Abstract
Matrix metalloproteases (MMPs) are a group of zinc-dependent endopeptidases that can degrade every component of the extracellular matrix. Under normal circumstances, the levels of MMPs are tightly regulated at both transcriptional and posttranscriptional levels. However, they are up-regulated in pathological states such as inflammation. Previous investigations have suggested that MMP-12 (metalloelastase) may be an important mediator in the pathogenesis of chronic lung injury. In this study we investigated the role of metalloelastase in the pathogenesis of acute lung injury using mice containing a targeted disruption of the metalloelastase gene. Neutrophil influx into the alveolar space in metalloelastase-deficient animals was reduced to ∼50% of that observed in parent strain mice following the induction of injury by immune complexes. In addition, lung permeability in metalloelastase-deficient mice was ∼50% of that of injured parent strain animals with normal levels of metalloelastase and this was correlated with histological evidence of less lung injury in the metalloelastase-deficient animals. Collectively, the data suggest that metalloelastase is necessary for the full development of acute alveolitis in this model of lung injury. Further, the data suggest that reduced injury in metalloelastase-deficient mice is due in part to decreased neutrophil influx into the alveolar space.
The matrix metalloproteinases (MMPs) are a group of zinc-dependent proteases that are active at physiological pH and collectively have the ability to degrade every component of the extracellular matrix. Overexpression of MMPs has been implicated in the pathogenesis of several diseases, such as bullous pemphigoid, 1 cancer, 2,3 emphysema, 4 acute respiratory distress syndrome, 5 and asthma. 6 The mechanisms of action and the roles of individual MMPs in the pathophysiology of these and other conditions have yet to be clearly defined due to a lack of selective metalloproteinase inhibitors. Tissue inhibitors of metalloproteinases (TIMPs) have been used which inhibit all MMP activities, and studies with TIMPs suggest a role for MMPs (as a group) in the pathophysiology of acute lung inflammation. 7 Recently, mice with genetic deletions of individual MMPs have been developed. It is hoped that the use of these animals will facilitate the understanding of the roles of individual MMPs in disease pathophysiology.
Murine metalloelastase is a 22-kd (active form) member of the MMP family. 8,9 This enzyme, the primary elastase enzyme of the macrophage, can hydrolyze a broad spectrum of extracellular matrix and has been shown to be necessary for macrophage-mediated proteolysis of the extracellular matrix during invasion. 10 Previous studies have also suggested that metalloelastase may be necessary for disease development in a murine model of emphysema, 11 and it has been implicated in the pathogenesis of human abdominal aortic aneurysms and rabbit aortic lesion development. 12 The engineering of a mouse strain deficient in metalloelastase facilitated the investigation of the specific role of this MMP in an acute immune complex-mediated murine model of lung injury. Because previous studies suggested a role for metalloelastase in chronic inflammatory injury, we used metalloelastase-deficient mice to explore what role this enzyme may play in a model of acute lung injury induced by immune complexes. Because other MMPs, such as gelatinase B, are also up-regulated in acute lung injury 7 and no selective metalloelastase inhibitors are available, the use of these metalloelastase-deficient mice allows for the precise delineation of the role of metalloelastase in the complex pathophysiology of the acute lung injury process.
Materials and Methods
Metalloelastase-Deficient Mice
Mice possessing a homozygous deletion of exons 1 and 2 of metalloelastase 9,10 were kindly provided by Dr. Steven Shapiro of the Departments of Medicine and Cell Biology at the Washington University School of Medicine (St. Louis, MO). They were bred and maintained on a 129SvEv background. Mice used in these studies were 5 to 9 weeks of age and housed under specific pathogen-free conditions.
IgG Lung Injury Model
All procedures involving animals were performed following review and approval by the University Committee on Use and Care of Animals (University of Michigan, Ann Arbor, MI). The immune complex-induced lung injury model is described in recent publications. 7,13 Mice were anesthetized with a mixture (9:1 v/v) of ketamine and xylazine (Fort Dodge Labs, Fort Dodge, IA). Tracheotomies were performed and 60 μl of a 5 mg/ml rabbit anti-bovine serum albumin (BSA) antibody solution was instilled into the lungs. In addition, 100 μl of a 2.5 mg/ml BSA solution was introduced intravenously. Animals were sacrificed after 4 hours by interperitoneal injection of 2 mg/kg ketamine. They were then exsanguinated and their thoracic cavities opened to expose the lungs and trachea. A Leur-lok syringe with a 21-gauge needle was then fed into a tracheal incision and secured with a suture, and lungs were initially lavaged with 0.7 ml of sterile saline. The retrieved bronchoalveolar lavage (BAL) fluid from this lavage was centrifuged to remove cells, subsequently used for zymograms, and the protein quantified for permeability analysis. The lungs were then repeatedly lavaged with a total of 2 ml of sterile phosphate-buffered saline (PBS). Cell pellets obtained from all lavages were resuspended in PBS (pH 7.5) and counted using a hemocytometer.
Western Blot Analysis
A rabbit polyclonal antibody raised against the catalytic region of metalloelastase (Dr. Steven Shapiro, Washington University School of Medicine) was used to probe a blot containing proteins from 30 μl of murine macrophage culture fluid from saline treated 129SvEv parent strain and metalloelastase-deficient mice. The blot was blocked by incubating it in 1 × T-TBS [Tween-Tris buffered saline; 1.5 mol/L NaCl, 10 mmol/L Tris, 1% Tween 20, 5% (w/v) nonfat dried milk]. It was then exposed to a polyclonal antibody raised against the catalytic region (encoded by exons 1 and 2) of metalloelastase-12 for 1 hour. The blot was then washed three times in Tris-buffered saline for 10 minutes each time and then incubated with a goat anti-rabbit IgG antibody coupled to horseradish peroxidase (Sigma Chemical Co., St. Louis, MO.) in T-TBS. Autoradiography was facilitated by using Phototope (New England Biolabs, Beverley, MA) reagents following the manufacturer’s protocol.
Substrate-Embedded Enzymography
SDS-PAGE substrate-embedded enzymography (zymography) was used to assess gelatinase A (MMP-2) and gelatinase B (MMP-9) activities. 14 Briefly, SDS-PAGE gels were prepared from 30:1 acrylamide:bis, with the incorporation of gelatin (1 mg/ml) before casting. The gelatin gels were routinely 7.5% acrylamide, with the final concentrations of the other components of the gels as follows: 325 mmol/L Tris-HCl (pH 8.8), 0.1% SDS, 0.05% ammonium persulfate, and 0.05% TEMED. Denatured but non-reduced samples and standards were then electrophoresed at constant voltage of 150 V in an ice bath under non-reducing conditions. Gels were removed and subjected to the following wash protocol: twice for 15 minutes in 50 mmol/L of Tris buffer (pH 7.4) containing 1 mmol/L Ca2+ and 0.5 mmol/L Zn2+ with 2.5% Triton X-100; and once for 5 minutes in Tris buffer alone. Gels were incubated overnight in Tris buffer with 1% Triton X-100 and stained with Coomassie brilliant blue 250-R. Zones of enzyme activity were scanned and quantitated. A volume of 25 μl of undiluted specimen (BAL fluid) was used for this assay.
Permeability Measurement
After injury, BAL fluids (described above) from parent strain and metalloelastase gene-deleted mice were used to assess lung vasculature permeability. Total protein content was assayed by BCA protein assay. Briefly, samples were diluted and BAL protein levels were determined for injured and control animals using the BCA protein assay (Pierce, Rockford, IL) and compared to a BSA standard curve. Protein concentration was determined using a BSA standard curve.
Histology
Four hours after induction of the lung injury, mice were sacrificed and the lungs, heart, and associated vasculature were removed en bloc. The lungs were inflated with 4% paraformaldehyde, embedded in paraffin, and 5-μm sections were mounted and stained with hematoxylin and eosin.
Statistical Analysis
Significance was determined using Students’ t-test with a threshold of 0.05. The sample size used in experiments was a minimum of 5 animals per group per experiment with at least 10 animals used for each parameter tested.
Results
Metalloelastase Is Not Present in the Metalloelastase Gene-Deleted Mice
To confirm the presence of metalloelastase in the parent strain and its absence in metalloelastase-deficient animals, Western blotting was performed on supernatant from peritoneal macrophages stimulated with LPS and interferon-γ. A 22-kd protein corresponding to the reported molecular weight of fully processed metalloelastase was detected by Western blot using an antibody against the active site of murine metalloelastase in the parent strain wild-type animals. As expected, this band was absent in metalloelastase-deficient mice, indicating that these mice do not produce functional metalloelastase (data not shown).
Immune Complex-Induced Lung Injury in Wild-Type and Metalloelastase-Deficient Mice
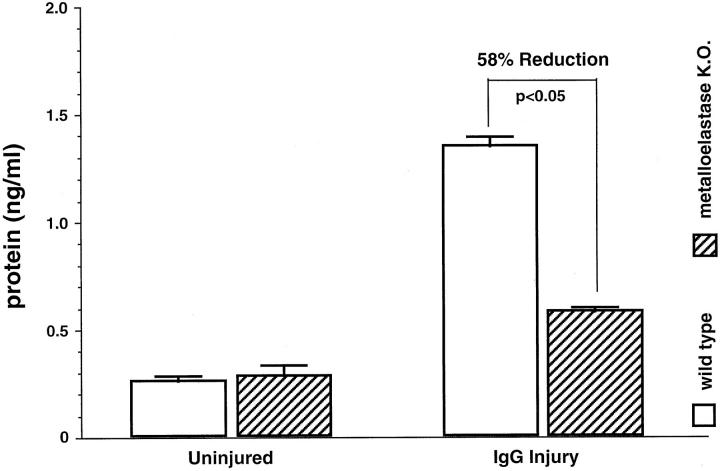
Acute lung injury was induced in the mice by IgG immune complexes, and the degree of lung injury was assessed by lung permeability changes, BAL leukocyte counts, and histological evidence of injury. Vascular permeability is a consistent marker of injury in this and several other models of lung injury. 15 After treatment for the prescribed time period, the animals were sacrificed and vascular permeability was assessed in BAL fluid by measuring total protein content. As Figure 1 ▶ shows, BAL fluid from uninjured parent strain mice and metalloelastase-deficient mice were similarly low in BAL protein. In contrast, BAL fluid protein levels from injured parent strain wild-type mice showed a marked increase in protein content (1.36 mg/ml) as compared to the saline treated parent strain group. BAL fluid from immune complex-injured metalloelastase gene-deleted mice had a 58% reduction in the quantity of protein (0.58 mg/ml versus 1.36 mg/ml), (P ≪0.05), in the BAL as compared to the injured wild-type mice. Thus, metalloelastase-deficient mice exhibit lower vascular permeability than the corresponding parent strain mice following formation of immune complexes in the alveolar wall.
Figure 1.
BAL protein quantitation in parent strain versus metalloelastase-deficient mice. BAL fluid was assessed for total protein four hours after injury. n = 5 for parent strain group and n = 5 for metalloelastase-deficient mice.
Histological Examination of Injured Lungs
Histological examination of lungs from wild-type and metalloelastase-deficient mice was performed to qualitatively assess the extent of injury and the infiltration of leukocytes into the alveolar space. As Figure 2 ▶ illustrates, lungs from immune complex-injured wild-type animals showed an influx of neutrophils, along with evidence of significant injury with some intracellular hemorrhage and fibrin deposition. In contrast to the injured parent strain mice, lungs from metalloelastase-deficient animals had less injury with a much smaller degree of leukocyte influx, suggesting that the reduced injury in these animals is associated with a reduction of neutrophil recruitment into the alveoli.
Figure 2.

Representative histological alterations in the lungs of parent strain and metalloelastase-deficient animals injured by IgG immune complexes. A: The injured lungs of the parent strain mice show widespread alveolitis with neutrophilic infiltrates and some intraalveolar hemorrhage. B: The high power inset illustrates the presence of numerous neutrophils (arrows). C: The injured lungs of the metalloelastase-deficient animals show much less injury with less neutrophils. D: The high power inset shows rare neutrophils (arrow) with less injury than the parent strain mice. (hematoxylin and eosin; A and C, ×40; B and D, ×100).
Inflammatory Cell Infiltration into Metalloelastase-Deficient Mouse Lungs
To further assess the protective effect of metalloelastase deletion, we quantitated the numbers of BAL leukocytes infiltrating the lungs of parent strain and metalloelastase gene-deleted mice. As Figure 3 ▶ shows, both saline treated parent strain and saline treated metalloelastase-deficient mice exhibited minimal neutrophil influx. In contrast, significant neutrophil influx was observed in both parent strain and metalloelastase-deficient gene-deleted mice following immune complex injury, with the influx observed in the metalloelastase-deficient group being ∼50% of that of the injured parent strain group (P < 0.05). The metalloelastase-deficient mice exhibited neutrophil counts between that of the saline treated metalloelastase-deficient and the immune complex-treated parent strain mice, which correlated with the vascular permeability data. In contrast to the data with neutrophils, there was no significant difference in BAL macrophage counts between saline treated control animals and those that were injured (Figure 3B) ▶ .
Figure 3.
Leukocyte counts from the BAL fluid of metalloelastase-deficient and parent strain mice. A: Neutrophils. B: Macrophages. (n = 5 for both the control group and the metalloelastase-deficient group).
Gelatinase A and Gelatinase B Cannot Compensate for the Absence of Metalloelastase
To assess any compensatory effects of other MMPs in the metalloelastase-deficient animals, we examined gelatinase A and gelatinase B levels in the BAL fluid from control and injured knockout mice. The rationale for the analysis was based on our previous studies and those of others that have shown consistent up-regulation of gelatinase activates in inflammation including acute lung injury. 1,7 As shown in Figure 4 ▶ , gelatinase B levels in both saline-treated control and saline-treated, metalloelastase-deficient animals were similarly low. Gelatinase B levels increased significantly following injury (in both control and metalloelastase-deficient animals), and there was a modest increase in gelatinase A. The gelatinase levels in the injured knockout animals were actually slightly lower than that found in the parent strain, suggesting that gelatinase B does not play a compensatory role in the reduced lung injury elaborated in metalloelastase-deficient animals. Similarly, the levels of gelatinase A in the BAL fluid from injured control mice and gene-deleted mice were the same. Thus, neither gelatinase A or B seem to have the ability to compensate for the lack of metalloelastase in this model of acute alveolitis.
Figure 4.

Measurement of MMP-9 and MMP-2 levels in BAL. A: MMP-9, B: MMP-2, for control groups (n = 5) and metalloelastase-deficient groups (n = 5). Activity was assessed by gelatin zymography, quantitated by scanning densitometry of dried gels and averaged. Fold difference was calculated by dividing the average densitometry values of both negative group and the injured group value by the average activity of the negative group.
Discussion
MMPs, including metalloelastase, have long been suspected of playing a role in the pathogenesis of tissue injury seen in inflammation including lung injury. 4-7 However, the lack of selective inhibitors of the MMP activities in vivo has hampered the determination of the phlogistic role of the individual MMPs. Using mice selectively depleted of MMPs, such as metalloelastase or gelatinase B, has allowed for such an analysis. Using mice selectively depleted of metalloelastase, we found in this study that metalloelastase appears to be playing a role in the tissue injury observed in a model of acute lung injury. Previous studies have demonstrated a role for metalloelastase in the pathogenesis of a model of emphysema, 11 but this study is the first to implicate this enzyme in acute lung injury as well.
The source of the metalloelastase in these models appears to be the leukocytes, which correlates with our previous studies showing that neutrophils and macrophages appear to be the primary source of the MMPs up-regulated in lung injury, with metalloelastase from macrophages appearing to be the major source of metalloelastase in the lung emphysema model. 9,11,16 In fact, the major elastase activity in the macrophage is metalloelastase, which is up-regulated in cigarette smokers as well as patients with emphysema. 8,11,17
Our previous studies with this acute lung injury model have also identified oxidants as being involved in the initiation of the injury, in part by their up-regulation of the production of inflammatory mediators such as tumor necrosis factor-α, as well as their activation of MMPs, in particular gelatinase B, which is also up-regulated during acute lung injury. 7,16,18 Oxidants such as hydrogen peroxide also can upregulate MMP production through activation of AP-1 and oxidants can also directly activate MMP activities. 19,20 Thus emerging evidence suggests that MMP production is modulated by other inflammatory mediators and that the up-regulation and activation of MMPs in the lung is ultimately responsible for much of the resulting lung injury.
The mechanism of action of the MMPs in the pathogenesis of tissue injury has been primarily focused on their known ability to break down all types of extracellular matrix. The MMPs are felt to play important roles in the normal remodeling of the extracellular matrix. 21 In inflammation, the up-regulation of MMP activities is felt to induce breakdown of the extracellular matrix with the levels of the active MMPs in excess of the levels of the MMPs inhibitors. In fact, there is evidence in lung injury in humans, as well as experimental animals, that this is occurring. For example, in patients with asthma or adult respiratory distress syndrome there is an overproduction of MMPs in the lung as compared to levels of the TIMP MMP inhibitors. 5-6 Experimentally, we have also found that MMP and TIMP levels are both up-regulated in lung injury. 7,10 Finally, in studies with gelatinase B knockout animals we have shown less lung injury than control animals with no reduction in the degree of leukocyte influx, suggesting that gelatinase B is directly affecting the lung extracellular matrix. 13
In addition to their direct effects on extracellular matrix there is also evidence from this study, as well as others, that MMPs are also involved in the recruitment of inflammatory cells into the lung. In this study the metalloelastase-deficient animals had less neutrophil infiltration into the lungs as compared to the wild-type control animals. The study by Shapiro and colleagues 11 also found less macrophage accumulation in the metalloelastase-deficient animals in smoking induced emphysema. Other MMPs such as stromelysin 1 have also been associated with modulation of leukocyte infiltration into inflamed tissues. 13,22
There are a number of possible mechanisms by which MMPs, such as metalloelastase, could affect leukocyte migration. One possible mechanism is that of the modulation of leukocyte chemotactic factors. In our studies with TIMP inhibition of acute lung injury there were reductions in neutrophil recruitment associated with reductions in the levels of the neutrophil chemotactic peptide C5a that is known to play a critical role in the evolution of acute lung injury. 7 Likewise, in the emphysema model described above the reductions in macrophage accumulation in the metalloelastase-deficient mice were associated with reductions in the macrophage chemoattractant MCP-1. 11 This reduction in macrophages also was associated with less elastin and collagen fragments that are also known to be chemotactic. 23 There are also recent studies using a model of vertebral disk reabsorption that found that stromelysin 1 was necessary for the generation of a macrophage chemotactic activity and the subsequent infiltration of the disk tissue by the macrophages. 24
Another possible mechanism of MMP involvement in leukocyte infiltration is the role of MMPs in leukocyte infiltration through vessel walls. MMPs have long been implicated in cell migration that occurs during tissue regeneration, wound healing, and inflammation. In vitro, decreased neutrophil migration through Matrigel-coated filters has been reported in the presence of MMP inhibitors and the gelatinases in particular have been implicated in the transmigration of eosinophils and lymphocytes. 25-27 Metalloelastase has also been identified as a requirement for the infiltration of macrophages in a murine model of inflammation with less infiltration of a Matrigel substrate by macrophages from metalloelastase-deficient mice. 10 Studies are ongoing to determine whether metalloelastase or other MMPs such as the gelatinases are required for neutrophil infiltration.
A third possible mechanism of MMP involvement in lung injury is the modulation of biologically active mediators such as tumor necrosis factor-α and the interleukins. For example, in a model of chronic dermal inflammation, gelatinase B-deficient mice had less injury associated with higher levels of the anti-inflammatory cytokine interleukin-10. 28 Also, MMPs have the ability to shed biologically active molecules such as TNF-α, IL-1 and IL-6 from the surface of macrophages, which, in turn, are known to up-regulate MMP production by both these and parenchymal cells. 18 Thus, it is possible that MMPs, such as metalloelastase, can affect lung injury by altering the levels of pro- and anti-inflammatory cytokines.
In summary, these studies provide evidence that metalloelastase plays a significant role in the pathogenesis of a model of acute lung injury. Extending the observations on the role of metalloelastase in chronic lung injury this study provides additional evidence of an important role for this MMP in lung injury. Furthermore, this study supports the findings in the chronic lung injury model suggesting that metalloelastase plays an important role in leukocyte recruitment into the lung in addition to directly damaging the lung. Dissecting the role that MMPs appear to play in leukocyte recruitment could provide important new information on the mechanisms of this response.
Acknowledgments
We thank Dr. Steve Shapiro of the Washington University School of Medicine for his kind gift of knockout animals and antibodies for MMP Western blots.
Footnotes
Address reprint requests to Kent J. Johnson, M.D., Department of Pathology, The University of Michigan, 1301 Catherine Road, Box 0602, Ann Arbor, MI 48109. E-mail: kjjkjj@umich.edu.
Supported in part by National Institutes of Health grants HL42607 and CA60958 from the United States Public Health Service.
References
- 1.Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior BM: Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med 1988, 188:475-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM: Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 1997, 94:1402-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford HC, Matrisian LM: Tumor and stromal expression of matrix metalloproteinases and their role in tumor progression. Invasion Metastasis 1994, 14:234-245. [PubMed] [Google Scholar]
- 4.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT: Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest 1998, 78:1077-1087 [PubMed] [Google Scholar]
- 5.Pugin J, Verghese G, Widmer MC, Matthay MA: The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 1999, 27:304-312 [DOI] [PubMed] [Google Scholar]
- 6.Mautino G, Oliver N, Chanez P, Bousquest J, Canopy F: Increased release of matrix metalloproteinase 9 in bronchoalveolar lavage fluid and by alveolar macrophages of asthmatics. Am J Respir Cell Mol Biol 1997, 17:583-591 [DOI] [PubMed] [Google Scholar]
- 7.Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, Mueller WT, Johnson KJ, Ward PA: Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 1999, 162:3653-3662 [PubMed] [Google Scholar]
- 8.Banda MJ, Werb Z: Mouse macrophage elastase: purification and characterization as a metalloproteinase. Biochem J 1981, 193:589-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, Senior RM, Ley TJ: Molecular cloning, chromosomal localization and bacterial expression of a murine macrophage metalloelastase. J Biol Chem 1992, 267:4644-4671 [PubMed] [Google Scholar]
- 10.Shipley MJ, Wesselchmidt RL, Kobayashi DK, Ley TJ, Shapiro SD: Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA 1996, 93:3942-3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD: Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277:2002-2004 [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T: Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol fed rabbits. Am J Pathol 1998, 153:109-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner RL, Beltran L, Younkin EM, Lewis CS, Varani J, Johnson KJ: Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 2001, 24:1-8 [DOI] [PubMed] [Google Scholar]
- 14.Gibbs DF, Warner RL, Weiss SJ, Johnson KJ, Varani J: Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol 1999, 20:1136-1144 [DOI] [PubMed] [Google Scholar]
- 15.Schmal H, Shanley TP, Jones ML, Friedl HP, Ward PA: Role of macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol 1996, 156:1963-1972 [PubMed] [Google Scholar]
- 16.Mulligan MS, Desrochers PE, Chinnaiyan AM, Gibbs DF, Johnson KJ, Weiss SJ: In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinase-2. Proc Natl Acad Sci USA 1993, 90:11523-11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI: Neutral metalloproteinases produced by human mononuclear phagocytes: enzyme profile, regulation, and expression during cellular development. J Clin Invest 1990, 86:1496-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro SD, Senior RM: Matrix metalloproteinases: matrix degradation and more. Am J Respir Cell Mol Biol 1999, 20:1100-1102 [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan S, Meng SP, Ramasamy S, Harrison DG, Galis ZS: Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Invest 1996, 98:2572-2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkus R, Weiner LM, Daniel V: Role of oxidants and antioxidants in the induction of AP-1 and NF-kB and glutathione S-transferase gene expression. J Biol Chem 1996, 271:13422-13429 [DOI] [PubMed] [Google Scholar]
- 21.Matrisian LM: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990, 6:121-126 [DOI] [PubMed] [Google Scholar]
- 22.Pilcher BK, Wang M, Qin XJ, Parks WC, Senior RM, Welgus HG: Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann NY Acad Sci 1999, 878:12-24 [DOI] [PubMed] [Google Scholar]
- 23.Senior RM, Griffin GL, Mecham RP: Chemotactic activity of elastin-derived peptides. J Clin Invest 1980, 66:859-862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, Matrisian LM: Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest 2000, 105:133-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Declaux C, Delacourt C, d’Ortho MP, Boyer V, Lafuma C, Harf A: Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol 1996, 14:288-295 [DOI] [PubMed] [Google Scholar]
- 26.Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL: T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 1995, 154:4379-4389 [PubMed] [Google Scholar]
- 27.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM: Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 1997, 17:519-528 [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG: Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA 1999, 96:6885-6889 [DOI] [PMC free article] [PubMed] [Google Scholar]