Abstract
Studying the mechanism(s) of uterine relaxation is important and will be helpful in the prevention of obstetric difficulties such as preterm labour, which remains a major cause of perinatal mortality and morbidity. Multiple signalling pathways regulate the balance between maintaining relative uterine quiescence during gestation, and the transition to the contractile state at the onset of parturition. Elevation of intracellular cyclic AMP promotes myometrial relaxation, and thus quiescence, via effects on multiple intracellular targets including calcium channels, potassium channels and myosin light chain kinase. A complete understanding of cAMP regulatory pathways (synthesis and hydrolysis) would assist in the development of better tocolytics to delay or inhibit preterm labour. Here we review the enzymes involved in cAMP homoeostasis (adenylyl cyclases and phosphodiesterases) and possible myometrial substrates for the cAMP dependent protein kinase. We must emphasise the need to identify novel pharmacological targets in human pregnant myometrium to achieve safe and selective uterine relaxation when this is indicated in preterm labour or other obstetric complications.
Background
Preterm labour is a major reproductive health problem due to a high incidence of severe short- and long-term infant morbidity. In developed countries, social and medical advances have improved the survival rate of the preterm neonate; however, the incidence of preterm birth and related morbidity remain a serious challenge and the physiopathological mechanisms of preterm birth are still a mystery. The use of tocolytic drugs, including those that operate through cyclic AMP such as beta-mimetics, to inhibit uterine contractility in preterm labour is controversial because there is no evidence that currently available drugs improve long term neonatal outcome. Moreover, some tocolytics can cause serious side effects such as tachycardia, hypertension and pulmonary oedema. The potential of drugs of high uterine selectivity, e.g. oxytocin receptor antagonists, for the management of preterm labour is encouraging and further clinical trials are being undertaken. However, there is a need to identify novel pharmacological targets in myometrium to provoke safe and selective uterine relaxation when this is clinically indicated [1].
It is not known whether the cause of preterm labour is the premature loss of uterine quiescence (e.g. removal of inhibitory factors), or the induction of uterine contractility (e.g. release of stimulatory mediators) or a combination of both [2]. The second messenger cyclic adenosine monophosphate (cAMP) is known to promote the relaxation of smooth muscle, and is likely to be implicated in the maintenance of uterine quiescence [2]. Hence, the study of myometrial cAMP regulatory pathways will help to understand the mechanism of labour and highlight possible targets for the development of more specific and effective tocolytics for preterm labour.
Cyclic AMP signalling pathways
Cyclic AMP is a diffusible intracellular second messenger, which influences many physiological events, by transducing hormone and small molecule effects into activation of protein kinases, modulating calcium transport and regulating gene activation. Its function in the relaxation of uterine and other types of smooth muscle is believed to be via inhibition of calcium mobilization and the contractile apparatus [3], through the activation of cAMP-dependent protein kinase (PRKA), which phosphorylates target proteins such as myosin light chain kinase (MYLK) [4] and phospholipase C (PLC) [5] (Table 1 and Figure 1). However the identification of PRKA substrates in human myometrium is a challenging area of research and more information is required before the mechanism of cyclic nucleotide-induced relaxation is understood.
Table 1.
Potential protein kinase A substrates involved in the regulation of human uterine relaxation
| Physiological roles (see Shabb (2001) [38]) | Protein substrate (HUGO nomenclature) | References |
| Autophosphorylation | cAMP dependant protein kinase regulatory subunit type IIα (PRKAR2A) | Zakhary et al. (2000) [39] |
| cAMP signalling | β-2 adrenoceptor (ADRB2) | Daaka Y et al. (1997) [40]; Iyer et al. (2006) [41] |
| G protein coupled receptor kinase-2 (ADRBK1) | Houslay & Baillie (2006) [42] | |
| Phosphodiesterase 4 (PDE4) | Murthy et al. (2002) [43] | |
| Phosphoinositide and calcium signalling | InsP3 Type I receptor (ITPR1) | Straub et al. (2004) [44] |
| Phospholipase-C β3 (PLCB3) | Yue et al. (1998) [45] | |
| Phospholipase-C γ1 (PLCG1) | Park et al. (1992) [46] | |
| ATPase 2 (ATP2) | Tribe et al. (2000) [47] | |
| Regulators of G-protein signalling (RGS) | Suarez et al. (2003) [48] | |
| Thromboxane A2 receptor (TBXA1R) | Walsh et al. (2000) [49] | |
| Rho signalling | RhoA small GTP binding protein (RHOA) | Murthy et al. (2003) [50] |
| Gα13 | Manganello et al. (2003) [51] | |
| Smooth muscle contraction | Myosin light chain kinase (MYLK) | Hirano et al. (2004) [52] |
| Myosin binding subunit of myosin phosphatase (PPP1R12A) | Wooldridge et al. (2004) [53] |
Figure 1.
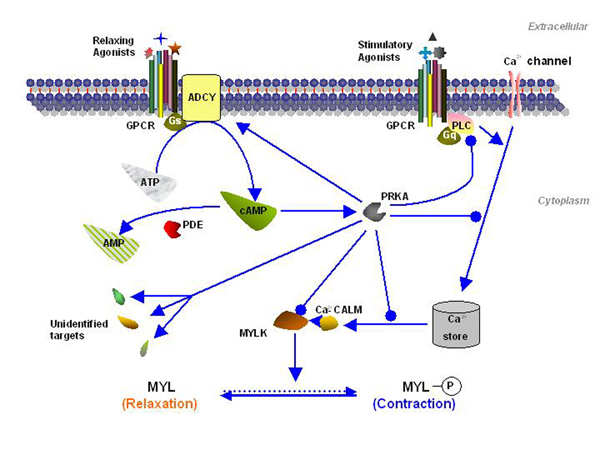
Cyclic AMP pathways in myometrial tissue. Activation of membrane receptors (GPCR) coupled to Gs activates adenylyl cyclase (ADCY) which converts ATP to cAMP. The levels of cAMP are tightly regulated by phosphodiesterases (PDE), especially PDE4 isoforms. It is thought that cAMP induces uterine relaxation via activation of a specific protein kinase (PRKA) which phosphorylates and inhibits myosin light chain kinase (MYLK). PRKA may also oppose the effect of stimulatory receptors which operate through the phospholipase C (PLC)/calcium pathway. However the precise targets for PRKA phosphorylation in human myometrium are under investigation ( stimulation,
stimulation,  inhibition)
inhibition)
The synthesis and catabolism of cAMP has been studied in many systems. Adenylyl cyclases (ADCY) catalyse the conversion of ATP to cAMP in response to the actions of hormones and drugs acting upon cell surface G protein coupled receptors. The cAMP formed is hydrolysed by a family of cyclic nucleotide phosphodiesterases (PDE). In myometrium ADCY activity is induced by endogenous agonists (catecholamines, prostaglandins, etc) operating through protein receptors e.g. ADRB2 (β2-adrenoceptor), PTGER2 (prostaglandin E2 receptor) coupled to Gs [6]. Activated ADCY removes one pyrophosphate molecule to convert ATP to cAMP and releases it into the cytoplasm. Cyclic AMP activates PRKA by binding to its catalytic subnits. Spatial and temporal changes in cAMP levels can be translated into compartmentalised responses by PRKA anchoring proteins which also bind other protein kinases and PDE isoforms to regulate a variety of signalling activities including feedback phosphorylation of ADCY [7]. The concentration of cAMP is reduced rapidly by PDE hydrolysis. Regulation of PDEs, especially PDE4, the largest myometrial PDE family, is an attractive area of study because of its potential as a new target for tocolysis. Long forms of PDE4 are involved in fast cAMP signalling loops through PRKA activation at conserved phosphorylation domains, while short forms of PDE4 are associated with long term responses promoted by cAMP such as gene transcription. Moreover, prostaglandin E2 up-regulates PDE activity by inducing the synthesis of PDE4 short isoforms [8].
Adenylyl cyclases and their regulators
Is there a specific myometrial cAMP generation system? This question is important because it would provide a target for pharmacological intervention. ADCY are a family of disparate enzymes directly involved in cAMP synthesis. The catalytic core of mammalian ADCY consists of a pseudosymmetric heterodimer composed of two highly conserved cytosolic regions, namely C1a and C2a, which can bind Gs and ATP. All isoforms of ADCY are activated by the α subunit of Gs (Gαs) and most are directly activated by the diterpene forskolin, which is a useful tool for functional studies [9,10]. The C1a and C2a regions alone are sufficient for cAMP generation, and linkage of portions of these cytosolic domains yields a soluble form of ADCY, which is fully activated by both Gαs and forskolin [11,12].
So far, nine mammalian membrane-bound isoforms of ADCY have been identified, and allocated into one of four subgroups, according to their modes of regulation [9] (table 2). All isoforms are activated by Gαs binding primarily to a hydrophobic, negatively charged pocket on the C2a domain [10]. Since the site of interaction of Gαs with ADCY is distal to the catalytic site, it has been suggested that the likely mechanism for Gαs activation involves a modulation of the structure of the active site by the induction of a conformational change. All ADCYs, except ADCY 9, which lacks the required serine and leucine residues, can be activated by forskolin, which binds in a narrow hydrophobic crevice between the C1a and C2a domains [13]. Activation of ADCYs by forskolin occurs even in the absence of a functional G protein, and is, therefore, a consequence of a direct action on the catalytic subunit of the enzyme [14].
Table 2.
Adenylyl cyclase (ADCY) isoforms
| Sub-family | Mode of regulation | Member | Chromosomal localization | Referen ces |
| 1 | Activated by calcium, Gαs, PRKA inhibited by Gαi/o and Gβγ | ADCY1 | 7p12 | Villacres et al. (1993) [54] |
| ADCY3 | 5p15 | Diel et al. (2006) [55] | ||
| ADCY8 | 2p22-24 | Steiner et al. (2006) [56] | ||
| 2 | Activated by Gαs and Gβγ | ADCY2 | 14q11.2 | Nguyen & Watts (2006) [57] |
| ADCY4 | 3q13.2-q21 | Wang & Burns (2006) [58] | ||
| ADCY7 | 12q12-13 | Guzman et al. (2005) [59] | ||
| 3 | Activated by Gαs, Inhibited by Gαi/o, Gβγ calcium, PRKA | ADCY5 | 16q12-13 | Iwami et al. (1995) [60] |
| ADCY6 | 8q24 | Chen et al. (1997) [61] | ||
| 4 | Activated by Gαs | ADCY9 | 16p13 | Hacker et al. (1998) [62] |
Responses of ADCYs to other modulators can be very different. For example, Group 1 cyclases (ADCY1, ADCY3, ADCY8) are stimulated by calcium/calmodulin (Ca2+-CALM) and by PRKA but are inhibited by the α subunits of proteins of the Gαi/o family and also by G protein βγ subunits (Gβγ). In contrast, Group 2 isoforms (ADCY2, ADCY4, and ADCY7) are activated by Gβγ and insensitive to Ca2+-CALM [15]. Group 3 isoforms (ADCY5 and ADCY6) are similar to Group 1, in that they are inhibited by Gαi/o and Gβγ, but they are also inhibited by the pertussis toxin-insensitive inhibitory G-protein Gαz, by low concentrations of calcium, and by PRKA phosphorylation at Ser-674 [16]. ADCY9 belongs in a separate group because it is insensitive to either calcium or Gαi/o/Gβγ, but is inhibited by the phosphatase calcineurin [17]. The effects of protein kinase C (PRKC) on ADCY are very complex and cut across groups. For instance PRKC phosphorylation enhances the activities of ADCY2 and ADCY7 (Group 2) as well as ADCY5 (Group 3). On the other hand phosphorylation by PRKC reduces the activities of ADCY4 (Group2) and ADCY6 (Group 3) [9,15]. In pregnancy there is upregulation of group 2 and group 3 isoforms in myometrium [18], but the question whether there are dominant ADCY isoforms coupled to specific myometrial receptors or whether there is promiscuity in the coupling of GPCR/Gαs complexes to several ADCY isoforms remains unanswered. The development of sensitive fluorescent tags to study molecular proximity in real time will help answer this question in myometrial cells.
Phosphodiesterases and their regulators
The phosphodiesterases (PDE) multi-gene family of enzymes catalyze the hydrolysis of 3',5'-cyclic nucleotides, and so occupy a key position in modulating intracellular cyclic nucleotide levels [19]. In myometrial tissue, the activity of PDE enzymes is significantly inhibited during pregnancy [20], probably as a consequence of the high progesterone levels [21], suggesting that cAMP-PDE inhibition may be part of the mechanism for myometrial relaxation and pregnancy maintenance.
At present, more than 14 PDE genes have been cloned in mammals and 21 gene products have been identified. PDE isoforms are grouped in 11 distinct but related families (PDE1 to PDE11), according to sequence homology, substrate specificity, inhibitor sensitivity and function. Within these families alternative splicing or mRNA transcription initiation result in additional diversity producing around 100 mRNA products, but it is not clear how many of these are actually translated into different proteins [19,22]. The protein isoforms all share a common structural pattern: a C-terminus whose function is unclear; a conserved catalytic core, which determines substrate specificity and inhibitor sensitivity; and a divergent N-terminus responsible for the enzyme's targeting [19,22].
PDE subfamilies are differentially expressed in human tissues, and only PDE1-5 subtypes have been detected in human term myometrium [23]. PDE1 subtypes are of mixed substrate specificity for cyclic nucleotides (they tend to hydrolyse either cAMP or cGMP) and contain auto-inhibitory substrate-like sequences; such inhibition is relieved by Ca2+/CALM [24]. PDE1 isoforms are clearly regulated by PRKA and are involved in cell proliferation [25].
PDE2 and PDE5 have similar structures as both of them contain allosteric cyclic nucleotide binding sites for cGMP. The binding of cGMP to PDE2 stimulates cAMP hydrolysis to mediate cross-talk between cGMP and cAMP pathways [26,27]. PDE5 expression is inhibited by hCG in human myometrium [28], and some evidence suggested that PDE5 may play a role in myometrial cell proliferation and cGMP dependent potassium channel regulation. High doses of sildenalfil, a specific PDE5 inhibitor, relax human pregnant myometrium in vitro, but this effect is unlikely to be of tocolytic value [29].
PDE3 has two isoforms PDE3A and PDE3B, which contain a conserved catalytic site for both cAMP and cGMP binding [30]. Functional studies suggest that PDE3 is involved in vascular smooth muscle contractions, heart function, platelet aggregation, oocyte maturation and cell metabolism [31].
PDE4 is the largest PDE subfamily, consisting two classes of isoforms, long or short PDE4, depending upon the presence or absence of upstream conserved regions. Both classes of PDE4 have a much higher affinity for cAMP than for cGMP [32]. In human myometrial cell, the major source (up to 75%) of PDE activity is provided by PDE4 isoforms and membrane targeting can influence the maximal activity of the enzyme and its sensitivity [23,33]. Early functional studies of PDE4 showed that specific inhibition with rolipram leads to inhibition of the spontaneous contraction of myometrial strips [34,35]. However, rolipram has significant side effects, particularly nausea and vomiting. Cilomilast, originally designed as a novel therapy for asthma with fewer side effects, produces concentration-dependent inhibition of the spontaneous contractions of term myometrium [36]. PDE4 selective inhibitors reduce the incidence of inflammation-induced preterm delivery in mice [37]. A better understanding of the regulation of PDE4 isoforms in pregnant human myometrium may be a useful approach to develop tocolytics of high uterine selectivity [33].
Conclusion
The role of cAMP as an important mediator in the regulation of myometrial function is beyond dispute. Much is known about the pathways for cAMP synthesis and hydrolysis. However, the mechanisms of action of cAMP and its associated kinase PRKA in myometrial cells are not fully understood. Several candidate PRKA substrates have been proposed based on studies in other types of smooth muscle. More research into the identification of physiological PRKA substrates in pregnant human myometrium and into their regulation by phosphorylation will clarify the role of cAMP in pregnancy maintenance, and provide novel targets for uterine-selective therapeutic intervention.
List of abbreviations used
ADCY, adenylyl cyclases; ADRB2, β2-adrenoceptor; CALM, calmodulin; cAMP, cyclic AMP (cyclic adenosine monophosphate); GPCR, G protein-coupled receptor; MYL, myosin light chain kinase; PDE, cyclic nucleotide phosphodiesterase; PLC, phospholipase C; PRKA, cAMP-dependent protein kinase; PRKC, protein kinase C; PTGER2, prostaglandin E2 receptor.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WY drafted the manuscript. ALB conceived the study, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
WY is a PhD student supported by the SAFE network of Excellence (LSHB-CT-2004-503243). Also we would like to thank Sarah A. Price and Edward Man-Lik Choi for unpublished material. We are grateful to Dr Ciara O'Sullivan and Dr Jorg Strutwolf for hosting SAFE workshops in Tarragona. Sponsorship by Ferring, PerkinElmer and Serono covered the publication cost.
This article has been published as part of BMC Pregnancy and Childbirth Volume 7, Supplement 1, 2007: Proceedings of the First and Second European Workshops on Preterm Labour of the Special Non-Invasive Advances in Fetal and Neonatal Evaluation Network of Excellence. The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2393/7?issue=S1.
Contributor Information
Wei Yuan, Email: Wei.Yuan@Bristol.ac.uk.
Andrés López Bernal, Email: A.LopezBernal@bristol.ac.uk.
References
- Keirse MJ. New perspectives for the effective treatment of preterm labor. Am J Obstet Gynecol. 1995;173:618–628. doi: 10.1016/0002-9378(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Price SA, López Bernal A. Uterine quiescence: the role of cyclic AMP. Exp Physiol. 2001;86:265–272. doi: 10.1113/eph8602182. [DOI] [PubMed] [Google Scholar]
- Sanborn BM. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture. Exp Physiol. 2001;86:223–237. doi: 10.1113/eph8602179. [DOI] [PubMed] [Google Scholar]
- Word RA. Myosin phosphorylation and the control of myometrial contraction/relaxation. Semin Perinatol. 1995;19:3–14. doi: 10.1016/S0146-0005(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Sanborn BM, Ku CY, Shlykov S, Babich L. Molecular signaling through G-protein-coupled receptors and the control of intracellular calcium in myometrium. J Soc Gynecol Investig. 2005;12:479–487. doi: 10.1016/j.jsgi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal A, Europe-Finner GN, Phaneuf S, Watson SP. Preterm labour: a pharmacological challenge. Trends Pharmacol Sci. 1995;16:129–133. doi: 10.1016/S0165-6147(00)89000-8. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends Biochem Sci. 1997;22:217–224. doi: 10.1016/S0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- Mehats C, Tanguy G, Dallot E, Cabrol D, Ferre F, Leroy MJ. Is up-regulation of phosphodiesterase 4 activity by PGE2 involved in the desensitization of beta-mimetics in late pregnancy human myometrium? J Clin Endocrinol Metab. 2001;86:5358–5365. doi: 10.1210/jc.86.11.5358. [DOI] [PubMed] [Google Scholar]
- Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, Suzuki Y, Defer N. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Mol Cell Endocrinol. 1997;128:179–194. doi: 10.1016/S0303-7207(97)04013-6. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sprang SR. The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr Opin Struct Biol. 1998;8:713–719. doi: 10.1016/S0959-440X(98)80090-0. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science. 1995;268:1769–1772. doi: 10.1126/science.7792604. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. Interaction of Gsalpha with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22265–22271. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- Hurley JH. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- Seamon K, Daly JW. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981;256:9799–9801. [PubMed] [Google Scholar]
- Taussig R, Gilman AG. Mammalian membrane-bound adenylyl cyclases. J Biol Chem. 1995;270:1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Cali JJ. Molecular diversity of the adenylyl cyclases. Adv Second Messenger Phosphoprotein Res. 1998;32:53–79. doi: 10.1016/s1040-7952(98)80005-0. [DOI] [PubMed] [Google Scholar]
- Antoni FA, Palkovits M, Simpson J, Smith SM, Leitch AL, Rosie R, Fink G, Paterson JM. Ca2+/calcineurin-inhibited adenylyl cyclase, highly abundant in forebrain regions, is important for learning and memory. J Neurosci. 1998;18:9650–9661. doi: 10.1523/JNEUROSCI.18-23-09650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SA, Pochun I, Phaneuf S, Lopez Bernal A. Adenylyl cyclase isoforms in pregnant and non-pregnant human myometrium. J Endocrinol. 2000;164:21–30. doi: 10.1677/joe.0.1640021. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Kofinas AD, Rose JC, Meis PJ. Changes in cyclic adenosine monophosphate-phosphodiesterase activity in nonpregnant and pregnant human myometrium. Am J Obstet Gynecol. 1987;157:733–738. doi: 10.1016/s0002-9378(87)80040-6. [DOI] [PubMed] [Google Scholar]
- Kofinas AD, Rose JC, Koritnik DR, Meis PJ. Progesterone and estradiol concentrations in nonpregnant and pregnant human myometrium. Effect of progesterone and estradiol on cyclic adenosine monophosphate-phosphodiesterase activity. J Reprod Med. 1990;35:1045–1050. [PubMed] [Google Scholar]
- Bushnik T, Conti M. Role of multiple cAMP-specific phosphodiesterase variants. Biochem Soc Trans. 1996;24:1014–1019. doi: 10.1042/bst0241014. [DOI] [PubMed] [Google Scholar]
- Leroy MJ, Lugnier C, Merezak J, Tanguy G, Olivier S, Le Bec A, Ferre F. Isolation and characterization of the rolipram-sensitive cyclic AMP-specific phosphodiesterase (type IV PDE) in human term myometrium. Cell Signal. 1994;6:405–412. doi: 10.1016/0898-6568(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Sonnenburg WK, Seger D, Kwak KS, Huang J, Charbonneau H, Beavo JA. Identification of inhibitory and calmodulin-binding domains of the PDE1A1 and PDE1A2 calmodulin-stimulated cyclic nucleotide phosphodiesterases. J Biol Chem. 1995;270:30989–31000. doi: 10.1074/jbc.270.52.30989. [DOI] [PubMed] [Google Scholar]
- Ang KL, Antoni FA. Reciprocal regulation of calcium dependent and calcium independent cyclic AMP hydrolysis by protein phosphorylation. J Neurochem. 2002;81:422–433. doi: 10.1046/j.1471-4159.2002.00903.x. [DOI] [PubMed] [Google Scholar]
- Martins TJ, Mumby MC, Beavo JA. Purification and characterization of a cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from bovine tissues. J Biol Chem. 1982;257:1973–1979. [PubMed] [Google Scholar]
- Wu AY, Tang XB, Martinez SE, Ikeda K, Beavo JA. Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J Biol Chem. 2004;279:37928–37938. doi: 10.1074/jbc.M404287200. [DOI] [PubMed] [Google Scholar]
- Belmonte A, Ticconi C, Dolci S, Giorgi M, Zicari A, Lenzi A, Jannini EA, Piccione E. Regulation of phosphodiesterase 5 expression and activity in human pregnant and non-pregnant myometrial cells by human chorionic gonadotropin. J Soc Gynecol Investig. 2005;12:570–577. doi: 10.1016/j.jsgi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mehats C, Schmitz T, Breuiller-Fouche M, Leroy MJ, Cabrol D. Should phosphodiesterase 5 selective inhibitors be used for uterine relaxation? Am J Obstet Gynecol. 2006;195:184–185. doi: 10.1016/j.ajog.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Scapin G, Patel SB, Chung C, Varnerin JP, Edmondson SD, Mastracchio A, Parmee ER, Singh SB, Becker JW, Van der Ploeg LH, et al. Crystal structure of human phosphodiesterase 3B: atomic basis for substrate and inhibitor specificity. Biochemistry. 2004;43:6091–6100. doi: 10.1021/bi049868i. [DOI] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehats C, Oger S, Leroy MJ. Cyclic nucleotide phosphodiesterase-4 inhibitors: a promising therapeutic approach to premature birth? Eur J Obstet Gynecol Reprod Biol. 2004;117:S15–17. doi: 10.1016/j.ejogrb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Leroy MJ, Cedrin I, Breuiller M, Giovagrandi Y, Ferre F. Correlation between selective inhibition of the cyclic nucleotide phosphodiesterases and the contractile activity in human pregnant myometrium near term. Biochem Pharmacol. 1989;38:9–15. doi: 10.1016/0006-2952(89)90142-1. [DOI] [PubMed] [Google Scholar]
- Bardou M, Cortijo J, Loustalot C, Taylor S, Perales-Marin A, Mercier FJ, Dumas M, Deneux-Tharaux C, Frydman R, Morcillo EJ, et al. Pharmacological and biochemical study on the effects of selective phosphodiesterase inhibitors on human term myometrium. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:457–463. doi: 10.1007/s002109900092. [DOI] [PubMed] [Google Scholar]
- Oger S, Mehats C, Barnette MS, Ferre F, Cabrol D, Leroy MJ. Anti-inflammatory and utero-relaxant effects in human myometrium of new generation phosphodiesterase 4 inhibitors. Biol Reprod. 2004;70:458–464. doi: 10.1095/biolreprod.103.023051. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Souil E, Herve R, Nicco C, Batteux F, Germain G, Cabrol D, Evain-Brion D, Leroy MJ, Mehats C. PDE4 inhibition prevents preterm delivery induced by an intrauterine inflammation. J Immunol. 2007;178:1115–1121. doi: 10.4049/jimmunol.178.2.1115. [DOI] [PubMed] [Google Scholar]
- Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem Rev. 2001;101:2381–2411. doi: 10.1021/cr000236l. [DOI] [PubMed] [Google Scholar]
- Zakhary DR, Fink MA, Ruehr ML, Bond M. Selectivity and regulation of A-kinase anchoring proteins in the heart. The role of autophosphorylation of the type II regulatory subunit of cAMP-dependent protein kinase. J Biol Chem. 2000;275:41389–41395. doi: 10.1074/jbc.M004212200. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Iyer V, Tran TM, Foster E, Dai W, Clark RB, Knoll BJ. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol. 2006;147:249–259. doi: 10.1038/sj.bjp.0706551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS. Phosphodiesterase-4 gates the ability of protein kinase A to phosphorylate G-protein receptor kinase-2 and influence its translocation. Biochem Soc Trans. 2006;34:474–475. doi: 10.1042/BST0340474. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Makhlouf GM. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol. 2002;282:C508–517. doi: 10.1152/ajpcell.00373.2001. [DOI] [PubMed] [Google Scholar]
- Straub SV, Wagner LE, 2nd, Bruce JI, Yule DI. Modulation of cytosolic calcium signaling by protein kinase A-mediated phosphorylation of inositol 1,4,5-trisphosphate receptors. Biol Res. 2004;37:593–602. doi: 10.4067/s0716-97602004000400013. [DOI] [PubMed] [Google Scholar]
- Yue C, Dodge KL, Weber G, Sanborn BM. Phosphorylation of serine 1105 by protein kinase A inhibits phospholipase Cbeta3 stimulation by Galphaq. J Biol Chem. 1998;273:18023–18027. doi: 10.1074/jbc.273.29.18023. [DOI] [PubMed] [Google Scholar]
- Park DJ, Min HK, Rhee SG. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992;267:1496–1501. [PubMed] [Google Scholar]
- Tribe RM, Moriarty P, Poston L. Calcium homeostatic pathways change with gestation in human myometrium. Biol Reprod. 2000;63:748–755. doi: 10.1095/biolreprod63.3.748. [DOI] [PubMed] [Google Scholar]
- Suarez VR, Park ES, Hankins GD, Soloff MS. Expression of regulator of G protein signaling-2 in rat myometrium during pregnancy and parturition. Am J Obstet Gynecol. 2003;188:973–977. doi: 10.1067/mob.2003.240. [DOI] [PubMed] [Google Scholar]
- Walsh MT, Foley JF, Kinsella BT. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for prostacyclin-mediated desensitization. J Biol Chem. 2000;275:20412–20423. doi: 10.1074/jbc.M907881199. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1006–1016. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- Manganello JM, Huang JS, Kozasa T, Voyno-Yasenetskaya TA, Le Breton GC. Protein kinase A-mediated phosphorylation of the Galpha13 switch I region alters the Galphabetagamma13-G protein-coupled receptor complex and inhibits Rho activation. J Biol Chem. 2003;278:124–130. doi: 10.1074/jbc.M209219200. [DOI] [PubMed] [Google Scholar]
- Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2+ sensitivity in vascular smooth muscle. J Smooth Muscle Res. 2004;40:219–236. doi: 10.1540/jsmr.40.219. [DOI] [PubMed] [Google Scholar]
- Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Xia Z, Bookbinder LH, Edelhoff S, Disteche CM, Storm DR. Cloning, chromosomal mapping, and expression of human fetal brain type I adenylyl cyclase. Genomics. 1993;16:473–478. doi: 10.1006/geno.1993.1213. [DOI] [PubMed] [Google Scholar]
- Diel S, Klass K, Wittig B, Kleuss C. Gbetagamma activation site in adenylyl cyclase type II. Adenylyl cyclase type III is inhibited by Gbetagamma. J Biol Chem. 2006;281:288–294. doi: 10.1074/jbc.M511045200. [DOI] [PubMed] [Google Scholar]
- Steiner D, Saya D, Schallmach E, Simonds WF, Vogel Z. Adenylyl cyclase type-VIII activity is regulated by G(betagamma) subunits. Cell Signal. 2006;18:62–68. doi: 10.1016/j.cellsig.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Watts VJ. Dexamethasone-induced Ras protein 1 negatively regulates protein kinase C delta: implications for adenylyl cyclase 2 signaling. Mol Pharmacol. 2006;69:1763–1771. doi: 10.1124/mol.105.019133. [DOI] [PubMed] [Google Scholar]
- Wang HY, Burns LH. Gbetagamma that interacts with adenylyl cyclase in opioid tolerance originates from a Gs protein. J Neurobiol. 2006;66:1302–1310. doi: 10.1002/neu.20286. [DOI] [PubMed] [Google Scholar]
- Guzman L, Romo X, Grandy R, Soto X, Montecino M, Hinrichs M, Olate J. A Gbetagamma stimulated adenylyl cyclase is involved in Xenopus laevis oocyte maturation. J Cell Physiol. 2005;202:223–229. doi: 10.1002/jcp.20102. [DOI] [PubMed] [Google Scholar]
- Iwami G, Akanuma M, Kawabe J, Cannon PJ, Homcy CJ, Ishikawa Y. Multiplicity in type V adenylylcyclase: type V-a and type V-b. Mol Cell Endocrinol. 1995;110:43–47. doi: 10.1016/0303-7207(95)03514-8. [DOI] [PubMed] [Google Scholar]
- Chen Y, Harry A, Li J, Smit MJ, Bai X, Magnusson R, Pieroni JP, Weng G, Iyengar R. Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation. Proc Natl Acad Sci USA. 1997;94:14100–14104. doi: 10.1073/pnas.94.25.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker BM, Tomlinson JE, Wayman GA, Sultana R, Chan G, Villacres E, Disteche C, Storm DR. Cloning, chromosomal mapping, and regulatory properties of the human type 9 adenylyl cyclase (ADCY9) Genomics. 1998;50:97–104. doi: 10.1006/geno.1998.5293. [DOI] [PubMed] [Google Scholar]


