Abstract
An innovative avenue for anti-inflammatory therapy is inhibition of neutrophil extravasation by potentiating the action of endogenous anti-inflammatory mediators. The glucocorticoid-inducible protein annexin 1 and derived peptides are effective in inhibiting neutrophil extravasation. Here we tested the hypothesis that an interaction with the receptor for formylated peptide (FPR), so far reported only in vitro, could be the mechanism for this in vivo action. In a model of mouse peritonitis, FPR antagonists abrogated the anti-migratory effects of peptides Ac2-26 and Ac2-12, with a partial reduction in annexin 1 effects. A similar result was obtained in FPR (knock-out) KO mice. Binding of annexin 1 to circulating leukocytes was reduced (>50%) in FPR KO mice. In vitro, annexin binding to peritoneal macrophages was also markedly reduced in FPR KO mice. Finally, evidence of direct annexin 1 binding to murine FPR was obtained with HEK-293 cells transfected with the receptor. Overall, these results indicate a functional role for FPR in the anti-migratory effect of annexin 1 and derived peptides.
The process of leukocyte movement outside the blood vessels is a hallmark of the host inflammatory reaction. Cell migration into inflamed tissues is finely regulated such that several soluble or cell-anchored mediators are involved in promoting this process. These include adhesion molecules, 1,2 and leukocyte activators: they act in concert to induce and promote the time-dependent process of cell rolling, adhesion and emigration (or diapedesis) that characterize the initial steps of cell egress from vessels into tissues. 3 The overall process of cell migration into inflamed tissues must be finely regulated and controlled, such that overshooting may lead to tissue injury and chronic inflammation. 4,5 It is therefore not surprising that a series of check-points are placed at different levels of the path that takes a leukocyte from an environment of rapid flowing blood to the almost static extravascular space. For instance, only a fraction of leukocytes rolling on the inflamed endothelium will become firmly adherent. 6 Similarly, a relevant proportion of adherent cells that are apparently ready to begin the emigration or diapedesis process, actually detach from the endothelium and return to the blood flow. 7,8 Therefore, endogenous mediators able to counteract the action of the pro-inflammatory mediators exist and operate at different phases of the leukocyte extravasation process. These include adenosine, nitric oxide, lipoxin A4, and annexin 1 (ANXA1). 9,10
ANXA1 (previously referred to as lipocortin 1) is a 37-kd member of the annexin superfamily of proteins 11 particularly abundant in neutrophils, where it represents up to 4% of the cytosolic proteins. 12 On adhesion to monolayers of endothelial cells in vitro ANXA1 is externalized onto the cell surface of neutrophils with the function to down-regulate cell emigration through the endothelial cells. 13 Several laboratories, including our own, have demonstrated the anti-migratory action of exogenous and, more importantly, endogenous ANXA1 both in acute 14 and chronic 15 models of inflammation. The anti-migratory property of the full-length protein is retained by peptides drawn from the N-terminus region, such as peptide Ac2-26. 16 The target of endogenous ANXA1 and exogenously administered ANXA1 or peptides seems to be the adherent leukocyte: the net result of their action is leukocyte detachment from the vessel wall rejoining the blood stream. 8,17
The cellular mechanism for the anti-migratory action displayed by ANXA1 and its mimetic has been until recently elusive. In a recent in vitro study, Walther and colleagues 18 reported the existence of a functional interaction between ANXA1-derived peptides and the receptor for formylated peptides (FPR) on human neutrophils, as measured with calcium flux assay and L-selectin shedding. FPR belongs to the group of seven transmembrane domain G-protein-linked receptors, and it is activated by formylated peptides: the downstream effect is neutrophil or monocyte/macrophage activation. 19,20 Importantly, FPR is relatively up-stream of several other receptors for leukocyte activators, and in vitro FPR activation can cause their rapid desensitization. 21 Peptide Ac2-26 did not compete with FMLP, however FPR antagonists block its in vitro effects. 18 In the present study we have addressed the question of FPR involvement in the inhibitory action of ANXA1-derived peptides on the process of neutrophil extravasation in vivo. FPR knock-out (KO) mice 22 were used to test the efficacy and binding capacity also of full-length ANXA1 protein.
Materials and Methods
Animals and Model of Inflammation
FPR KO mice, recently described, 22 have been back-crossed with C57BL/6 for six generations, and C57BL/6 mice (purchased from Banton and Kingsman, Hull, UK) were used as wild-type controls in the experiments. All animals were fed on a standard chow pellet diet with free access to water, maintained on 12-hour light/dark cycles, and housed for 1 week before experimentation. Animal work was performed according to Home Office regulations (guidance on the operation on animals was from the Scientific Procedures act 1986).
Zymosan peritonitis was induced as previously reported. 23 Briefly, mice were injected intraperitoneally with zymosan A (1 mg in 0.5 ml of sterile saline). Animals were sacrificed 4 hours later by carbon-dioxide exposure and peritoneal cavities were lavaged with 3 ml of phosphate-buffered saline (PBS) containing 3 mmol/L ethylenediaminetetraacetic acid. Aliquots of the lavage fluid were then stained with Turk’s solution (0.01% crystal violet in 3% acetic acid) and differential cell counts performed using a Neubauer hemocytometer and a light microscope (Olympus B061, JENCONS-PLS, Leighton Buzzard, UK).
Reagents
Human recombinant ANXA1, peptide Ac2-26 (Ac-AMVSEFLKQAWFIENEEQEYVQTVK) or Ac2-12 (Ac-AMVSEFLKQAW) were obtained as described. 17 The doses used were chosen from a previous study. 17 The FPR antagonists Boc1 (N-t-butoxycarbonyl-MLP) and Boc2 (N-t-butoxycarbonyl-PLPLP) 18 and other materials were obtained from Sigma-Aldrich Co. (Poole, UK). In vivo, compounds were administered intravenously 15 minutes before zymosan.
ANXA1 Binding
In Vitro
Mice received 20 μg of ANXA1 intravenously at time 0, and were bled by cardiac puncture 5 minutes later. ANXA1 bound on the cell surface was measured using a whole blood staining protocol using 10 μg/ml of monoclonal antibody (mAb) 1B. 24-26 Flow cytometry analysis allowed the identification of the monocyte and polymorphonuclear cell population, and the measurement of fluorescence intensity (green channel) associated with either population.
In Vivo
Because radiolabeling protocols cause ANXA1 degradation, we developed an indirect method to assess ANXA1 binding to leukocytes. 25 An estimation of binding affinity was made using a flow cytometric approximation of Scatchard analysis, in which free ANXA1 is calculated from total ANXA1 added to each tube less the amount bound to the cells. 26 The protocol used has already been described. 25 The macrophage population was identified by flow cytometry for the higher values in forward and side scatter characteristics. 23 HEK 293 cells expressing mouse FPR have already been fully characterized for their response to FMLP. 27,28 The ANXA1 binding assay was performed as described above, and the effect of mouse FPR expression on the binding capacity displayed by the cells was determined.
Statistical Analysis
Comparisons between groups were made using one-way analysis of variance followed by Bonferroni posthoc test. A P value <0.05 was considered significant.
Results
Effect of FPR Antagonism or FPR Deficiency on the Anti-Migratory Actions of ANXA1 and ANXA1-Derived Peptides
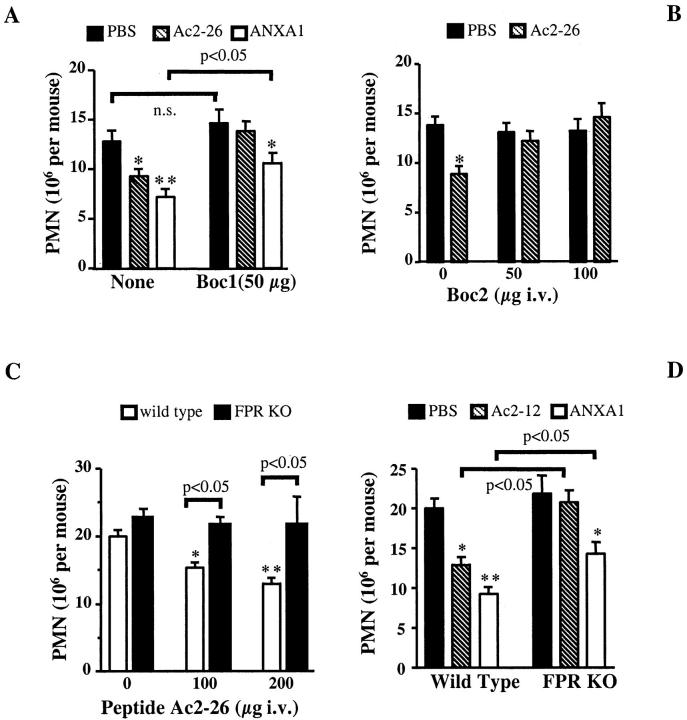
Figure 1A ▶ shows that the intense 4 hours polymorphonuclear leukocyte (PMN) peritoneal infiltration induced by zymosan was inhibited by peptide Ac2-26 and full-length ANXA1, as previously reported. 16 Co-injection of the FPR antagonist Boc1 (50 μg) abrogated the inhibition exerted by peptide Ac2-26, and significantly attenuated that afforded by ANXA1 (Figure 1A) ▶ . The FPR antagonist Boc2 was as active as Boc1 on peptide Ac2-26 (Figure 2A) ▶ .
Figure 1.
Analysis of ANXA1 and derived peptides anti-migratory activity after treatment with FPR antagonists or in FPR KO mice. A: Mice were treated intravenously with 200 μg of peptide Ac2-26, 10 μg of ANXA1, or 100 μl of PBS, alone or together with 50 μg of Boc1, 15 minutes before zymosan. Data are means ± SEM of 10 to 12 mice per group. B: Mice received peptide Ac2-26 (200 μg i.v.) or PBS (100 μl) alone or with the reported doses of Boc2. Data are mean ± SEM of six mice per group. Wild-type or FPR KO mice were treated intravenously with the reported doses of peptide Ac2-26 (C), or with 100 μg of peptide Ac2-12 or 10 μg of ANXA1 (D). Controls received PBS alone. In all cases, after 15 minutes mouse peritoneal cavities were inflamed with zymosan (1 mg) and cell influx was quantified 4 hours later. *, P < 0.05; **, P < 0.01 versus respective PBS group.
Figure 2.
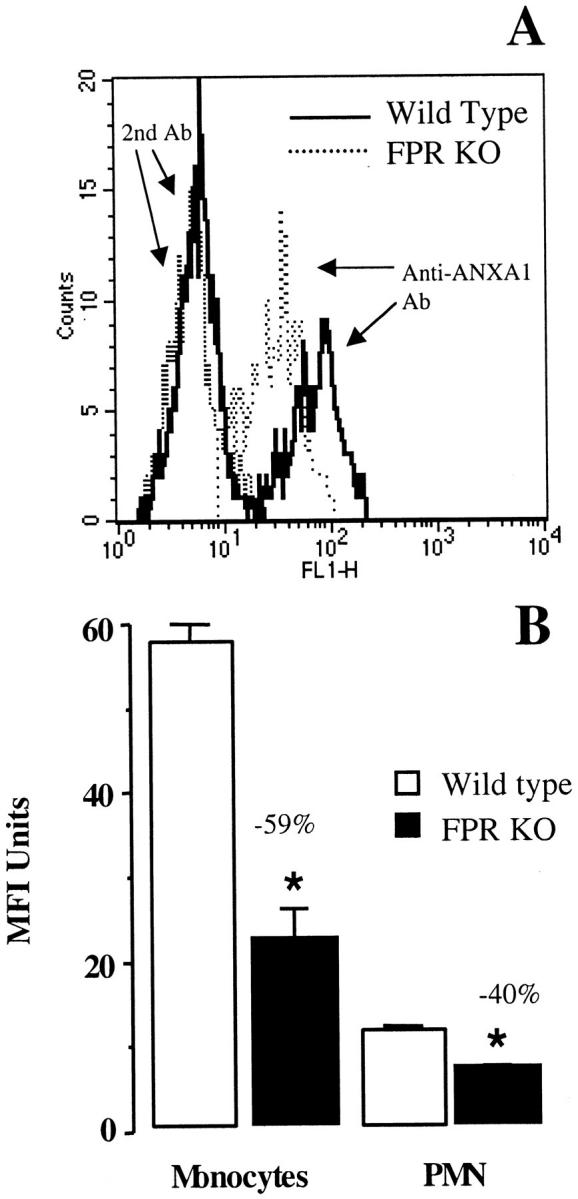
In vivo ANXA1 binding to circulating leukocytes. A: Representative histograms showing monocyte-associated fluorescence as detected 5 minutes after intravenous treatment with 20 μg of ANXA1 in wild-type and FPR KO mice. Fluorescence because of the second antibody alone is also shown. B: As in A, but data are means ± SEM of four distinct mice. Results obtained for the PMN population are also shown. No binding to lymphocytes was detected (not shown). *, P < 0.05 versus respective wild-type group.
Whereas there was no difference in the extent of PMN accumulation into the inflamed peritoneal cavities of wild-type and FPR KO mice, the dose-dependent inhibition produced by peptide Ac2-26 was diminished in FPR KO mice (Figure 1C) ▶ . In this set of experiments, the effect of the shorter peptide Ac2-12 was also tested. Both Ac2-12 and full-length ANXA1 significantly reduced PMN influx in wild-type mice: the shorter peptide was no longer active in FPR KO mice, whereas the inhibition afforded by ANXA1 was again reduced (Figure 1D) ▶ .
ANXA1 Binding to Circulating Leukocytes
The existence of saturable binding sites for ANXA1 on human 29 and mouse 30 leukocytes has long been known, however the nature of these sites has so far remained elusive. 25 ANXA1 injection into the tail vein of wild-type mice resulted in a detectable binding to circulating monocytes and PMN, as measured ex vivo (Figure 2, A and B) ▶ . There was a marked and significant reduction (40 to 60%) in the extent of binding measured after intravenous injection in FPR KO mice. Figure 2A ▶ shows a representative histogram with ANXA1 bound on circulating monocytes, whereas Figure 2B ▶ shows the cumulative data for four distinct mice.
ANXA1 Binding to Mouse FPR
Because residual binding and anti-migratory activity were displayed by ANXA1 in FPR KO mice, binding studies were performed in vitro on peritoneal macrophages. Figure 3A ▶ confirms the existence of a saturable binding to these cells, 30 and shows a pronounced reduction in cells taken from FPR KO mice. Nonetheless residual-binding activity is present. Out of four distinct experiments, approximate kd 110 ± 20 nmol/L and 250 ± 20 nmol/L could be calculated in wild-type and KO mice. Finally, we sought direct evidence for ANXA1 interaction with mouse FPR. Importantly, HEK-293 cells transfected with mouse FPR displayed higher binding capacity for the protein than control cells (Figure 3B) ▶ .
Figure 3.
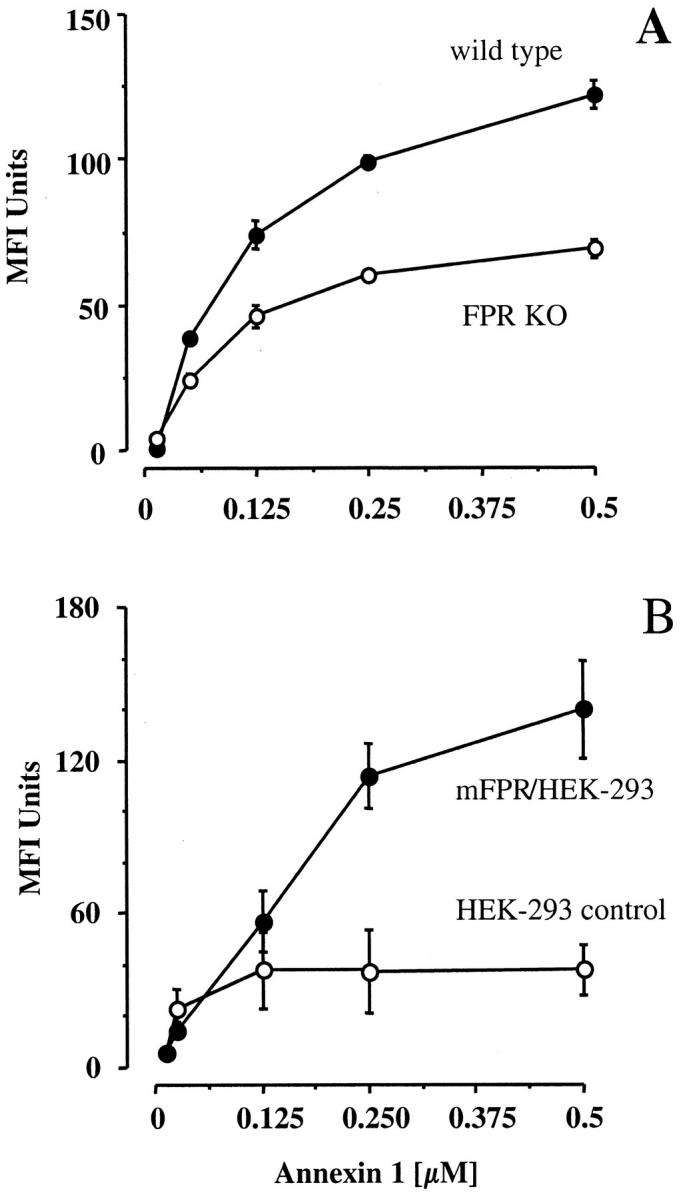
Characterization of ANXA1 binding in vitro. A: Reported is a saturation concentration-response curve for ANXA1 binding to peritoneal MØ collected from wild-type or FPR KO mice (pool of four animals), as determined in vitro. Data are means ± SEM of triplicate determinations, and are representative of three distinct experiments. B: ANXA1 binding to 293 control cells and to 293 cells transfected with mouse FPR is shown. Data are from three experiments performed in duplicate.
Discussion
The glucocorticoid-inducible protein ANXA1 is an endogenous mediator able to down-regulate the process of leukocyte extravasation, and therefore it is one of the mediators responsible for anti-inflammation. 9,10 Identification of the molecular mechanism(s) responsible for ANXA1 anti-migratory activity may lead to the identification and design of novel anti-inflammatory therapeutics. In our previous studies we have identified in the ANXA1 N-terminus as the region retaining this property. 9,16 It has recently been shown that ANXA1-derived N-terminus peptides activate FPR in in vitro settings. 18 In the present study we demonstrate that FPR is functionally linked to the in vivo anti-migratory actions displayed not only by ANXA1-derived peptides, but also in the actions of the full-length protein.
This study started by testing the effect of two FPR antagonists in an in vivo model of PMN extravasation. Both Boc1 and Boc2 exerted a potent antagonism on the anti-migratory action displayed by peptide Ac2-26. Although these antagonists are relatively selective for FPR (in vitro they do not alter the effect of platelet-activating factor on human and mouse neutrophils; data not shown), this finding was validated by using FPR KO mice. These mice are more susceptible to Listeria monocytogenes infection than wild-type controls, and succumb at a higher rate after bacterial infection. In addition, their acute inflammatory reaction to thioglycollate is not different from that of control animals. 22 Here, the response to peritoneal zymosan injection was not different between wild-type and FPR KO mice. Overall these data provide in vivo relevance to the finding of Walther and colleagues, 18 who tested peptide Ac2-26 on human neutrophils and HeLa cells transfected with human FPR. However, in vitro the activity was retained by the region spanning amino acids 19 to 25 (EYVQTVK). In addition, data for full-length ANXA1 were not shown. 18 In our hands, peptide Ac2-12 (given at a dose equimolar to 200 μg of peptide Ac2-26) was able to inhibit (∼40%) neutrophil extravasation, and a more pronounced effect was measured with recombinant ANXA1. Both these responses were significantly altered in FPR KO mice, with a significant residual activity being measured for ANXA1. The efficacy of the shorter peptide validated our previous study in which direct observation of mesenteric microvessels was conducted. 17 Future studies are required to address this discrepancy on the sequence of the ANXA1 N-terminus required for FPR activation.
The fact that ANXA1 retained some degree of efficacy in Boc-treated and FPR KO mice clearly indicated the existence of another receptor or mechanism of action. This may not be surprising in view of the length of ANXA1 (346 amino acids; molecular weight, 37 kd). 31 The existence of specific and saturable ANXA1 binding sites on human monocytes and neutrophils was originally described by Goulding and colleagues 25 reporting the existence of a binding protein with an apparent molecular weight of 15 kd. ANXA1 binding to mouse circulating leukocytes and peritoneal macrophages has also been characterized. 16 To test the hypothesis formulated above we performed ANXA1 binding assay to leukocytes of FPR KO mice. In vivo administration of ANXA1 resulted in a detectable binding to peripheral circulating leukocytes. As expected, 26 the highest binding was observed to monocytes with a lower but still above basal binding to circulating neutrophils. No binding to lymphocytes was measured (data not shown). A significant reduction in ANXA1 binding was measured on circulating monocytes and neutrophils of FPR KO mice. These data are of importance as they link ANXA1 binding to FPR in vivo to the anti-migratory action of the protein. Importantly, no changes in neutrophil response to the chemokine macrophage inflammatory protein-1α 22 or to the FPR2 agonist serum amyloid protein type A 27,28 have been reported in FPR KO mice, suggesting (in absence of specific antibodies) that at least the receptors used by these chemoattractants are not altered by deletion of the mouse FPR gene.
The apparently modest binding to PMN is expected because adhesion to endothelial cells augments ANXA1 binding capacity to this leukocyte type. 26 Importantly FPR can be exported on the neutrophil cell surface on adhesion because it is contained in few cytoplasmic granules and vesicles. 32 Because ANXA1 is predominantly contained in gelatinase granules, 33 we propose that PMN adhesion to the endothelium triggers externalization of ANXA1 and FPR, and therefore activates the entire pathway. The in vivo data here presented suggest that interaction with FPR is the mechanism responsible for the leukocyte detachment phenomenon promoted by endogenous and exogenous ANXA1, and peptides Ac2-26 and Ac2-12. 8,17
In conclusion, this study provides the first in vivo evidence that FPR is functionally involved in the in vivo inhibitory effects of ANXA1 and derived peptido-mimetics. We also report for the first time reduced ANXA1 binding in the absence of a specific receptor. On the other hand, strong indication that other receptor(s) and/or mechanism(s) must be involved in the action of full-length ANXA1 is also provided. For instance direct interaction between ANXA1 and very late antigen-4 on a human monocytic cell line has recently been demonstrated. 34 In view of the lack of direct competition between peptide Ac2-26 and formyl-Met-Leu-Phe in vitro binding assays, 18 and the fact that formyl-Met-Leu-Phe and ANXA1 are clearly able to produce opposite effects in inflammation, it is likely that we have so far only scraped the surface of a complex and fascinating biological system.
Acknowledgments
We thank Dr. N. J. Goulding for semiquantitative analysis of the annexin 1 binding, Mr. Richard Thompson for expert help in maintaining the FPR KO colony, and Dr. Q. Choudhury for maintaining the HEK-293 cell cultures.
Footnotes
Address reprint requests to Dr. Mauro Perretti, The William Harvey Research Institute, Division of Pharmacology, St. Bartholomew’s and The Royal London SMD, Charterhouse Square, London EC1M 6BQ, United Kingdom. E-mail: m.perretti@qmw.ac.uk.
Supported by the Arthritis Research Campaign United Kingdom (fellowship P0569 to M. P.).
References
- 1.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994, 76:301-314 [DOI] [PubMed] [Google Scholar]
- 2.Cronstein BN, Weissmann G: The adhesion molecules of inflammation. Arthritis Rheum 1993, 36:147-157 [DOI] [PubMed] [Google Scholar]
- 3.Panés J, Granger DN: Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology 1998, 114:1066-1090 [DOI] [PubMed] [Google Scholar]
- 4.Edwards SW, Hallet MB: Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today 1997, 18:320-324 [DOI] [PubMed] [Google Scholar]
- 5.Epstein FH: Tissue destruction by neutrophils. N Engl J Med 1989, 320:365-376 [DOI] [PubMed] [Google Scholar]
- 6.Von Andrian UH, Hansell P, Chambers JD, Berger EM, Filho IT, Butcher EC, Arfors K-E: L-selectin function is required for β2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am J Physiol 1992, 263:H1034-H1044 [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka K, Katori M: Detachment of polymorphonuclear leukocytes adhered on venular endothelial cells. Microcirc Ann 1992, 8:129-130 [Google Scholar]
- 8.Mancuso F, Flower RJ, Perretti M: Leukocyte transmigration, but not rolling or adhesion, is selectively inhibited by dexamethasone in the hamster post-capillary venule. Involvement of endogenous lipocortin 1. J Immunol 1995, 155:377-386 [PubMed] [Google Scholar]
- 9.Perretti M: Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci 1997, 18:418-425 [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Takano T, Chiang N, Gronert K, Clish CB: Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis. Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am J Respir Crit Care Med 2000, 161:S95-S101 [DOI] [PubMed] [Google Scholar]
- 11.Raynal P, Pollard HB: Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta 1994, 1197:63-93 [DOI] [PubMed] [Google Scholar]
- 12.Rosales JL, Ernst JD: Calcium-dependent neutrophil secretion: characterization and regulation by annexins. J Immunol 1997, 159:6195-6202 [PubMed] [Google Scholar]
- 13.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ: Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med 1996, 22:1259-1262 [DOI] [PubMed] [Google Scholar]
- 14.Perretti M, Flower RJ: Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol 1993, 150:992-999 [PubMed] [Google Scholar]
- 15.Yang Y, Hutchinson P, Morand EF: Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum 1999, 42:1538-1544 [DOI] [PubMed] [Google Scholar]
- 16.Perretti M, Ahluwalia A, Harris JG, Goulding NJ, Flower RJ: Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse: a qualitative comparison with an anti-CD11b monoclonal antibody. J Immunol 1993, 151:4306-4314 [PubMed] [Google Scholar]
- 17.Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M: Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci USA 1998, 95:14535-14539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther A, Riehemann K, Gerke V: A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell 2000, 5:831-840 [DOI] [PubMed] [Google Scholar]
- 19.Lala A, Sojar HT, De Nardin E: Expression and purification of recombinant human N-formyl-L-leucyl-L-phenylalanine (FMLP) receptor: generation of polyclonal antibody against FMLP receptor. Biochem Pharmacol 1997, 54:381-390 [DOI] [PubMed] [Google Scholar]
- 20.Prossnitz ER, Ye RD: The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther 1997, 74:73-102 [DOI] [PubMed] [Google Scholar]
- 21.Ali H, Richardson RM, Haribabu B, Snyderman R: Chemoattractant receptor cross-desensitization. J Biol Chem 1999, 274:6027-6030 [DOI] [PubMed] [Google Scholar]
- 22.Gao JL, Lee EJ, Murphy PM: Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med 1999, 189:657-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Getting SJ, Flower RJ, Perretti M: Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol 1997, 120:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepinsky RB, Sinclair LK, Dougas I, Liang C-M, Lawton P, Browning JL: Monoclonal antibodies to lipocortin-1 as probes for biological functions. FEBS Lett 1990, 261:247-252 [DOI] [PubMed] [Google Scholar]
- 25.Goulding NJ, Pan L, Wardwell K, Guyre VC, Guyre PM: Evidence for specific annexin I-binding proteins on human monocytes. Biochem J 1996, 316:593-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Euzger H, Flower RJ, Goulding NJ, Perretti M: Differential modulation of annexin I binding sites on monocytes and neutrophils. Med Inflamm 1999, 8:53-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartt JK, Barish G, Murphy PM, Gao JL: N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med 1999, 190:741-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang TS, Wang JM, Murphy PM, Gao JL: Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem Biophys Res Commun 2000, 270:331-335 [DOI] [PubMed] [Google Scholar]
- 29.Goulding NJ, Luying P, Guyre PM: Characteristics of lipocortin 1 binding to the surface of human peripheral blood leucocytes. Biochem Soc Trans 1990, 18:1237-1238 [DOI] [PubMed] [Google Scholar]
- 30.Perretti M, Flower RJ, Goulding NJ: The ability of murine leukocytes to bind lipocortin 1 is lost during acute inflammation. Biochem Biophys Res Commun 1993, 192:345-350 [DOI] [PubMed] [Google Scholar]
- 31.Wallner BP, Mattaliano RJ, Hession C, Cate RL, Tizard R, Sinclair LK, Foeller C, Chow EP, Browning JL, Ramachandran KL, Pepinsky RB: Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature 1986, 320:77-81 [DOI] [PubMed] [Google Scholar]
- 32.Sengeløv H, Boulay F, Kjeldsen L, Borregaard N: Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J 1994, 299:473-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ, Goulding NJ: Annexin I is stored within gelatinase granules of human neutrophils and mobilised on the cell surface upon adhesion but not phagocytosis. Cell Biol Int 2000, 24:163-174 [DOI] [PubMed] [Google Scholar]
- 34.Solito E, Romero IA, Marullo S, Russo-Marie F, Weksler BB: Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the α4β1 integrin. J Immunol 2000, 165:1573-1581 [DOI] [PubMed] [Google Scholar]