Abstract
A member of the p21-activated protein kinase (PAK) family, γ-PAK has cytostatic properties and is activated by cellular stresses such as hyperosmolarity or DNA damage. We report herein that γ-PAK is associated in vivo with the nonreceptor protein tyrosine kinase c-Abl. γ-PAK phosphorylates c-Abl on sites located in the kinase domain, in a region that is implicated in protein–protein interactions and in subcellular localization. Activation of γ-PAK in human embryonic kidney 293T cells by cotransfection with constitutively active Cdc42 induces activation of c-Abl, resulting in increased phosphotyrosine levels. Cotransfection of c-Abl and γ-PAK elicits phosphorylation of γ-PAK on tyrosine and down-regulation of γ-PAK activity, promoting accumulation of inactive γ-PAK. γ-PAK is also phosphorylated in vitro by c-Abl. γ-PAK activity is regulated by ubiquitination and proteolysis in vivo, as shown by immunoblotting with an anti-ubiquitin antibody in the presence of proteasome inhibitors. In summary, we describe a functional interaction between γ-PAK and c-Abl in which γ-PAK stimulates c-Abl tyrosine kinase activity and c-Abl phosphorylates and down-regulates γ-PAK, suggesting the existence of a negative feedback loop between c-Abl and γ-PAK.
The family of p21-activated protein kinases (PAK) comprises three highly conserved isoforms: α-PAK (PAK1), β-PAK (PAK3), and γ-PAK (PAK2, PAK I; refs. 1–4). PAKs are composed of a C-terminal catalytic domain and an N-terminal regulatory domain. The regulatory domain contains a p21-binding site for Cdc42 and Rac1 that leads to autophosphorylation and activation (1–4). α-PAK is activated by growth factors such as platelet-derived growth factor (5) and epidermal growth factor (6) and by insulin (7). α-PAK participates in the control of actin cytoskeletal dynamics (8, 9). In contrast, γ-PAK is activated in response to stimuli leading to cytostasis, such as hyperosmolarity (10) and DNA damage induced by ionizing and UV radiation or chemotherapeutic drugs (11). In addition, γ-PAK (but not α- or β-PAK) is activated in response to apoptotic stimuli, such as anti-Fas, C2 ceramide, or tumor necrosis factor, and by caspase cleavage followed by autophosphorylation (12–14). Expression of the catalytic domain of γ-PAK induces some of the morphological and biochemical changes characteristic of programmed cell death.
γ-PAK has cytostatic properties, as shown by microinjection of the active enzyme into early frog embryos, resulting in arrest of cell cleavage (15). The activity of a γ-PAK homolog is high in frog oocytes and is decreased following fertilization and in early embryogenesis (15). A γ-PAK homolog (X-PAK) has been implicated in the negative control of G2/M transition in Xenopus (16, 17). Expression of wild-type γ-PAK (γ-PAKwt) in mammalian cells, but not the kinase-inactive mutant K278R, inhibits cell division.¶ Thus, γ-PAK appears to be a potent cytostatic protein kinase that is also involved in apoptosis.
The nonreceptor tyrosine protein kinase c-Abl is a 150-kDa enzyme that, in its mutated forms, has been implicated in the induction of different types of leukemias (18, 19). c-Abl contains different domains and features that allow for the transduction of a variety of cellular signals. These domains and features include Src homology (SH) 3 and SH2 domains, a tyrosine kinase catalytic domain, proline motifs known to bind to SH3-containing proteins such as Crk or Nck, DNA-binding domains, and actin-binding domains. c-Abl is autophosphorylated, phosphorylated by other protein kinases, and myristoylated.
Nuclear c-Abl has been shown to be activated in response to DNA damage (20). Abl is associated with the retinoblastoma protein (pRB), and phosphorylation of pRB at the G1/S boundary induces c-Abl release, resulting in activation of Abl during S phase (21). The significance of this c-Abl activation is unknown, as c-Abl is not required for many of the physiological responses to DNA damage (19, 22). c-Abl has cytostatic and cytotoxic properties (23, 24), although the mechanism of growth inhibition is unknown, and c-Abl−/−-deficient cells do not appear to have defects in DNA repair or cell-cycle progression (25).
Recently, the cytoplasmic form of c-Abl was shown to be activated by growth factor receptors such as platelet-derived growth factor and epidermal growth factor (26). Cells that lack c-Abl are defective in their ability to reorganize the cytoskeleton in response to growth factor stimulation. The functions of c-Abl in the cytoplasm also remain undefined. A portion of c-Abl is associated with actin and may be implicated in the control of the actin cytoskeleton. In addition, both PAK and Abl have been implicated in axon guidance in Drosophila (2, 3, 19).
Here, we report that γ-PAK and c-Abl are associated in vivo. γ-PAK phosphorylates c-Abl in vitro, and activation of γ-PAK by Cdc42 induces activation of c-Abl in vivo. In addition, c-Abl induces tyrosine phosphorylation and accumulation of inactive γ-PAK in transiently transfected cells. These data indicate that γ-PAK has a role in the control of c-Abl activity and suggest the existence of a negative feedback loop between c-Abl and γ-PAK.
Experimental Procedures
Materials.
Cell culture media and reagents were purchased from GIBCO/BRL. Superfect reagent was from Qiagen (Chatsworth, CA). Diphenylcarbamoyl chloride (DPCC)-treated trypsin was from Sigma, and sequencing-grade trypsin and histone 4 (H4) were from Roche Molecular Biochemicals. Protein A/G-agarose, normal IgG, peroxidase-coupled secondary antibodies, and antibodies against γ-PAK (N-19), c-Abl (K12), and phosphotyrosine (PY99) were purchased from Santa Cruz Biotechnology. Antibody to the hemagglutinin epitope (HA)-tag (HA.11) was from Babco (Richmond, CA). Rabbit polyclonal antibody PEX5 recognized the Abl carboxyl terminus as described (27). Mouse monoclonal antibody to c-Abl (8E9) was from PharMingen. Antiphosphotyrosine antibody (4G10) was from Upstate Biotechnology (Lake Placid, NY). Antibody to ubiquitin (Ubi-1) was from Zymed. [γ-32P]ATP was purchased from NEN. The proteasome inhibitors, lactacystin and Z-Leu-Leu-norleucinal (LLnL), were from Biomol. Cellulose TLC sheets were from Selecto Scientific (Suwanee, GA), and silica gel TLC sheets were from EM Science. Cdc42N17 and Cdc42L61 were kindly provided by J. S. Gutkind (National Institutes of Health, Bethesda). Gluthatione S-transferase (GST)-Cdc42 and caspase 3 were expressed in Escherichia coli and purified as described (14, 28).
Cell Culture and Transient Transfection.
PCDNA3.1/wild-type PAK, Cdc42, and γ-PAK K278R (kinase-inactive) constructs were described elsewhere (28). Cdc42N17 and Cdc42L61 were subcloned into the PCDNA3.1 vector; both γ-PAK and Cdc42 were expressed as HA-tagged proteins. PSRα c-Abl (27) and GST-Crk (26) constructs were described previously.
Exponentially growing human embryonic kidney (HEK) 293T cells were transfected by using Superfect reagent. The amount of DNA transfected was normalized to 5 μg by addition of the empty PCDNA3.1 vector. At 24 h after transfection, cells were harvested, frozen in liquid nitrogen, and stored at −70°C.
Immunoprecipitation and Western Blotting.
Cells were thawed in RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) containing phosphatase inhibitors (2 mM Na3VO4/10 nM okadaic acid) and protease inhibitors (40 μg/ml leupeptin/40 μg/ml pepstatin/40 μg/ml aprotinin/0.5 mM phenylmethylsulfonyl fluoride). After 20 min on ice, the lysates were centrifuged at 13,000 × g for 15 min, and the insoluble pellet was discarded. Protein concentrations were determined by using the Bradford reagent. For immunoprecipitation, 500 μg of protein (diluted to 500 μl with RIPA buffer containing inhibitors) was incubated for 2 h at 4°C with 1 μg of the specified antibody and for 1 h more with 30 μl of protein A/G-agarose [1:1 (vol/vol) slurry solution]. Immunoprecipitates were collected by centrifugation at 2,040 × g, washed three times with RIPA buffer plus inhibitors, and boiled for 5 min with electrophoresis sample buffer. Following SDS/PAGE, proteins were detected by Western blotting as described elsewhere (11).
Preparation of Cell Extracts.
Cells were thawed in 0.5 ml of freshly prepared lysis buffer A containing 50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/5 mM MgCl2/1 mM EDTA/1 mM EGTA/10 mM β-mercaptoethanol/1% Nonidet P-40, phosphatase inhibitors (50 mM NaF/5 mM Na4P2O7/2 mM Na3VO4/10 nM okadaic acid), and protease inhibitors (40 μg/ml leupeptin/40 μg/ml pepstatin/40 μg/ml aprotinin/0.5 mM phenylmethylsulfonyl fluoride). After 10 min on ice, the lysate was centrifuged at 16,000 × g for 10 min at 4°C. The insoluble pellet was discarded, and the protein concentration in the supernatant was determined by Bradford assay.
Assay for c-Abl Activity.
c-Abl activity was determined with the substrate GST-Crk following immunoprecipitation with K12 antibody (1 μg) from cell extracts (100 μg of protein) and extensive washing as described (26), with slight modifications. The immunoprecipitates were assayed with 0.5 μg of GST-Crk in a final volume of 20 μl of c-Abl phosphorylation buffer (20 mM Tris⋅HCl, pH 7.5/10 mM MgCl2/1 mM DTT/1 μM [γ-32P]ATP/2,000 cpm/pmol) for 45 min at room temperature. Phosphorylation of Crk was analyzed after SDS/PAGE by using a PhosphorImager system (Molecular Dynamics).
Assay for γ-PAK Activity.
γ-PAK activity was assayed following immunoprecipitation from cell lysates with N19 or anti-HA antibodies with H4 as a substrate (11). Following SDS/PAGE, phosphorylation of H4 was analyzed by using a PhosphorImager system.
Phosphorylation of c-Abl by γ-PAK in Vitro.
Full-length c-Abl and GST–γ-PAK were expressed in insect cells. c-Abl fragments 593–730, SH3 (amino acids 41–132), SH2 (amino acids 132–230), or SH2–SH1 K290R (amino acids 137–671) (29) were expressed as GST proteins in E. coli. Full-length c-Abl immunoprecipitated from cell lysates, as described below, or c-Abl fragments (1.0 μg) were phosphorylated with γ-PAK (0.2 μg), following activation with Cdc42(GTPγS) or caspase cleavage, as described elsewhere (11, 14). Phosphorylation was carried out for 30 min at 30°C in 25 μl of γ-PAK phosphorylation buffer and analyzed as described above.
Phosphorylation of γ-PAK by c-Abl.
HEK 293T cells were transfected with c-Abl and lysed in lysis buffer B (50 mM Tris⋅HCl, pH 7.0/150 mM NaCl/10% glycerol/1% Triton X-100/1.5 mM MgCl2/1 mM EGTA) containing protease and phosphatase inhibitors. The lysate was incubated with K12 antibody at 4°C overnight and for an additional 2 h with 30 μl of protein A/G agarose [1:1 (vol/vol) slurry solution], and immunoprecipitation was as described (26). Assays were carried out in 25 μl of c-Abl phosphorylation buffer with GST–γ-PAK (0.3 μg) for 30 min at 30°C. Following SDS/PAGE, phosphotyrosine was detected by Western blotting with PY99 antibody.
Phosphopeptide Mapping and Phosphoamino Acid Analysis.
Abl 593–730 and Abl SH2–SH1 K290R phosphorylated with [γ-32P]ATP and γ-PAK were analyzed by SDS/PAGE as described above. Protein in the gel slices was digested with diphenylcarbamoyl chloride-treated trypsin, and tryptic phosphopeptide mapping and phosphoamino acid analysis were carried out as described (28).
Analysis of Tryptic Phosphopeptides by MS.
Abl 593–730 (1.0 μg) was incubated with nonradioactive ATP in the presence and absence of γ-PAK. The protein band was digested with sequencing-grade trypsin (0.2 μg) in 25 μl of NH4HCO3, pH 8.0. Tryptic digests were analyzed by surface-enhanced laser-desorption-ionization MS (SELDI) by using a SELDI-protein chip system (Ciphergen, Palo Alto, CA).
Results
γ-PAK Associated with c-Abl in Vivo.
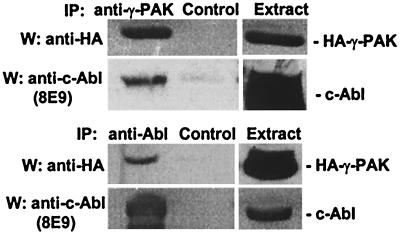
To determine whether γ-PAK and c-Abl were associated in vivo, HEK 293T cells were transiently transfected with c-Abl and HA-tagged γ-PAK; 24 h after transfection, the cells were lysed in the presence of protease and phosphatase inhibitors. γ-PAK was immunoprecipitated from the extracts by using the polyclonal antibody N19, with normal rabbit IgG as a control. The immunoprecipitates were probed for γ-PAK and c-Abl by Western blotting with anti-HA-tag and anti-Abl 8E9, respectively. Fig. 1 (Upper) shows that c-Abl was present in γ-PAK immunoprecipitates but not in the control immunoprecipitates. The inverse experiment was carried out by using the K12 polyclonal antibody to immunoprecipitate c-Abl (Fig. 1 Lower); Western blots showed that γ-PAK was present in the c-Abl immunoprecipitate. To confirm the results, two different antibodies were used to immunoprecipitate γ-PAK (anti-HA) or c-Abl (Pex5); similar results were obtained with these antibodies (data not shown). Thus, it was concluded that γ-PAK and c-Abl were associated in vivo when over-expressed.
Figure 1.
Coimmunoprecipitation of c-Abl and γ-PAK. c-Abl and HA-γ-PAK were transfected into 293T cells. Lysates (500 μg) were immunoprecipitated with anti-γ-PAK (N19) and control antibody (Upper) or anti-cAbl (K12) and control antibody (Lower), and the immunoprecipitates were analyzed by Western blotting as indicated; normal rabbit IgG was the control. Western blots of 10% of the original cell extracts used for immunoprecipitation (50 μg of total protein) are shown for reference.
γ-PAK Phosphorylated c-Abl in Vitro.
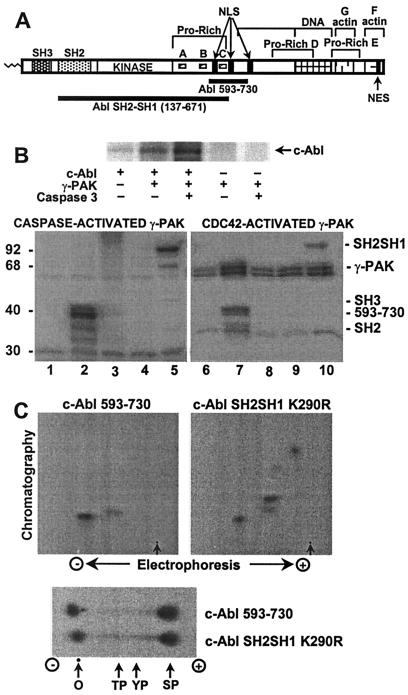
The physical association of γ-PAK and c-Abl suggested that a substrate relationship may exist for these kinases. To test whether γ-PAK could phosphorylate c-Abl, the c-Abl tyrosine kinase was expressed in insect cells and immunoprecipitated from cell extracts by using the K12 antibody (see Fig. 2A for c-Abl structure). After washing extensively with RIPA buffer, c-Abl immunoprecipitates were incubated with [γ-32P]ATP and inactive γ-PAK or γ-PAK activated by cleavage with caspase 3. Fig. 2B (Upper) shows that a protein with the same molecular weight as c-Abl was phosphorylated by active γ-PAK in the c-Abl immunoprecipitates. The phosphorylation of c-Abl was due to γ-PAK and not to a contaminating protein kinase present in c-Abl immunoprecipitates, as shown with controls using nonactivated γ-PAK or c-Abl alone. Similar results were obtained with Cdc42-activated γ-PAK (data not shown).
Figure 2.
Phosphorylation of c-Abl by γ-PAK in vitro. (A) A cartoon of the c-Abl domains SH3, SH2, kinase, and the C-terminal domain. The latter contains proline-rich sequences A, B, C, D, and E; three nuclear localization signals (NLS); one nuclear export signal (NES); and DNA-binding and actin-binding domains. The Crk/CrkL family of CF adaptor proteins binds to proline-rich sequences A and B, whereas the Nck and Abi adaptors bind to proline-rich sequence C. (B, Upper) c-Abl expressed in insect cells was immunoprecipitated and phosphorylated with γ-PAK activated by caspase 3 as described in Experimental Procedures and analyzed by SDS/PAGE. (B, Lower) GST-fusion proteins of c-Abl fragments were phosphorylated with activated γ-PAK and subjected to SDS/PAGE. Lanes 1 and 6, no substrate; lanes 2 and 7, c-Abl 593–730; lanes 3 and 8, c-Abl SH2; lanes 4 and 9, c-Abl SH3; lanes 5 and 10, c-Abl SH2–SH3 K290R. (C, Upper) Tryptic phosphopeptide mapping was carried out on Abl 593–730 and Abl SH2–SH1 K290R phosphorylated by γ-PAK. Arrows indicate the origin. (C, Lower) Phosphoamino acid analysis of Abl 593–730 and Abl SH2–SH1 K290R phosphorylated by γ-PAK. In all instances, radiolabeled phosphate was detected by using a PhosphorImager system. O, origin; TP, phosphothreonine; YP, phosphotyrosine; SP, phosphoserine.
To study further the phosphorylation of c-Abl by γ-PAK, different domains of the c-Abl tyrosine kinase were expressed in E. coli as GST-fusion proteins and incubated with active γ-PAK and [γ-32P]ATP. As shown in Fig. 2B (Lower), γ-PAK, activated by Cdc42 or by cleavage with caspase 3, phosphorylated two different peptides, Abl 593–730 and kinase-inactive Abl SH2–SH1 K290R (amino acids 137–671), whereas Abl SH3 (amino acids 41–132) and SH2 (amino acids 132–230) domains were not phosphorylated. Abl 593–730 was of interest because it contained the sequence KKKKKTAPTPPKRSSSF (). This sequence includes the nuclear localization signal and the Nck/Abi binding site (29). A putative γ-PAK phosphorylation site (KRXS) exists in this region at Ser 619 (30). Phosphopeptide maps of c-Abl 593–730 showed one major phosphopeptide (Fig. 2C Upper left). The same phosphopeptide was obtained with Abl SH2–SH1 K290R (which also contained the putative γ-PAK phosphorylation site), along with two additional major phosphopeptides (Fig. 2C Upper right). This suggested that an additional γ-PAK phosphorylation site or sites were located in the SH1 catalytic domain. Thus, γ-PAK phosphorylated c-Abl on two different domains, 593–730 and the SH1 kinase domain. Phosphoamino acid analysis showed that phosphate was incorporated exclusively on serine (Fig. 2C Lower).
To determine the position of the phosphorylated residues, nonphosphorylated and phosphorylated c-Abl 593–730 were partially digested with trypsin, and the molecular weight of the resulting peptides was determined by matrix-assisted laser desorption ionization/time of flight spectrometry. From a comparison of the molecular weights of the phosphopeptides obtained from tryptic digestion of the phosphorylated and nonphosphorylated protein, it was possible to show phosphate in the region containing three adjacent serines at positions 618–620, which corresponded to the expected γ-PAK phosphorylation site KRXS.
Cotransfection of γ-PAK- and Cdc42-Induced Activation of c-Abl in Vivo.
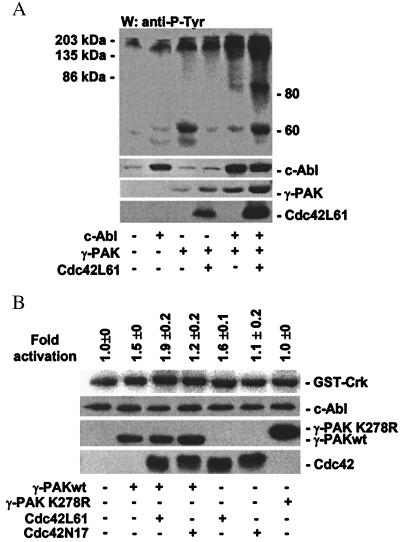
To determine whether expression of active γ-PAK altered intracellular protein phosphotyrosine levels in the presence or absence of c-Abl, γ-PAK, constitutively active Cdc42L61, and c-Abl were transfected into 293T cells, and cell lysates were analyzed by Western blotting with antiphosphotyrosine antibody. Transfection with c-Abl alone showed a slight increase in phosphorylation at the position of c-Abl (Fig. 3A). Transfection with γ-PAK alone or with Cdc42L61 induced an increase in tyrosine phosphorylation primarily in the 135- to 200-kDa range. Cotransfection of c-Abl with γ-PAK significantly enhanced the amount of phosphotyrosine at this position. When c-Abl, γ-PAK, and active Cdc42 were cotransfected, a phosphoprotein migrating at 150 kDa, as well as two protein bands migrating around 80 and 60 kDa, was observed. The latter is likely to be p62 Dok (31).
Figure 3.
Effects of γ-PAK on c-Abl activity and on phosphotyrosine levels. (A) c-Abl, HA-γ-PAK, and active HA-Cdc42L61 were transfected into 293T cells and analyzed by Western blotting with a mixture of PY99 and 4G10 antiphosphotyrosine antibodies. c-Abl, γ-PAK, and Cdc42L61 were detected by Western blotting with 8E9 or anti-HA antibody. (B) HA-γ-PAKwt, HA-γ-PAK K278R, and mutants of HA-Cdc42 were transfected into 293T cells. Endogenous c-Abl tyrosine kinase was assayed following immunoprecipitation of c-Abl with K12 antibodies by using GST-Crk as substrate. Lysates were simultaneously analyzed for c-Abl, γ-PAK, and Cdc42 proteins by Western blotting. Mean activation ± SEM of three independent experiments is given.
To determine whether γ-PAK could alter the tyrosine kinase activity of c-Abl in vivo, γ-PAKwt or the inactive γ-PAK mutant K278R was transiently transfected into HEK 293T cells along with constitutively active Cdc42L61 or inactive Cdc42N17. Endogenous c-Abl kinase activity was assayed after immunoprecipitation by using GST-Crk as a substrate. Fig. 3B shows transfection of γ-PAKwt alone induced a consistent 1.5-fold activation of c-Abl, whereas the γ-PAK kinase-inactive mutant K278R had no effect on c-Abl activity. Cotransfection of constitutively active Cdc42 (Cdc42L61) with γ-PAKwt enhanced c-Abl activity about 2-fold as compared with the activity of the control. Because there was less than 100% transfection efficiency (40–70%), the fold activation observed on transfection with γ-PAK and active Cdc42 most likely represents an underestimate of the activation of endogenous c-Abl. Cotransfection of the inactive Cdc42 mutant Cdc42N17 with γ-PAK had a slight effect on c-Abl activity and acted more as a dominant-negative mutant. Inactive Cdc42N17 or γ-PAK K278R alone had no effect on c-Abl activity. Cdc42L61 alone was able to induce c-Abl activation (1.6-fold), suggesting that the small G protein directly activated endogenous PAK or acted through other downstream effectors.
c-Abl-Induced Accumulation of Inactive γ-PAK.
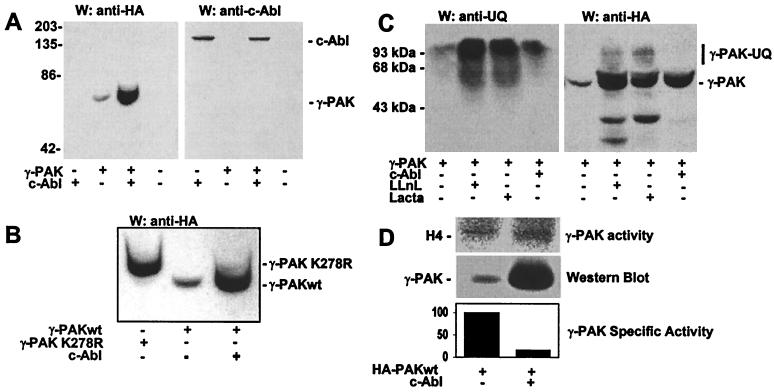
Following transient transfection of γ-PAK, the levels of recombinant γ-PAKwt protein were less than those of endogenous γ-PAK, as observed by Western blotting with antibodies against total γ-PAK and the HA epitope in recombinant γ-PAK.¶ In contrast, levels of γ-PAK K278R protein were 8-fold higher than the recombinant wild-type protein (Z. Huang and J.A.T., unpublished results). Cotransfection of c-Abl with γ-PAKwt induced a significant accumulation of γ-PAK protein, as shown by Western blotting (Fig. 4A Left). The levels of γ-PAK were 17-fold higher than when γ-PAK was transfected alone. There was no increase in the level of c-Abl protein coexpressed with γ-PAK (Fig. 4A Right). The increased levels of γ-PAK in cells cotransfected with c-Abl resembled the expression levels of the kinase-inactive mutant K278R (Fig. 4B). This mutant, in contrast to γ-PAKwt, accumulated in 293T cells on transfection, reaching levels similar to those of other proteins expressed using the same vector (data not shown).
Figure 4.
c-Abl induces γ-PAK accumulation and inactivation. (A) Western blots of lysates from cells transfected with c-Abl, HA-γ-PAK, or both enzymes using anti-HA antibodies (Left) or anti-Abl antibodies (Right). (B) Western blots of lysates of cells transfected with HA-γ-PAK K278R, HA-γ-PAKwt, or HA-γ-PAKwt and c-Abl using anti-HA antibodies. (C) Western blots using anti-ubiquitin (Left) or anti-HA antibodies (Right) of total extracts from cells transfected with HA-γ-PAK and treated for 9 h with 50 μM LLnL or 10 μM lactacystin (Lacta). Nontreated cells and cells coexpressing c-Abl are shown for comparison. UQ, ubiquitin. (D) Following immunoprecipitation with HA antibody from cells transfected with HA-γ-PAK alone or with c-Abl, γ-PAK activity was assayed with H4. γ-PAK protein was detected simultaneously by Western blotting with anti-HA antibody. Mean activation of three independent experiments is shown.
To determine whether the steady-state level of γ-PAK in exponentially growing cells was mediated by down-regulation through the ubiquitin/proteasome system, 293T cells transfected with γ-PAK were incubated with the proteasome inhibitors LLnL or lactacystin. Both inhibitors induced an accumulation of ubiquitin-containing proteins, as shown by Western blotting with anti-ubiquitin (Fig. 4C Left). LLnL and lactacystin also caused an accumulation of γ-PAK as shown by Western blotting with anti-HA-tag (Fig. 4C Right). The levels of γ-PAK obtained with the proteasome inhibitors were similar to the levels of γ-PAK induced by cotransfection with c-Abl. In addition, higher molecular weight forms of γ-PAK were observed as a result of ubiquitination (Fig. 4C Right). The inhibitors did not lead to changes in the accumulation of γ-PAK K278R, and no higher molecular weight forms of K278R were observed (data not shown). The results indicate that γ-PAK can be down-regulated by the ubiquitin/proteasome pathway and that γ-PAKwt can be stabilized by c-Abl.
c-Abl stabilization of γ-PAK could be the result of inactivation of γ-PAK by the tyrosine kinase. To test this hypothesis, the activity of γ-PAK was measured in extracts of cells transfected with γ-PAKwt alone or cotransfected with c-Abl (Fig. 4D). By using H4 as substrate, γ-PAK activity was increased 2.9-fold on cotransfection of c-Abl, with a 17-fold increase in the amount of γ-PAK protein; thus, c-Abl caused a 6-fold decrease in the specific activity of γ-PAK. It was concluded that c-Abl induced inactivation of γ-PAK or interfered with its activation by other cellular factors.
c-Abl Phosphorylated γ-PAK on Tyrosine.
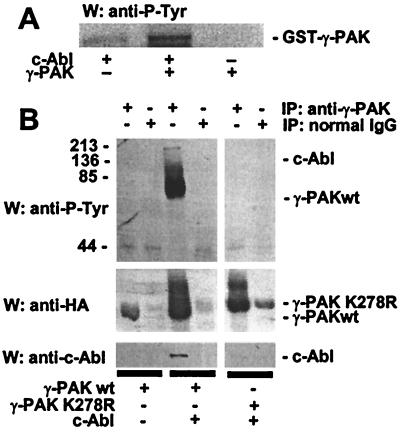
To determine whether c-Abl could phosphorylate γ-PAK in vitro, 293T cells were transfected with c-Abl, and the tyrosine kinase was immunoprecipitated from cell extracts by using the K12 antibody. c-Abl immunoprecipitates were incubated with GST–γ-PAK and ATP, and phosphotyrosine was detected by Western blotting with the phosphotyrosine antibody PY99. As seen in Fig. 5A, γ-PAK was phosphorylated by c-Abl; no phosphotyrosine was detected in the absence of c-Abl.
Figure 5.
c-Abl induces tyrosine phosphorylation of γ-PAK. (A) GST–γ-PAK was phosphorylated by using immunoprecipitates from 293T cells transfected with c-Abl and analyzed by Western blotting with antiphosphotyrosine antibody (PY99). (B) HA-γ-PAKwt, HA-γ-PAK K278R, and c-Abl were transfected into 293T cells, immunoprecipitated with anti-γ-PAK N19 antibodies or normal rabbit IgG, and analyzed by Western blotting with a mixture of PY99 and 4G10 (Upper), with anti-HA antibodies (Middle), or with anti-c-Abl antibodies (8E9, Lower). The positions of γ-PAK and c-Abl are shown.
To determine whether c-Abl induced tyrosine phosphorylation of γ-PAK in vivo, γ-PAK was immunoprecipitated from 293T cells transfected with γ-PAK alone or with c-Abl. No phosphotyrosine was detected in immunoprecipitates from cells transfected with γ-PAK alone (Fig. 5B). When γ-PAK was immunoprecipitated from cells cotransfected with c-Abl, two bands of phosphotyrosine were detected at the positions of c-Abl and γ-PAK. Interestingly, when γ-PAK K278R was cotransfected with c-Abl, no phosphotyrosine was detected in the immunoprecipitates of γ-PAK at the position of K278R or the position of c-Abl. When the immunoprecipitates of K278R were analyzed by Western blotting with anti-Abl antibodies, no c-Abl was detected (Fig. 5B), although similar levels of c-Abl were observed in cells cotransfected with γ-PAKwt or γ-PAK K278R (data not shown). These results suggested that γ-PAKwt could be phosphorylated on tyrosine when complexed with c-Abl, and that kinase-inactive γ-PAK did not interact with c-Abl and was not phosphorylated on tyrosine.
Discussion
c-Abl and γ-PAK are coimmunoprecipitated from transiently transfected cells, suggesting that c-Abl and γ-PAK interact directly and/or as part of a larger oligomeric complex. In vitro, c-Abl expressed in insect cells is phosphorylated by γ-PAK, and γ-PAK is phosphorylated by c-Abl. When γ-PAK and Cdc42 are transfected into 293T cells, up to a 2.2-fold increase in endogenous c-Abl activity is observed in immunoprecipitates of c-Abl. An inactive mutant of Cdc42 (Cdc42N17) acts as a dominant-negative mutant when cotransfected with γ-PAK. Transfection of active Cdc42L61 alone activates c-Abl 1.6-fold; this can be explained by activation of endogenous PAK. Consistent with activation of c-Abl tyrosine kinase activity, cellular phosphotyrosine levels are significantly increased when c-Abl is coexpressed with γ-PAK and are enhanced further by cotransfection with γ-PAK and active Cdc42. In contrast, the kinase-inactive mutant γ-PAK K278R does not form a complex with c-Abl and has no effect on c-Abl activity.
Only a fraction of c-Abl is associated with γ-PAK; thus, only a fraction of total c-Abl would be activated by γ-PAK. Coexpression of c-Abl and γ-PAK induces accumulation of γ-PAK to levels 17-fold higher than with γ-PAK alone. This suggests that the amount of γ-PAK protein is tightly regulated in vivo. In contrast, the kinase-inactive mutant K278R alone accumulates to levels 4- to 5-fold higher than the wild-type enzyme, suggesting that only active or activatable γ-PAK is down-regulated. By using specific inhibitors of the proteasome, we have shown that this down-regulation is due to the ubiquitin–proteasome system. The observation that γ-PAK accumulates in cells coexpressing c-Abl at levels similar to or greater than those of kinase-inactive γ-PAK suggests that c-Abl can function to inhibit the protein kinase activity of γ-PAK, thus indirectly avoiding targeting of γ-PAK for degradation. When γ-PAK activity expressed in 293T cells is compared with that from cells coexpressing c-Abl and γ-PAK, the specific activity of γ-PAK is reduced 6-fold in the presence of c-Abl. These results suggest a feedback loop between activation of c-Abl by γ-PAK and inactivation of γ-PAK by c-Abl.
Direct control of γ-PAK activity by c-Abl is indicated by the observation that γ-PAK is phosphorylated on tyrosine when coexpressed with c-Abl. The fact that kinase-inactive γ-PAK is not phosphorylated on tyrosine under the same conditions, nor does it interact with c-Abl, argues that phosphorylation of γ-PAK by c-Abl leads directly to a decrease in the specific activity of γ-PAK. The fact that kinase-inactive γ-PAK K278R does not interact with Abl and is not phosphorylated or ubiquitinated can be explained by the significant structural changes between the inactive and active forms as identified by differences in migration on SDS/PAGE and by the partial x-ray crystallographic structure of α-PAK (PAK1), which reveals a multistage activation switch (32).
γ-PAK has cytostatic properties, as shown by injection of subfemtomolar amounts of γ-PAK into one cell of two-cell frog embryos (15) and by inhibition of cell growth on expression of active γ-PAK but not K278R.¶ γ-PAK is activated during apoptosis and has some apoptotic activity (12, 13). For these reasons, it is critical that γ-PAK activity be tightly regulated. This can occur by inhibition of γ-PAK activity through association with c-Abl, and possibly other proteins, and by degradation via the proteasome.
γ-PAK phosphorylates c-Abl on serine in the region of residues 618–620, which contains the expected γ-PAK phosphorylation site KRXS as predicted from substrate specificity studies with heptapeptides (30). Upstream of this region are proline-rich (PXXP) motifs that bind proteins such as c-Crk, Grb2, Nck, Abi-1, and Abi-2. The Abl C-terminal domain is also phosphorylated by other protein kinases such as p34Cdc2 and protein kinase C (19). The Abi adaptor binds to c-Abl and may stabilize the inactive form of c-Abl, or it may block access to substrates; mutated Abi activates the transforming properties of c-Abl (29). The phosphorylation of c-Abl by γ-PAK would introduce negative charges in the positively charged region that is immediately upstream of one of the two Abi-binding sites, which could change the affinity for Abi. In support of this possibility, a recent study has shown that the binding affinity of α-PAK for the Nck SH3 domain is significantly reduced by phosphorylation of serine residues within the Nck binding site at the amino terminus of α-PAK (33).
In addition to phosphorylating the Abi/Nck-binding site on c-Abl, γ-PAK phosphorylates the c-Abl protein kinase domain. Phosphorylation of the c-Abl catalytic domain by γ-PAK may alter the c-Abl kinase activity. Although γ-PAKwt has some basal activity when extracted from transfected 293T cells, the protein kinase depends on other factors to become fully active in vivo; to activate γ-PAK, we cotransfected an endogenously active form of Cdc42 (Cdc42L61) with the protein kinase. This action increased the levels of endogenous c-Abl activity by about 2-fold, indicating that active γ-PAK induced c-Abl activation in vivo. Alternatively, γ-PAK phosphorylation of serine in the c-Abl protein kinase domain and in region 618–620 may positively regulate c-Abl (e.g., by disrupting the Abl–Abi interaction), thus allowing other signaling pathways to fully activate the tyrosine kinase. Phosphorylation may alter its subcellular localization by inhibiting nuclear localization and increasing the c-Abl tyrosine kinase in the cytosol, where it could be activated by growth factor and adhesion receptors. These possibilities point to potential functional interactions between protein serine and tyrosine kinases.
Acknowledgments
We thank Dr. J. S. Gutkind, National Institutes of Health, Bethesda, for clones of Cdc42 mutants, Dr. Zhongdong Huang and Barbara Walter for advice and technical assistance, and Ciphergen for MS analysis of c-Abl. This research was supported by National Cancer Institute Grants F32CA73185 (to P.A.Z.) and CA70940 (to A.M.P.) and National Institutes of Health Grant GM26738 (to J.A.T.).
Abbreviations
- PAK
p21-activated protein kinase
- GST
gluthatione S-transferase
- LLnL
Z-Leu-Leu-Leu-norleucinal
- HA
hemagglutinin epitope
- γ-PAKwt
wild-type γ-PAK
- SH
Src homology
- HEK
human embryonic kidney
Footnotes
Huang, Z. & Traugh, J. A. (1999) FASEB J. 13, A1578 (abstr.).
References
- 1.Manser E, Lim L. Prog Mol Subcell Biol. 1999;22:115–133. doi: 10.1007/978-3-642-58591-3_6. [DOI] [PubMed] [Google Scholar]
- 2.Daniels R H, Bokoch G M. Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia S, Cerione R A. Trends Cell Biol. 1999;9:350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 4.Roig J, Traugh J A. In: Vitamins and Hormones. Litwack G, editor. Vol. 62. San Diego: Academic; 2000. , in press. [Google Scholar]
- 5.Dharmawardhane S, Sanders L C, Martin S S, Daniels R H, Bokoch G M. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galisteo M L, Chernoff J, Su Y C, Skolnik E Y, Schlessinger J. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 7.Tsakiridis T, Taha C, Grinstein S, Klip A. J Biol Chem. 1996;271:19664–19667. doi: 10.1074/jbc.271.33.19664. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z S, Manser E, Chen X Q, Chong C, Leung T, Lim L. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards D C, Sanders L C, Bokoch G M, Gill G N. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- 10.Roig J, Huang Z, Lytle C, Traugh J A. J Biol Chem. 2000;275:16933–16940. doi: 10.1074/jbc.M001627200. [DOI] [PubMed] [Google Scholar]
- 11.Roig J, Traugh J A. J Biol Chem. 1999;274:31119–31122. doi: 10.1074/jbc.274.44.31119. [DOI] [PubMed] [Google Scholar]
- 12.Rudel T, Bokoch G M. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 13.Lee N, MacDonald H, Reinhard C, Halenbeck R, Roulston A, Shi T, Williams L T. Proc Natl Acad Sci USA. 1997;94:13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter B N, Huang Z, Jakobi R, Tuazon P T, Alnemri E S, Litwack G, Traugh J A. J Biol Chem. 1998;273:28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- 15.Rooney R D, Tuazon P T, Meek W E, Carroll E J, Hagen J J, Gump E L, Monnig C A, Lugo T, Traugh J A. J Biol Chem. 1996;271:21498–21504. doi: 10.1074/jbc.271.35.21498. [DOI] [PubMed] [Google Scholar]
- 16.Faure S, Vigneron S, Doree M, Morin N. EMBO J. 1997;16:5550–5561. doi: 10.1093/emboj/16.18.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure S, Vigneron S, Galas S, Brassac T, Delsert C, Morin N. J Biol Chem. 1999;274:3573–3579. doi: 10.1074/jbc.274.6.3573. [DOI] [PubMed] [Google Scholar]
- 18.Pendergast A M. Curr Opin Cell Biol. 1996;8:174–181. doi: 10.1016/s0955-0674(96)80063-9. [DOI] [PubMed] [Google Scholar]
- 19.Van Etten R A. Trends Cell Biol. 1999;9:179–186. doi: 10.1016/s0962-8924(99)01549-4. [DOI] [PubMed] [Google Scholar]
- 20.Kharbanda S, Ren R B, Pandey P, Shafman T D, Feller S M, Weichselbaum R R, Kufe D W. Nature (London) 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 21.Wang J Y. Curr Opin Genet Dev. 1993;3:35–43. doi: 10.1016/s0959-437x(05)80338-7. [DOI] [PubMed] [Google Scholar]
- 22.Takao N, Mori R, Kato H, Shinohara A, Yamamoto K-I. J Biol Chem. 2000;275:725–728. doi: 10.1074/jbc.275.2.725. [DOI] [PubMed] [Google Scholar]
- 23.Wen S T, Jackson P K, Van Etten R A. EMBO J. 1996;15:1583–1595. [PMC free article] [PubMed] [Google Scholar]
- 24.Sawyers C L, Mclaughlin J, Goga A, Havlik M, Witte O. Cell. 1994;77:121–131. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 25.Koleske A J, Gifford A M, Scott M L, Nee M, Bronson R T, Miczek K A, Baltimore D. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 26.Plattner R, Kadlec L, DeMali K A, Kazlauskas A, Pendergast A M. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendergast A M, Muller A J, Havlik M H, Clark R, McCormick F, Witte O N. Proc Natl Acad Sci USA. 1991;88:5927–5931. doi: 10.1073/pnas.88.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobi R, Huang Z, Walter B N, Tuazon P T, Traugh J A. Eur J Biochem. 2000;267:4456–4464. doi: 10.1046/j.1432-1327.2000.01488.x. [DOI] [PubMed] [Google Scholar]
- 29.Dai Z H, Pendergast A M. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- 30.Tuazon P T, Spanos W C, Gump E L, Monnig C A, Traugh J A. Biochemistry. 1997;36:16059–16064. doi: 10.1021/bi9717845. [DOI] [PubMed] [Google Scholar]
- 31.Yamanishi Y, Baltimore D. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 32.Lei M, Lu W, Meng W, Parrini M-C, Eck M J, Mayer B J, Harrison S C. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z-S, Manser E, Lim L. Mol Cell Biol. 2000;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]