between intensity of MAP-2 staining and the characteristics of the melanocytic lesions studied (P = 0.0039).
Figure 6.
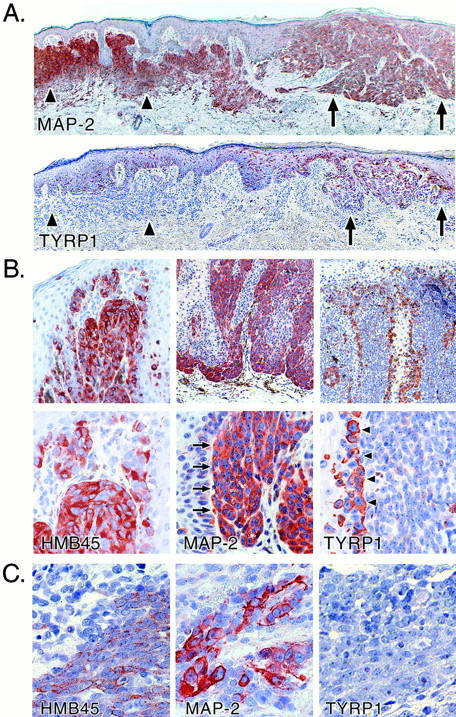
Immunohistochemical analysis of MAP-2 expression in melanocytic lesions. A: Primary malignant melanoma (superficial spreading type, Breslow thickness, 0.5 mm; Clark level III) arising in association with a melanocytic nevus. Upper panel: MAP-2. Lower panel: TYRP1. Arrowheads demarcate the approximate boundaries of the melanocytic nevus and the arrows indicate the melanoma. At this stage, the invasive melanoma cells are decorated as intensely as the nevus cells for MAP-2. Staining for TYRP1 is strong in the in situ melanoma but weak in the invasive disease and negative in the intradermal nevus cells. B: Primary malignant melanoma (Breslow thickness, 1.14 mm; Clark level III). Staining of invasive melanoma cells with anti-MAP-2 mAb is indicated by arrows and TYRP1 staining of tumor cells at the junction of the in situ and dermal invasive components of the tumor is highlighted by arrowheads. Note the reciprocal expression of TYRP1 and MAP-2. HMB45 staining helps to confirm melanocytic differentiation. Metastases of this melanoma, one excised from the left arm and another from the brain, stained negative or weakly positive for MAP-2, respectively (data not shown). C: The reciprocal relation between expression of MAP-2 and TYRP-1 is also illustrated in metastatic melanoma. HMB45 staining confirmed melanocytic differentiation of the metastatic lesion.
In cutaneous primary melanomas consisting of both in situ and extensive invasive components, most of the invasive component was strongly positive for MAP-2, whereas malignant melanocytes at the dermal-epidermal junction showed weak staining (Figure 6B ▶ ; lower panels show higher magnification). Metastatic lesions excised from the left arm and brain from the same patient showed negative or weakly positive staining, respectively, for MAP-2 (data not shown). TYRP1 expression was, conversely, restricted mostly to the cells in the in situ and junctional components. Cells within the tumor nests either showed weak TYRP1 expression or were TYRP1-negative (Figure 6B) ▶ .
A limited number of primary superficial-spreading malignant melanomas and associated nevi were also studied for expression of additional neuronal markers, specifically neuron-specific enolase, neurofilament protein p68, neural adhesion molecule (CD56/N-CAM), and low-affinity nerve growth factor receptor (p75NGFR). Significant expression of neuron-specific enolase was found in both nevus and melanoma components. Whereas no expression of neurofilament protein p68 or CD56/N-CAM could be detected in either component, p75NGFR expression was found to be restricted to malignant melanocytes in situ. All primary melanomas tested showed expression of the melanocytic markers gp100 and MelanA/MART-1 (data not shown).
In the small percentage of metastatic melanomas scored positive for MAP-2 immunoreactivity, the staining was heterogeneous. A few isolated cells and/or clusters showing intense cytoplasmic staining were observed with adjacent cells devoid of MAP-2 expression (Figure 6C) ▶ . Tumor cells within metastatic lesions, including those stained positive with mAb HMB45, were negative for TYRP1 expression (Figure 6C) ▶ .
Discussion
We have shown earlier that treatment of human melanoma cells in vitro with the differentiation-inducing agent HMBA results in growth inhibition, dendritic morphology, and selective repression of the melanocytic marker TYRP1. This in vitro phenotype induced by treatment with the differentiation inducer is particularly interesting in that the repression of TYRP1 occurs independently of tyrosinase and other tyrosinase-related proteins and also MITF, the melanocyte-specific transcription factor known to be critical for activation of TYRPs. 12,13,19,20 Our attempts to understand the changes in gene expression that accompany this phenotype revealed induction of the neuronal marker MAP-2 in melanoma cells. Immunohistochemical analyses showed abundant expression of MAP-2 in melanocytic nevi and primary melanomas. The MAP-2 expression pattern in vivo showed transition from weak or negative MAP-2 staining of melanocytes and primary melanoma cells in the epidermis to intensely stained invasive melanoma cells in the dermis. Interestingly, expression of TYRP1 in cutaneous melanocytic lesions showed a pattern that suggests a reciprocal relationship between this melanocyte differentiation marker and neuronal marker MAP-2.
MAPs are a heterogeneous group of proteins associated with microtubules and are known to regulate the stability of microtubules, primarily in axons and dendrites of neurons. Although MAPs are expressed most abundantly in neuronal cells, certain MAPs such as MAP-1 and MAP-4 are widely expressed in nonneuronal cells. 21-23 For example, MAP-1 is expressed in many cell types including mouse and human melanoma cells (our unpublished observations). 24 Expression of MAP-2, one of the most extensively studied MAPs, is restricted to neurons and MAP-2 expression is used as marker for neuronal differentiation. 25-27 MAP-2 is localized primarily in the dendrites but not in axons. Multiple isoforms of MAP-2, which arise from alternative splicing of mRNA, have been characterized. 10 Interestingly, this splicing event seems to be developmentally regulated. Thus, whereas three isoforms, MAP-2a and MAP-2b (both ∼280 kd) and MAP-2c (∼70 kd) are found during neuronal differentiation, only the 280-kd MAP-2a and MAP-2b isoforms are found in adult neurons. 28 Juvenile and mature MAP-2 isoforms are known to induce distinct patterns of outgrowth. 29 Expression or induction of the developmentally regulated 6-kb transcript for the juvenile 70-kd MAP-2c isoform in nonneuronal cells, specifically in neural crest derived cells, therefore suggests possible activation of pathways of neuronal differentiation. In this context, it is worth noting that transduction of cultured chick retinal pigment epithelial cells with retrovirus expressing neuroD, a helix-loop-helix transcription factor, results in their transdifferentiation to photoreceptor-like cells and expression of MAP-2. 30
The ability of neoplastic melanocytes, especially dermal nevus cells, to differentiate along pathways of other neural crest-derived cell types has been well documented. 2,31,32 Thus, in melanocytic nevi, the terminal differentiation of type C nevus cells deep within the dermis results in Schwann cell-like morphology and activation of Schwann cell markers. 2,31,32 Cellular and stromal interactions within the dermis are thought to provide the signals for such trans-differentiation of melanocytes. Only rarely do malignant melanocytes follow the Schwann cell pathway of differentiation, even when they are localized within similar environment as nevus cells. 2 When they do, desmoplastic neurotropic melanomas are produced, and these lesions are distinctive in that they tend to metastasize much later in their course than conventional malignant melanomas. 33 In contrast, most malignant melanomas seem to acquire characteristics more similar to neurons as evident by the expression of intermediate filament protein peripherin and other neuronal markers. 4-7 These observations have led to the hypothesis that during malignant progression, cutaneous melanocytes follow divergent differentiation pathways. 2 Our observations on the activation of neuronal MAP-2 in primary cutaneous melanoma are consistent with this proposal. Yet, the majority of nevi also express MAP-2, which suggests that neural differentiation in neoplastic melanocytes in the dermis parallels or precedes Schwannian pathway of differentiation. Interestingly, among other neuronal markers tested in this study, only neuron-specific enolase showed a pattern of staining similar to MAP-2.
In vitro experiments on possible signaling pathways involved in MAP-2 induction in melanoma cells showed that activation of protein kinase C and cAMP pathways, which are known to be important for maintenance of melanocytic differentiation, was not sufficient for MAP-2 induction. It seems that HMBA-activated pathways, which also down-regulate the melanocyte differentiation marker TYRP1, allowed induction of MAP-2. But activators of either protein kinase C or cAMP pathways could facilitate this induction. HMBA is a hybrid polar compound shown to induce terminal differentiation in mouse erythroleukemia cells (MEL) and a variety of human tumor cells. 34 Although the effects of HMBA have been investigated extensively, the mechanism(s) by which it induces differentiation is not clear. 35-38 For example, in MEL cells cAMP-dependent protein kinase A and protein kinase Cε have been implicated in the mechanism of HMBA action. On the other hand, although HMBA mimics the effect of thyrotropin by increasing thyroglobulin production in thyroid cells in primary culture, the action of HMBA is reported to occur without increasing cAMP. 35 In contrast to the more potent inducer of differentiation, suberoylanilide hydroxamic acid, the mechanism of action of HMBA on MEL cells does not seem to involve inhibition of histone deacetylases. 37 Our observation that the selective repression of TYRP1, which occurs concomitantly with MAP-2 up-regulation in melanoma cells, by HMBA is not mediated by inhibition of histone deacetylases is consistent with this finding (unpublished observation).
The biological significance of MAP-2 expression in melanocytes remains to be understood. MAP-2 in neurons is known to be enriched within dendrites, 8 and induction of MAP-2 in vitro by HMBA is accompanied by extensive dendritic morphology, suggesting that MAP-2 is involved in the formation of dendrites. This notion is supported by the observation that when MAP-2c is expressed in nonneuronal cells, microtubules in the transfected cells form long, stable bundles that promote outgrowth of processes. 39 Indeed, when expressed in nonneuronal NIH3T3 cells, MAP-2 seems to retain the ability to associate selectively with microtubules and other cytoskeletal elements and produce distinct cellular processes. 40
This raises the question—what are the consequences of stabilizing microtubules in melanocytes during the early stages of tumor progression? Microtubules and other cytoskeletal elements play a critical role in such diverse cellular processes ranging from cell motility, signal transduction, and mitosis. 21 For example, phosphorylation-regulated association of MAP-2 isoforms with intracellular signaling proteins such as Src and Grb2, has recently been demonstrated. These associations have been suggested to play a role in modulation of neuronal cytoskeleton by extracellular signals. 41 It is possible that abundant expression of MAP-2 during the terminal differentiation of dermal nevus cells leads to microtubule stabilization and consequent withdrawal from cell cycle and senescence or apoptosis. Thus, malignant melanocytes that express MAP-2 may be inhibited from further tumor progression. Those that escape this control may continue to proliferate and metastasize. The focal MAP-2 expression in some metastases (Figure 6C) ▶ suggests that potential control of this differentiation can be reinstated at all stages of tumor progression, a finding that could have therapeutic implications.
Expression of MAP-2 and resultant changes in the dynamics of microtubule organization has implications for neuronal differentiation and progression of melanocytic neoplasms. Because an increasing number of agents that target microtubule organization are being tested for their anti-cancer activity, understanding regulation of MAP-2 expression and other microtubule stabilizing cellular proteins in melanoma cells will be important for designing appropriate therapeutic strategies. 42,43
Acknowledgments
We thank Dr. Doug Case of Department of Public Health Sciences of Wake Forest University School of Medicine for help with the statistical analysis.
Footnotes
Address reprint requests to Vijayasaradhi Setaluri, Ph.D., Departments of Dermatology and Cancer Biology, Wake Forest University School of Medicine, Winston-Salem, North Carolina 27157. E-mail: setaluri@wfubmc.edu.
Supported by National Institutes of Health grants AR44617 (to V. S.) and NS30985 (to J. A. H.) and by a Dermatology Foundation Dermik Laboratory Research Fellowship (to D. F.).
References
- 1.Le Douarin NM: The Neural Crest. 1982. Cambridge University Press, Cambridge
- 2.Reed JA, Finnerty B, Albino AP: Divergent cellular differentiation pathways during the invasive stage of cutaneous malignant melanoma progression. Am J Pathol 1999, 155:549-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangueza OP, Requena L: Neoplasms with neural differentiation: a review.: Part II: malignant neoplasms. Am J Dermatopathol 1998, 20:89-102 [DOI] [PubMed] [Google Scholar]
- 4.Prieto VG, McNutt NS, Lugo J, Reed JA: The intermediate filament peripherin is expressed in cutaneous melanocytic lesions. J Cutan Pathol 1997, 24:145-150 [DOI] [PubMed] [Google Scholar]
- 5.Khare VK, Albino AP, Reed JA: The neuropeptide/mast cell secretagogue substance P is expressed in cutaneous melanocytic lesions. J Cutan Pathol 1998, 25:2-10 [DOI] [PubMed] [Google Scholar]
- 6.Lammerding-Koppel M, Noda S, Blum A, Schaumburg-Lever G, Rassner G, Drews U: Immunohistochemical localization of muscarinic acetylcholine receptors in primary and metastatic malignant melanomas. J Cutan Pathol 1997, 24:137-144 [DOI] [PubMed] [Google Scholar]
- 7.Dhillon AP, Rode J, Leathem A: Neurone specific enolase: an aid to the diagnosis of melanoma and neuroblastoma. Histopathology 1982, 6:81-92 [DOI] [PubMed] [Google Scholar]
- 8.Matus A: Microtubule-associated proteins: their potential role in determining neuronal morphology. Ann Rev Neurosci 1988, 11:29-44 [DOI] [PubMed] [Google Scholar]
- 9.Kosik KS, Caceres A: Tau protein and the establishment of an axonal morphology. J Cell Sci 1991, 15(Suppl):69-74 [DOI] [PubMed] [Google Scholar]
- 10.Graner CC, Matus A: Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol 1988, 106:779-783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifkind RA, Richon VM, Marks PA: Induced differentiation, the cell cycle, and the treatment of cancer. Pharmacol Ther 1996, 69:97-102 [DOI] [PubMed] [Google Scholar]
- 12.Fang D, Setaluri V: Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1. Biochem Biophys Res Commun 1999, 256:657-663 [DOI] [PubMed] [Google Scholar]
- 13.Fang D, Kute T, Setaluri V: Regulation of tyrosinase-related protein 2 (TYRP2) in human melanocytes: relationship to growth and dendritic morphology. Pigment Cell Res 2001 14:132–139 [DOI] [PubMed]
- 14.Elder DE, Rodeck U, Thurin J, Cardillo F, Clark WH, Stewart R, Herlyn M: Antigenic profile of tumor progression stages in human melanocytic nevi and melanomas. Cancer Res 1989, 49:5091-5096 [PubMed] [Google Scholar]
- 15.Rodeck U, Herlyn M, Menssen HD, Furlanetto RW, Koprowsk H: Metastatic but not primary melanoma cell lines grow in vitro independently of exogenous growth factors. Int J Cancer 1987, 40:687-690 [DOI] [PubMed] [Google Scholar]
- 16.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M: Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res 1997, 7(Suppl 2):S35-S42 [PubMed] [Google Scholar]
- 17.Houghton AN, Real FX, Davis LJ, Cordon-Cardo C, Old LJ: Phenotypic heterogeneity of melanoma. : Relation to the differentiation program of melanoma cells. J Exp Med 1987, 165:812-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey TE, Takahashi T, Resnick LA, Oettgen HF, Old LJ: Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci USA 1976, 73:3278-3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayasaradhi S, Doskoch PM, Wolchok J, Houghton AN: Melanocyte differentiation marker gp75, the brown locus protein, can be regulated independently of tyrosinase and pigmentation. J Invest Dermatol 1995, 105:113-119 [DOI] [PubMed] [Google Scholar]
- 20.Goding CR: Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev 2000, 14:1712-1728 [PubMed] [Google Scholar]
- 21.Kobayashi N, Mundel P: A role of microtubules during the formation of cell processes in neuronal and non-neuronal cells. Cell Tissue Res 1998, 291:163-174 [DOI] [PubMed] [Google Scholar]
- 22.Wiche G, Briones E, Koszka C, Artileb U, Krepler R: Widespread occurrence of polypeptides related to microtubule-associated proteins (MAP-1 and MAP-2) in non-neuronal cells and tissues. EMBO J 1984, 3:991-998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP: Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci 1997, 110:3055-3064 [DOI] [PubMed] [Google Scholar]
- 24.Sato C, Nishizawa K, Nakamura H, Komagoe Y, Shimada K, Ueda R, Suzuki S: Monoclonal antibody against microtubule associated protein-1 produces immunofluorescent spots in the nucleus and centrosome of cultured mammalian cells. Cell Struct Funct 1983, 8:245-254 [DOI] [PubMed] [Google Scholar]
- 25.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, Nedergaard M, Goldman SA: In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med 2000, 6:271-277 [DOI] [PubMed] [Google Scholar]
- 26.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX: Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 2000, 75:991-1003 [DOI] [PubMed] [Google Scholar]
- 27.Tojima T, Yamane Y, Takahashi M, Ito E: Acquisition of neuronal proteins during differentiation of NG108–15 cells. Neurosci Res 2000, 37:153-161 [DOI] [PubMed] [Google Scholar]
- 28.Ludin B, Matus A: The neuronal cytoskeleton and its role in axonal and dendritic plasticity. Hippocampus 1993, 3:61-71 [PubMed] [Google Scholar]
- 29.Leclerc N, Baas PW, Garner CC, Kosik KS: Juvenile and mature MAP2 isoforms induce distinct patterns of process outgrowth. Mol Biol Cell 1996, 7:443-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan RT, Wang SZ: Differential induction of gene expression by basic fibroblast growth factor and neuroD in cultured retinal pigment epithelial cells. Vis Neurosci 2000, 17:157-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Hirose T, Hasegawa T, Seki K, Nakanishi H, Hizawa K: Ultrastructural heterogeneity of acquired intradermal melanocytic nevus cells. Ultrastruct Pathol 1996, 20:255-261 [DOI] [PubMed] [Google Scholar]
- 32.Brocker EB, Magiera H, Herlyn M: Nerve growth and expression of receptor for nerve growth factor in tumors of melanocyte origin. J Invest Dermatol 1991, 96:662-665 [DOI] [PubMed] [Google Scholar]
- 33.Skelton HG, Smith KJ, Laskin WB, McCarthy WF, Gagnier JM, Graham JH, Lupton GP: Desmoplastic malignant melanoma. J Am Acad Dermatol 1995, 32:717-725 [DOI] [PubMed] [Google Scholar]
- 34.Rifkind RA, Richon VM, Marks PA: Induced differentiation, the cell cycle, and the treatment of cancer. Pharmacol Ther 1996, 69:97-102 [DOI] [PubMed] [Google Scholar]
- 35.Aouani A, Samih N, Amphoux-Fazekas T, Mezghrani A, Mykhaylov S, Hovsepian S, Lombardo D, Fayet G: Hexamethylene bisacetamide (HMBA) increases thyroglobulin levels in porcine thyroid cells without increasing cyclic-AMP. Horm Metab Res 1999, 31:402-405 [DOI] [PubMed] [Google Scholar]
- 36.Leng L, Yu F, Dong L, Busquets X, Osada S, Richon VM, Marks PA, Rifkind RA: Differential modulation of protein kinase C isoforms in erythroleukemia during induced differentiation. Cancer Res 1993, 53:5554-5558 [PubMed] [Google Scholar]
- 37.Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA: A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA 1998, 95:3003-3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheele JS: cAMP-dependent phosphorylation and hexamethylene-bis-acetamide induced dephosphorylation of p19 in murine erythroleukemia cells. Mol Cell Biochem 1998, 185:55-63 [DOI] [PubMed] [Google Scholar]
- 39.Weisshar B, Doll T, Matus A: Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2(MAP2c). Development 1992, 116:1151-1161 [DOI] [PubMed] [Google Scholar]
- 40.Colella R, Lu C, Hodges B, Wilkey DW, Roisen FJ: GM1 enhances the association of neuron-specific MAP2 with actin in MAP2-transfected 3T3 cells. Brain Res Dev Brain Res 2000, 121:1-9 [DOI] [PubMed] [Google Scholar]
- 41.Lim RWL, Halpain S: Regulated association of microtubule-associated protein 2 (MAP2) with Src and Grb2: evidence for MAP2 as a scaffolding protein. J Biol Chem 2000, 275:20578-20587 [DOI] [PubMed] [Google Scholar]
- 42.Lopes NM, Miller HP, Young ND, Bhuyan BK: Assessment of microtubule stabilizers by semiautomated in vitro microtubule protein polymerization and mitotic block assays. Cancer Chemother Pharmacol 1997, 41:37-47 [DOI] [PubMed] [Google Scholar]
- 43.Veitia R, David S, Barbier P, Vantard M, Gounon P, Bissery MC, Fellous A: Proteolysis of microtubule associated protein 2 and sensitivity of pancreatic tumours to docetaxel. Br J Cancer 2000, 83:544-549 [DOI] [PMC free article] [PubMed] [Google Scholar]