Abstract
Background
Hormonal alterations during development have lifelong effects on the prostate gland. Endogenous estrogens, including 17β-estradiol (E2), and synthetic estrogenic endocrine disruptors, such as bisphenol A (BPA), have similar effects on prostate development. Increasing exposure to estrogens within the low-dose, physiologic range results in permanent increases in the size and androgen responsiveness of the prostate, whereas exposure within the high-dose, pharmacologic range has the opposite effects.
Objectives
We tested the hypothesis that the low-dose effects of estrogens on the developing prostate are associated with increased expression of androgen receptor (Ar) and estrogen receptor 1 (α) (Esr1) genes in mesenchyme cells.
Methods
Ar and Esr1 mRNA levels were quantified in primary cultures of fetal mouse prostate mesenchyme cells treated with E2 and BPA.
Discussion
Ar and Esr1 mRNA expression increased in response to E2, with thresholds of 0.001 and 0.037 nM, respectively; and in response to BPA, with a threshold of 1 nM for both mRNAs. We did not observe the expected inhibition of Ar mRNA expression by pharmacologic levels of E2 relative to unexposed cells.
Conclusions
The observed induction of gene expression occurred at concentrations within the range of free E2 previously shown to permanently increase prostate size, thus supporting the involvement of direct effects of estrogens on gene expression in prostate mesenchyme. The effects of BPA occurred within the range of concentrations currently measured in human serum, demonstrating the vulnerability of developing tissues to xenoestrogens.
Keywords: 17β-estradiol, androgen receptor gene, bisphenol A, dose–response relationship, estrogen receptor 1 (α) gene, prostate, sexual differentiation
During fetal life, alterations in normal prostate gland development can produce permanent changes that persist throughout adulthood and may increase the risk of disease in later life (Ho et al. 2006; Risbridger et al. 2005). The prostate differentiates from the cranial region of the urogenital sinus (UGS) (Marker et al. 2003). In humans, the first epithelial buds are observed in the lateral region of the UGS during the tenth week of gestation in a pattern that shows a remarkable similarity to that of bud development in mice and rats during the early phase of gland genesis (Timms et al. 1994). Prostate ductal budding begins on gestation day (GD) 17 in mice (2 days before birth) (Timms et al. 1994). Prostate development is dependent on 5α-dihydrotestosterone (DHT) production from testosterone within the UGS mesenchyme (Marker et al. 2003). Androgen receptor expression in prostatic mesenchyme is required for directing growth and branching morphogenesis of epithelial buds, presumably by induction of paracrine factors secreted by mesenchyme (Cunha and Donjacour 1987; Kokontis and Liao 1999). During development, prostatic epithelial cells exhibit little androgen binding, and androgen receptor protein expression in epithelium is not required for differentiation (Cunha and Donjacour 1987; Prins and Birch 1995; Timms et al. 1999). Therefore, fetal mouse UGS mesenchyme cells provide an informative model of endocrine control of prostate development.
There is now considerable evidence that estrogens modulate the activity of androgens in regulating prostate development. The UGS mesenchyme in mice and rats responds to estrogens via estrogen receptor 1 (α ), whereas in the human prostate estrogen receptor 2 (β ) may mediate most responses to estrogens during development (Adams et al. 2002; Prins et al. 1998). Prostatic growth and androgen receptor ligand-binding activity are permanently decreased in response to high, pharmacologic doses of both natural and xenobiotic estrogens during development (Prins and Birch 1995; Rajfer and Coffey 1978; vom Saal et al. 1997). In contrast, increases in prenatal estrogen levels within the physiologic range (the normal range for endogenous estradiol) stimulate prostate development, leading to permanently increased prostate size and androgen receptor ligand-binding activity (Gupta 2000; Timms et al. 1999; vom Saal et al. 1997).
Estrogenic endocrine disruptors have the potential to alter prostate development in a manner similar to that of endogenous estradiol. In this study, we chose to examine bisphenol A (BPA), the monomer used to make polycarbonate plastic and as an additive in many other plastic products. BPA is produced in excess of 6 billion pounds per year, and the potential for human exposure is great due to leaching from plastic and plastic-lined metal food and beverage containers, as well as from dental sealants (Takao et al. 2002; Welshons et al. 2006).
We have proposed that one mechanism by which fetal estrogen exposure stimulates prostate development is by increasing prostatic androgen receptor gene [Ar; GenBank accession no. X53779 (Benson et al. 2007)] expression, thereby increasing the androgen responsiveness of the developing prostate, leading to enhanced gland genesis and growth (Richter et al. 2005; vom Saal et al. 1997). In the present study we sought to determine whether the endogenous hormone 17β-estradiol (E2), within its physiologic range, and the manmade estrogenic endocrine disruptor BPA, within the range measured in human serum (Schönfelder et al. 2002), directly influence Ar and estrogen receptor 1 (α ) (Esr1; GenBank accession no. NM_007956.2) gene expression at the transcriptional level in fetal mouse UGS mesenchyme.
Materials and Methods
Animals, housing, mating, and organ collection
CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and bred at the University of Missouri in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Animals were housed on corn-cob bedding in standard polypropylene cages. They received water purified by ion exchange and carbon filtration from glass bottles. Pregnant and lactating females were fed Purina 5008 chow (Purina Mills, St. Louis, MO). After being weaned, animals were fed Purina 5001 chow (Ralston Purina). Rooms were maintained at 25 ± 2°C under a 12 hr:12 hr light:dark cycle. Animals were treated humanely and with regard for alleviation of suffering. Animal procedures were approved by the University of Missouri Animal Care and Use Committee and conformed to the NIH Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996).
Tissue collection, primary cell culture, and dosing
Timed-pregnant females were killed on GD17 (mating = GD0) by CO2 asphyxiation, and fetuses were removed from the uterine horns. The bladder and UGS were removed from male fetuses as previously described (Timms et al. 1999; vom Saal et al. 1997). The prostatic region of the UGS was removed from the bladder at the bladder neck, and mesenchymal cells were isolated as described by Gupta (1999). Briefly, UGS tissue was disrupted by digestion with 3 mg collagenase type I/mL (Sigma Chemical Co., St. Louis, MO) for 30–50 min at 37°C in a shaking water bath followed by manual pipetting. Clumps of epithelium were allowed to settle out, and suspended mesenchymal cells were collected and cultured in complete medium [RPMI-1640 without phenol red (Gibco, Grand Island, NY) supplemented with 2 mM l-glutamine, 100 U penicillin G sodium/mL, 100 mg streptomycin sulfate/mL, and 0.25 mg fungizone/mL] with 10% (vol/vol) fetal bovine serum (FBS; U.S. Bio-Technologies, Parkerford, PA). Cells were grown to 95% confluence and then passaged by digestion with 0.05% trypsin in 0.53 mM EDTA (Gibco) for 5 min at room temperature. Cell viability was assayed with alamarBlue (BioSource International, Camarillo, CA) according to the manufacturer’s instructions.
We characterized the cell-type composition of the UGS cell primary cultures by immunofluorescent staining of cytokeratins with mouse anti-pan-cytokeratin clone PCK-26 fluorescein isothiocyanate conjugate (Sigma), and co-staining of the mesenchymal cell marker vimentin with goat anti-vimentin (Sigma) and rabbit anti-goat Cy3 conjugate (Sigma) (Prins et al. 1991).
During experimental treatments with E2, BPA, tamoxifen, and raloxifene, FBS was charcoal-stripped to remove all hormones, and cells were maintained in a constant background of 690 pM DHT (200 pg/mL). Cells were treated with DHT rather than testosterone to control for potential treatment effects on the intracellular concentration of this high-affinity ligand for the androgen receptor, which is formed from testosterone in UGS mesenchyme cells in vivo, and also to avoid the intracellular metabolism of testosterone to E2 by aromatase; DHT is not a substrate for aromatase (Kokontis and Liao 1999). First passage cells were seeded onto 24-well plates at 7 × 104 cells/well in estrogen-free complete medium with 5% (vol/vol) charcoal-stripped FBS, 5% (vol/vol) charcoal-stripped horse serum (Sigma), 690 pM DHT (Steraloids, Wilton, NH), and ITS supplement (insulin-transferrin-selenium; Cambrex, Walkersville, MD) for final concentrations of 10 μg insulin/mL, 10 μg transferrin/mL, and 10 ng selenium/mL. Cells were maintained in this estrogen-free medium for 3 days, with one medium change, before the start of treatments. E2, BPA, and tamoxifen were obtained from Sigma. Raloxifene (LY 156,758) was obtained from Eli Lilly (Indianapolis, IN). During treatments with E2 and BPA, cells were grown for 4 days, and the medium was changed every day, except where noted. The concentration of E2 in culture medium during treatments was measured by radio-immunoassay as previously described by vom Saal et al. (1990).
Real time RT-PCR measurement of gene expression
Total RNA was isolated with the RNAqueous kit (Ambion, Austin, TX) according to the manufacturer’s instructions. Total RNA was quantified by absorbance at 260 nm. Expression of specific mRNAs were measured by one-step real-time reverse transcription-polymerase chain reaction (RT-PCR) as described by Bustin (2000), with the TaqMan EZ RT-PCR kit (PE Applied Biosystems, Foster City, CA) on the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems). The concentrations of Mn2+, probe, and primers were optimized for each primer/probe set. Primer/probe sets for Ar, vimentin (Vim; GenBank accession no. NM_011701.3), and acidic ribosomal phosphoprotein P0 (Arbp; GenBank accession no. NM_007475.2) were designed using Primer Express software (PE Applied Biosystems) and are shown in Table 1. Primers were designed to span exon boundaries in order to prevent amplification of genomic DNA. Primers were synthesized by Invitrogen (Carlsbad, CA), and probes were synthesized by PE Applied Biosystems. The primer/probe set for Esr1 was TaqMan Gene Expression Assay ID Mm00433149_m1 (PE Applied Biosystems), which spans Esr1 exons 3–4.
Table 1.
Sequences of primers and probes for real time RT-PCR assays.
| Gene | Sequence (5′ → 3′) | 5′ position in CDS | Exon boundary |
|---|---|---|---|
| Ar | |||
| Forward | TGTCAAAAGTGAAATGGGACC | 1494 | |
| Reverse | TGGTACTGTCCAAACGCATGT | 1567 | 1–2 at 1553 |
| Probe | TGGATGGAGAACTACTCCGGACCTTATGGG | 1516 | |
| Arbp | |||
| Forward | GAGATTCGGGATATGCTGTTGG | 289 | 2–3 at 302 |
| Reverse | GGCGATGGCACCAGCC | 351 | |
| Probe | CAATAAGGTGCCAGCTGCTGCTCG | 312 | |
| Vim | |||
| Forward | GCACCCTGCAGTCATTCAGA | 602 | |
| Reverse | CCACTTTCCGTTCAAGGTCAA | 673 | 3–4 at 660 |
| Probe | AGGATGTTGACAATGCTTCTCTGGCACG | 623 | |
CDS, coding sequence.
The relative concentrations of specific mRNAs in each sample were normalized to total RNA per well, as described by Bustin (2000) and Latil et al. (2001). Normalization to total RNA allowed for comparisons between independent experiments. In parallel experiments, total DNA per well was measured by fluorescence of Hoechst 33258 (Sigma), as described by Labarca and Paigen (1980). From these data, the average RNA/DNA ratio was calculated for each treatment; we used these values to convert the mRNA/total RNA measurements to mRNA/DNA to assess the effect of E2 and BPA on gene expression per cell.
Statistical analyses
Treatments were replicated in three wells per experiment and at least two, and in most cases more (up to 10), replicate experiments. Outliers were detected with Grubbs’ test (Grubbs 1969) and removed. Treatment effects were evaluated on untransformed data for RNA, and on reciprocals for DNA and gene expression, with the analysis of variance (ANOVA) general linear model procedure using SAS software (SAS Institute Inc., Cary, NC). We made planned comparisons of means for each treatment relative to controls using the least-squares means test only if the overall ANOVA showed significant treatment effects. To avoid inflation of error rates, we did not use multiple comparisons among all treatments. The criterion for statistical significance was p < 0.05.
Results
Characterization of UGS cells and nominal E2 concentration in primary culture
Consistent with previous reports (Gupta 1999), immunofluorescent staining for the mesenchymal cell marker vimentin revealed no epithelial cells in first passage cells treated for 5 days with 0.1 nM E2 or with no E2 (data not shown). The UGS primary cell cultures that we examined were thus homogenous populations of mesenchyme cells that retained mesenchymal differentiation markers throughout the incubation period.
After the initial administration of E2 in culture medium, the E2 concentration slowly decreased, presumably by metabolism, sequestration in cells, and/or binding to the tissueculture dish. In more detail, E2 was administered at a concentration of 1 nM, in the middle of the dose range in our experiment. The concentration of E2 in medium decreased by 2 hr to approximately 90% and by 18 hr to approximately 60% of the administered dose, and then remained stable through the remaining time period examined (up to 48 hr).
In the experiments we conducted, test chemicals were added to medium every 24 hr. Thus, at the midpoint of the dose–response curve tested, the actual E2 concentration in the culture medium was approximately 60% of the initial concentration at the time we collected the cells for analysis of mRNA. Measurement of DNA and RNA content and induction of gene expression confirmed that bioactive amounts of E2 were thus present at nominal concentrations < 0.001 nM (Figures 1 and 2).
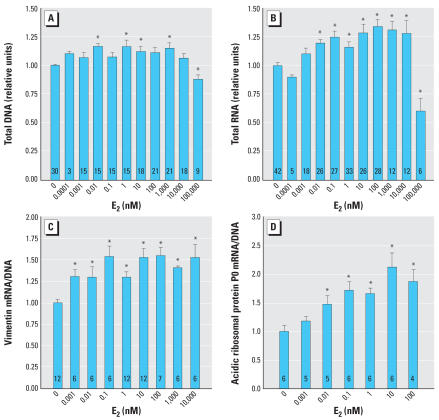
Figure 1.
Indicators of cell growth increase with E2 dose. (A) DNA content slightly increased with E2 in a dose-independent manner up to 10,000 nM and decreased significantly at 100,000 nM E2. (B) Total RNA content increased with E2 in a dose-dependent manner up to 10,000 nM and decreased significantly at 100,000 nM E2. (C and D) Gene expression of the cytoskeleton protein vimentin (C) and the ribosomal component acidic ribosomal phosphoprotein P0 (D) increased with E2 treatment. Units are fold induction relative to the control. Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05).
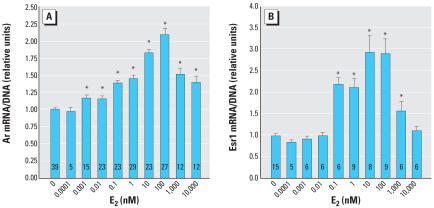
Figure 2.
Biphasic Ar and Esr1 gene expression responses induced by E2. (A) Ar mRNA expression increases with increasing dose of E2 up to a dose of 100 nM. A dose of 0.001 nM (0.27 pg/mL) is within the physiologic range of free E2 in mouse fetuses, and a significant response to this dose is consistent with prior in vivo findings (vom Saal et al. 1997). (B) Esr1 mRNA expression increases with increasing dose of E2 up to a dose of 100 nM. Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05).
E2 induces growth of UGS mesenchyme cells
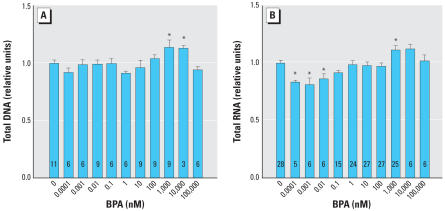
E2 treatment induced a small increase in cell growth and proliferation as indicated by DNA and RNA content at doses of 0.01–10,000 nM (Figure 1A, 1B). At 100,000 nM E2, inhibition of cell growth and proliferation was observed (Figure 1A, 1B). Cell viability was not affected at any E2 dose tested (data not shown). Subsequent experiments used a dose range of 0.0001–10,000 nM in order to avoid the cell growth–inhibiting effects of very high doses of E2. Relative total RNA was induced to a greater degree than DNA (Figure 1A, 1B). The housekeeping genes Vim, a component of the cytoskeleton in mesenchyme cells, and Arbp, a component of the ribosome, were examined to determine whether either could be used as a reference gene. However, both of these genes increased expression in response to E2, consistent with a general induction of cell growth (Figure 1C, 1D). Vimentin and acidic ribosomal phosphoprotein P0 exhibited differently shaped dose–response curves, suggesting differences in their mechanisms of transcriptional regulation by E2. Because neither house-keeping gene was an appropriate control gene, expression of specific mRNAs was normalized to DNA.
E2 induces the steroid receptor mRNAs Ar and Esr1
Ar mRNA expression was induced by E2 up to just over 2-fold above control levels (Figure 2A). The observed threshold of induction, 0.001 nM, is slightly higher than the measured free serum E2 concentration of 0.00077 nM or 0.21 pg/mL in male mouse fetuses on GD18 (vom Saal et al. 1997). The increase in Ar mRNA with E2 dose was monotonic up to 100 nM E2. At doses of ≥ 1,000 nM E2, Ar mRNA levels declined relative to the maximum observed induction at 100 nM E2. Inhibition of cell growth was only evident at 100,000 nM E2.
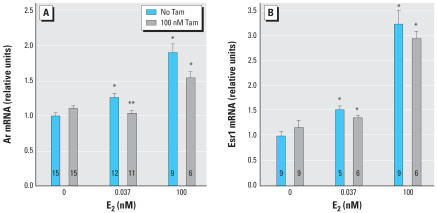
The induction of Ar mRNA by a physiologically relevant level of E2, 0.037 nM (10 pg/mL), was significantly inhibited by antiestrogen treatment (Figure 3A). The anti-estrogen raloxifene (100 nM) had similar effects to 100 nM tamoxifen (raloxifene data not shown). The inhibition of the Ar response to E2 by tamoxifen was overcome by addition of a pharmacologic dose of 100 nM E2, demonstrating that the inhibition by tamoxifen observed at 0.037 nM E2 is not due to cytotoxicity or other nonspecific effects (Figure 3A).
Figure 3.
Antiestrogen treatment inhibits E2-induced expression of Ar but does not significantly inhibit E2-induced expression of Esr1. (A) The antiestrogen tamoxifen (Tam) blocks induction of Ar mRNA by a physiologic dose of E2, and the inhibition by Tam is overcome by a pharmacologic dose of E2. (B) Tam does not significantly inhibit induction of Esr1 mRNA by a physiologic dose of E2, or by a pharmacologic dose of E2. Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05). **Significant differences between the same E2 treatment with and without Tam (p < 0.05).
Esr1 mRNA expression was induced by E2 by approximately 3-fold over the control, with a threshold at 0.037 nM (Figure 3B) and a peak at 10–100 nM E2 (Figure 2B). The induction of Esr1 mRNA by 0.037 nM E2 was not significantly inhibited by anti-estrogen treatment (Figure 3B).
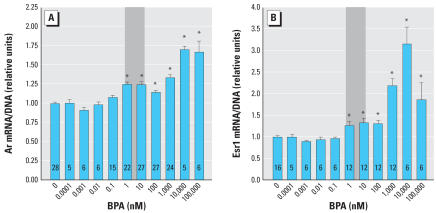
BPA acts as an estrogen agonist in UGS mesenchyme cells
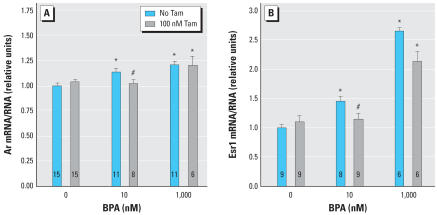
The effects of BPA on cell growth as indicated by DNA and RNA content (Figure 4) were much less pronounced than the effects of E2 (Figure 1). The dose–response curve for RNA was biphasic, with significant reductions in RNA content, but not DNA, at very low concentrations of BPA (Figure 4). Ar and Esr1 mRNA expression were induced by BPA treatment (Figure 5). The dose–response curves for BPA were shifted to the right by approximately 1,000-fold for Ar and approximately 30-fold for Esr1, relative to E2 (based on the significant stimulation of Esr1 in response to 0.037 nM E2; Figure 3B). As indicated in Figure 5A and 5B, a significant increase in both Ar and Esr1 transcription occurred at BPA concentrations within the range typically reported in human blood and tissues, including fetal blood (Schönfelder et al. 2002; Welshons et al. 2006). The induction of both Ar and Esr1 mRNAs by a physiologically relevant low dose of BPA (10 nM) was inhibited by a 100-nM dose of tamoxifen. For both genes, the inhibition by tamoxifen was overcome by a high dose of BPA (1,000 nM) (Figure 6).
Figure 4.
Indicators of cell growth in response to BPA. (A) Total DNA content was stable at low doses of BPA and significantly increased only at 1,000 nM. (B) Total RNA was significantly decreased at very low doses of BPA, 0.0001–0.001 nM, and significantly increased only at 1,000 nM. Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05).
Figure 5.
Gene expression of Ar and Esr1 are induced by the synthetic estrogen BPA. (A) Ar mRNA expression increases with increasing dose of BPA. (B) Biphasic Esr1 mRNA expression response increases with increasing dose of BPA up to a dose of 10,000 nM (10 μM or 2.28 μg/mL). Shaded areas represent the typical range of concentrations of unconjugated BPA measured in human serum (Welshons et al. 2006). Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05).
Figure 6.
Antiestrogen treatment inhibits BPA-induced expression of steroid receptors Ar and Esr1. (A) The antiestrogen tamoxifen (Tam) blocks induction of Ar mRNA by a 10-fold lower dose of BPA, and the inhibition by tamoxifen is overcome by a 10-fold higher dose of BPA. (B) Tam inhibits induction of Esr1 mRNA by a 10-fold lower dose of BPA, and the inhibition by Tam is overcome by a 10-fold higher dose of BPA. Error bars represent 1 SE. The number of replicates measured for each treatment is shown in each bar.
*Values significantly different from the control (p < 0.05). **Significant differences between the same BPA treatment with and without Tam (p < 0.05).
Discussion
The aims of the present study were to investigate whether there were direct effects on Ar and Esr1 gene activity that could be related to the previously observed stimulatory effect on prostate development caused by prenatal exposure to serum concentrations of bioactive E2 in fetal mice and the concentration of bioactive BPA currently measured in human fetal serum. We found that both the natural estrogen E2 and the synthetic estrogenic endocrine disruptor BPA stimulated increases in prostate Ar and Esr1 mRNAs. The stimulation occurred at physiologically relevant part-per-trillion doses of E2, and at parts-per-billion doses of BPA, which are within the range found in human fetal blood (reviewed by Welshons et al. 2006).
Exposure of male mouse and rat fetuses to slightly elevated estrogen levels results in permanent prostate enlargement and elevated androgen receptor levels in adulthood (Timms et al. 1999; vom Saal et al. 1997). Some confusion concerning effects of estrogen on the prostate has been created by studies in which only very high, pharmacologic (supraphysiologic) doses of estrogen were tested. Effects of pharmacologic doses of estrogenic chemicals on prostate development are not predictive of effects at physiologic doses. The dramatic effects of physiologic doses of estrogens have been revealed in studies in which pregnant mice were administered low doses of E2, the drugs diethylstilbestrol (DES) and ethinylestradiol, BPA, or the estrogenic insecticide methoxychlor, resulting in a permanent increase in prostate size in male offspring (Gupta 2000; Nagel et al. 1997; Thayer et al. 2001; vom Saal et al. 1997; Welshons et al. 1999). As the dose of E2 or DES was increased into the pharmacologic range, the stimulating effect observed at low doses disappeared and inhibition of prostate development occurred (Gupta 2000; Timms et al. 2005; vom Saal et al. 1997). Thus, the inhibitory effects of pharmacologic doses of estrogens on the developing prostate are opposite to effects of physiologic doses of the same estrogenic chemicals.
Available data on short-term effects of developmental estrogen exposures are consistent with the long-term effects observed in adulthood. For example, a high, pharmacologic dose of estradiol benzoate given to neonatal rats induced down-regulation of androgen receptor protein expression as early as postnatal day (PND) 6 (Prins and Birch 1995). In contrast, low, physiologically relevant doses of estrogenic chemicals (DES and BPA) fed to pregnant mice induced up-regulation of prostatic androgen receptor ligand binding activity in male offspring as early as PND3; this observation was replicated in organ culture of fetal mouse prostate treated with 0.1 pg/mL DES or 50 pg/mL BPA (Gupta 2000). The increase in prostate size in response to 50 pg/mL (0.22 nM) BPA in organ culture is below the threshold observed for stimulation of either Ar or Esr1 gene expression observed in the present study.
Our findings support the hypothesis that prenatal exposure to elevated estrogen levels permanently increases prostate size and androgen responsiveness at least in part by inducing Ar mRNA expression. Importantly, the effects on Ar mRNA expression occurred with a threshold at 0.001 nM E2 (0.28 pg/mL), consistent with concentrations previously shown to alter prostate development in vivo. Specifically, the total serum E2 concentration in male mouse fetuses on GD18 is approximately 0.35 nM (94 pg/mL), and the free (unconjugated and unbound to plasma proteins) serum concentration of E2 is 0.00077 nM (0.21 pg/mL), or 0.2% of total serum E2 (vom Saal et al. 1997), similar to findings in rats (Montano et al. 1995). An increase in free serum E2 in male mouse fetuses (due to maternal administration of E2 via Silastic capsule) to 0.0012 nM (0.31 pg/mL) significantly increased prostate size and the number of prostatic androgen receptors in adulthood (vom Saal et al. 1997). Our results show that at these same physiologic doses, E2 acts directly on fetal UGS mesenchyme cells to increase Ar mRNA expression. This response was inhibited by the antiestrogens raloxifene (data not shown) and tamoxifen (Figure 3), suggesting that the induction of Ar mRNA by E2 is mediated through the classical genomic estrogen receptor pathway.
The differences in the shapes of the dose–response curves for Ar and Esr1 suggest that the receptors are regulated by distinct mechanisms. Distinct dose–response relationships were also noted for vimentin, acidic ribosomal protein P0, total RNA content, and DNA content. These findings are consistent with data from microarray studies demonstrating considerable diversity in dose–response relationships of different estrogen-responsive genes; in particular, as one moves across the dose–response curve, entirely different sets of genes are activated or inhibited (Coser et al. 2003; Shioda et al. 2006). Induction of Ar and Esr1 also displayed different responses to inhibition by anti-estrogens, in that Esr1 induction was not significantly inhibited by antiestrogen co-treatment (Figure 3). The threshold for effects on Esr1 expression was between 0.01 nM (2.3 ng/mL) and 0.037 nM (8.4 ng/mL) E2 (Figures 2B and 3B). This is above the normal range of free E2 in serum in male mouse fetuses. However, serum estradiol may underestimate estrogen levels in prostate tissue because cells in the developing prostate express aromatase, which metabolizes testosterone to E2 (Ellem and Risbridger 2006; Risbridger et al. 2003), and because estrogen receptor agonists, including xenoestrogens, exhibit additive effects in combination (Rajapakse et al. 2002). The induction of Esr1 expression by E2 suggests that estrogen exposure may create a positive feedback loop in the UGS, such that exposure to estrogens increases sensitivity to future or continuing exposure.
Although the observed effects of physiologic concentrations of E2 on Ar mRNA expression are consistent with established in vivo effects, our pharmacologic dose range (e.g., 100 nM) in vitro observations are not consistent with established in vivo effects (Gupta 2000; Prins and Birch 1995; vom Saal et al. 1997), which predicted a decline in Ar mRNA expression relative to control levels at this high dose of E2 (Figure 2A). The in vivo regulation of androgen receptors by pharmacologic doses of estrogens may thus involve systemic and posttranscriptional effects that are not observable in terms of Ar mRNA levels in isolated mesenchyme cells. The involvement of posttranscriptional mechanisms is supported by the observation that developmental exposure to pharmacologic doses of estrogens permanently up-regulates androgen receptor degradation by the proteasome (Woodham et al. 2003).
The behavior of BPA in this system is consistent with the established activity of BPA as an estrogen receptor agonist (reviewed by vom Saal and Hughes 2005; vom Saal and Welshons 2006; Welshons et al. 2006), which was first reported in 1936 (Dodds and Lawson). The weak effects of BPA on cell growth, measured as DNA and RNA content, are consistent with previous reports that the relative potency of BPA is greater in stimulating estrogen receptor–dependent gene transcription than in stimulating growth of uterine tissue (Nagel et al. 2001). There are several interesting differences between the dose–response curves of Ar and Esr1 in response to BPA compared with E2. Based on the thresholds of induction of gene expression, BPA is approximately 1,000-fold less potent than E2 for induction of Ar, but only about 30-fold less potent than E2 for induction of Esr1 (based on a threshold of Esr1 induction of 0.037 nM E2; Figure 3B). In addition, the shape of the dose–response curve for Esr1 differs between E2 and BPA; induction of Esr1 by BPA was inhibited by tamoxifen, whereas induction of Esr1 by E2 was not significantly inhibited by tamoxifen. These differences between E2 and BPA, which are seen in the Esr1 dose–response curves but not the Ar dose–response curves, underline the probability that distinct mechanisms are at work in the induction of Ar and Esr1 by estrogens.
Of great importance, the doses of BPA required for induction of both Ar and Esr1 are within the range of typical levels of BPA measured in human serum, which range from approximately 1 to 10 nM (Figure 5) (Schönfelder et al. 2002; Welshons et al. 2006). Because our experiments measured the response to BPA in the absence of other estrogens, they are likely to underestimate the induction of Ar and Esr1 expression in response to the additive mixture of endogenous estrogens, BPA, and other xenoestrogens to which humans are continuously exposed in our modern world (Colborn et al. 1993). The consequences of developmental induction of Ar and Esr1 for the adult phenotype of the prostate have not been directly examined, but exposure during fetal life to very low doses of BPA (2–50 μg/kg/day) permanently increases prostate size in mice (Gupta 2000; Nagel et al. 1997). Neonatal exposure to a 10 μg/kg/day dose of BPA results in precancerous lesions (prostate interepithelial neoplasia) in adult male rats, associated with epigenetic changes (Ho et al. 2006). The report of Ho et al. (2006) is consistent with the finding that estrogenic chemicals stimulate an abnormal rate of proliferation in basal epithelial cells in the primary ducts of the mouse fetal prostate (Timms et al. 2005). Basal cells are progenitor cells proposed to be involved in prostate cancer (Kirschenbaum et al. 2006). We are currently examining whether the permanent increase in prostate AR receptor protein in mice exposed during fetal life to low doses of estrogenic chemicals is caused by a permanent increase in expression of the Ar gene, and whether this is associated with a change in DNA methylation at the Ar gene.
Conclusions
Ar mRNA in mesenchyme cells isolated from fetal mouse prostate is up-regulated by E2 within its physiologic range, and by BPA within the range detected in human fetal serum. Induction of Ar mRNA by E2 or BPA was inhibited by antiestrogen co-treatment. Therefore, the low-dose effects of estrogens on prostatic Ar regulation are estrogen receptor–dependent, act at the transcriptional level, are mediated through local effects on UGS mesenchyme cells, and can be modeled in a primary cell culture system. In contrast, down-regulation of androgen receptor protein in response to high doses of estrogens in vivo likely includes systemic and post-transcriptional mechanisms. Esr1 mRNA is also up-regulated by E2 and BPA in a dose-dependent manner, suggesting the possibility of positive feedback in estrogen effects on the prostate. The induction of Esr1 by E2 is not significantly inhibited by antiestrogen treatment, suggesting the involvement of non-estrogen receptor–mediated mechanisms.
Taken together, these results are consistent with the hypothesis that prenatal exposure to elevated estrogen or xenoestrogen levels within the physiologic range results in an increase in androgen receptor and estrogen receptor 1 (α ) number in the developing prostate mesenchyme, which increases androgen and estrogen responsiveness and growth. The estrogen receptor–dependent induction of Ar by BPA confirms that this mechanism is not unique to E2 and underscores the vulnerability of the developing reproductive system to the additive effects of exogenous estrogenic endocrine disruptors.
Footnotes
Support was provided by S. Khan, G.S. Johnson, and D. Lubahn, and by grants ES-11283 (F.S.vS), 1F32ES-11549-01 (C.A.R.), and P01ES10535 (D. Lubahn) from the National Institute of Environmental Sciences (NIEHS).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, the National Institutes of Health, or the Public Health Service.
References
- Adams JY, Leav I, Lau KM, Ho SM, Pflueger SMV. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate. 2002;52:69–81. doi: 10.1002/pros.10103. [DOI] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. GenBank. Nucleic Acids Res. 2007;35:D21–D25. doi: 10.1093/nar/gkl986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coser KR, Chesnes J, Hur JY, Ray S, Isselbacher KJ, Shioda T. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc Natl Acad Sci USA. 2003;100:13994–13999. doi: 10.1073/pnas.2235866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Donjacour A. 1987. Stromal-epithelial interactions in normal and abnormal prostatic development. In: Current Concepts and Approaches to the Study of Prostate Cancer, Vol 239 (Coffey DS, Gardner WAJ, Bruchovsky N, Resnick MI, Karr JP, eds). New York:Alan R. Liss, Inc., 251–272. [PubMed]
- Dodds EC, Lawson W. Synthetic oestrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31:1–12. [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]
- Gupta C. Modulation of androgen receptor (AR)-mediated transcriptional activity by EGF in the developing mouse reproductive tract primary cells. Mol Cell Endocrinol. 1999;152:169–178. doi: 10.1016/s0303-7207(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224:61–68. doi: 10.1046/j.1525-1373.2000.22402.x. [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto JB, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epi-genetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources 1996. Guide for the Care and Use of Laboratory Animals. Washington, DC:National Academy Press.
- Kirschenbaum A, Liu XH, Yao S, Narla G, Friedman SL, Martignetti JA, et al. Sex steroids have differential effects on growth and gene expression in primary human prostatic epithelial cell cultures derived from the peripheral versus transition zones. Carcinogenesis. 2006;27:216–224. doi: 10.1093/carcin/bgi219. [DOI] [PubMed] [Google Scholar]
- Kokontis JM, Liao S. Molecular action of androgen in the normal and neoplastic prostate. Vitam Horm. 1999;55:219–307. doi: 10.1016/s0083-6729(08)60937-1. [DOI] [PubMed] [Google Scholar]
- Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Latil A, Bièche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, et al. Evaluation of androgen, estrogen (ERα and ERβ ), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253:165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Montano MM, Welshons WV, vom Saal FS. Free estradiol in serum and brain uptake of estradiol during fetal and neonatal sexual differentiation in female rats. Biol Reprod. 1995;53:1198–1207. doi: 10.1095/biolreprod53.5.1198. [DOI] [PubMed] [Google Scholar]
- Nagel SC, Hagelbarger JL, McDonnell DP. Development of an ER action indicator mouse for the study of estrogens, selective ER modulators (SERMs), and xenobiotics. Endocrinology. 2001;142:4721–4728. doi: 10.1210/endo.142.11.8471. [DOI] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Thayer KA, Dhar MD, Boechler M, Welshons WV. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bio-activity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997;105:70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Prins GS, Marmer M, Woodham C, Chang W, Kuiper G, Gustafsson J-Å, et al. Estrogen receptor-β messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology. 1998;139:874–883. doi: 10.1210/endo.139.3.5827. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xeno-estrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Invest Urol. 1978;16:186–190. [PubMed] [Google Scholar]
- Richter CA, Timms BG, vom Saal FS. 2005. Prostate development: mechanisms for opposite effects of low and high doses of estrogenic chemicals. In: Endocrine Disruptors: Effects on Male and Female Reproductive Systems (Naz RK, ed). New York:CRC Press, 379–410.
- Risbridger GP, Almahbobi GA, Taylor RA. Early prostate development and its association with late-life prostate disease. Cell Tissue Res. 2005;322:173–181. doi: 10.1007/s00441-005-1121-9. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Bianco JJ, Ellem SJ, McPherson SJ. Oestrogens and prostate cancer. Endocr Relat Cancer. 2003;10:187–191. doi: 10.1677/erc.0.0100187. [DOI] [PubMed] [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in human maternal–fetal–placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T, Chesnes J, Coser KR, Zou L, Hur J, Dean KL, et al. Importance of dosage standardization for interpreting transcriptomal signature profiles: evidence from studies of xenoestrogens. Proc Natl Acad Sci USA. 2006;103:12033–12038. doi: 10.1073/pnas.0605341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao Y, Lee HC, Kohra S, Arizono K. Release of bisphenol A from food can lining upon heating. J Health Sci. 2002;48:331–334. [Google Scholar]
- Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17α-ethinyl oestradiol. Hum Reprod. 2001;16:988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms BG, Mohs TJ, Didio LJA. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–1432. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- Timms BG, Petersen SL, vom Saal FS. Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses. J Urol. 1999;161:1694–1701. [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S. Paradoxical effects of maternal stress on fetal steroid and postnatal reproductive traits in female mice from different intrauterine positions. Biol Reprod. 1990;43:751–761. doi: 10.1095/biolreprod43.5.751. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, Thayer KA, Judy BM, vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Woodham C, Birch L, Prins GS. Neonatal estrogen down-regulates prostatic androgen receptor through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]