Abstract
Age-related changes in the hepatic sinusoid, called pseudocapillarization, may contribute to the pathogenesis of dyslipidaemia. Caloric restriction (CR) is a powerful model for the study of aging because it extends lifespan. We assessed the effects of CR on the hepatic sinusoid to determine whether pseudocapillarization is preventable and hence a target for the prevention of age-related dyslipidemia. Livers from young (6 months) and old (24 months) CR and ad libitum fed (AL) F344 rats were examined using electron microscopy and immunohistochemistry. In old age, there was increased thickness of the liver sinusoidal endothelium and reduced endothelial fenestration porosity. In old CR rats, endothelial thickness was less and fenestration porosity was greater than in old AL rats. Immunohistochemistry showed that CR prevented age-related decrease in caveolin-1 expression and increase in peri-sinusoidal collagen IV staining, but did not alter the age-related increase of von Willebrand’s factor. CR reduces age-related pseudocapillarization of the hepatic sinusoid and correlates with changes in caveolin-1 expression.
Keywords: liver sinusoidal endothelial cell, pseudocapillarization, caloric restriction, aging, caveolin-1, liver, fenestrations
INTRODUCTION
The implications of age-related impairment in liver function are well recognised (Le Couteur et al, 2005; Schmucker, 2005). One mechanism for this change is age-related alteration of the ultrastructure of the liver sinusoidal endothelium (Le Couteur et al, 2005). In old age, there is a 30–50% reduction in the area of the endothelium perforated by fenestrations (‘porosity’). This is associated with increased endothelial thickness and extracellular matrix in the space of Disse, including collagen and basal lamina (Cogger et al, 2003; Le Couteur et al, 2001; McLean et al, 2003; Warren et al, 2005). These ultrastructural changes are associated with increased expression of antigens not usually expressed in young healthy livers such as von Willebrands factor and collagen IV. This has been termed age-related pseudocapillarization.
There are several implications of age-related pseudocapillarization. The thickened endothelium and defenestration are likely to reduce the transfer of many substrates between the sinusoid and hepatocytes (Le Couteur et al, 2005), particularly lipoproteins (Hilmer et al, 2005; Le Couteur et al, 2002). We have shown that the loss of fenestrations in old age impedes the transfer of some lipoproteins from the blood to the hepatocytes, which provides a mechanism for age-related post prandial hypertriglyceridemia and impaired chylomicron remnant clearance (Hilmer et al, 2005). Therefore it is of therapeutic interest to determine whether pseudocapillarization is preventable through the effects of caloric restriction (CR).
CR increases longevity and changes delayed by CR are generally considered to be an integral part of the aging process (Ingram et al, 2004). Reducing caloric intake delays the onset of age-related diseases and increase maximum lifespan by between 20% and 40% in many species (Everitt et al, 2005). CR influences lipoprotein profiles and the onset of vascular disease in animal models (Zhu et al, 2004) and similar effects have replicated in short term studies in humans (Heilbronn et al, 2006).
We postulated that one mechanism for the effects of CR on lipoprotein metabolism and susceptibility to vascular disease might be related to its effects on the liver sinusoidal endothelium (Le Couteur et al, 2001). The liver sinusoidal endothelium is exquisitely sensitive to oxidative stress (Cogger et al, 2001; Cogger et al, 2004) and other toxic insults (McCuskey, 2006). Thus, it is plausible that the structure of the liver sinusoidal endothelium may be profoundly influenced by the quantity of the dietary load, with its concomitant oxidants delivered via the portal vein. Here we assessed whether CR reduces age-related pseudocapillarization of the liver sinusoidal endothelium.
MATERIALS AND METHODS
Young (6 months) and old (24 month) CR and AL Fisher F344 rats were obtained from the National Institute on Aging. CR was started at weaning and increased at 10% per week and reached 40% at 2.5 months. Ethical approval was from the Animal Care and Users Committee of the National Institute on Aging. Liver samples were obtained under anesthesia with pentobarbital. Electron microscopy was performed as previously described (Cogger et al, 2003; Le Couteur et al, 2001; Warren et al, 2005). Samples for scanning microscopy were examined using a Joel JSM 6380 scanning electron microscope. Ten random sinusoids were photographed from each liver. Analysis of fenestral diameter and frequency was made using Image J image analysis program. Total numbers of fenestrations counted were 2777 for young AL, 6291 young CR, 6290 for old AL and 5114 for old CR rats. For transmission microscopy, tissue was embedded in Spurrs resin. Twelve sinusoids randomly selected from each liver were viewed using a Zeiss 902 transmission electron microscope and captured with a Gatan BioScan Slow Scan CCD camera. Ten measurements from each of the twelve sinusoids of endothelial thickness were made from each sinusoid. Paraformaldehyde-fixed samples were stained with hematoxylin and eosin for light microscopy. Samples were also incubated with 0.1% Sirius red (a collagen stain). Immunohistochemistry was used to determine the expression of von Willebrand’s factor, collagen IV and caveolin-1. Antibodies used were mouse anti-human factor von Willebrands factor (Dako, Denmark), rabbit anti-human caveolin-1 (Santa Cruz, CA) and goat anti-human collagen IV (Zymed, CA).
Results of the image analysis are presented as the mean of the values for each field analysed ± standard error of the mean. P values reported are those derived from the Student-Newman-Keuls method if one-way ANOVA showed a significant difference (P<0.05) between the observations in the four groups. Two-way ANOVA was used to analyse the interaction between age and response to caloric restriction. Statistical calculations were performed using Sigmastat version 2.03 (SPSS Inc, CA).
RESULTS
The liver and body weights are shown in Table 1. The results of electron microscopy are shown in Table 2 and representative micrographs are shown in Figure 1. Old age was associated with reduced fenestration porosity in AL rats. CR rats had greater porosity than AL rats at both 6 and 24 months of age. Many fenestrations were noted that seemed to overlay stellate cells, giving the appearance of non-perforated fenestrations (Figure 2). These occurred most frequently in the old AL rats where they accounted for 15% of all fenestrations compared with 4% in young CR, 9% in young AL and 6% in old CR rats. Old age was associated with increased thickness of the liver endothelial cell in the AL rats but not in the old CR rats.
Table 1.
Liver and body weights for the young and old, CR and AL F344 rats.
| Parameter | Young AL (n=5) | Young CR (n=5) | Old AL (n=6) | Old CR (n=4) |
|---|---|---|---|---|
| Body weight (g) | 368 ± 12 | 214 ± 4 | 396 ± 21 | 291 ± 11 |
| Liver weight (g) | 10.9 ± 1.1 | 5.1 ± 0.4 | 13.1 ± 0.9 | 6.9 ± 0.6 |
| Liver weight (% of body weight) | 3.0 ± 0.2 | 2.4 ± 0.2 | 3.3 ± 0.1 | 2.4 ± 0.1 |
Table 2.
The effects of aging and CR on the liver sinusoidal endothelium determined by image analysis of electron microscopy. Results are presented as mean ± SEM of fields.
| Parameter | Young AL | Young CR | Old AL | Old CR |
|---|---|---|---|---|
| Porosity (%) | 3.4 ± 0.3 | 4.3 ± 0.2 | 2.4 ± 0.1 | 3.9 ± 0.3 |
| Fenestration frequency (number per μm2) | 8.0 ± 0.6 | 10.8 ± 0.8 | 6.3 ± 0.4 | 10.9 ± 0.6 |
| Fenestration diameter (nm) | 68 ± 1 | 67 ± 1 | 66 ± 2 | 62 ± 1 |
| Endothelial thickness (nm) | 180 ± 5 | 191 ± 5 | 211 ± 6 | 190 ± 7 |
[Porosity: one-way ANOVA P < 0.01, old AL was significantly less than young AL (P < 0.001) and old CR (P < 0.001). Young CR was significantly greater than young AL (P < 0.001). Endothelial thickness: one-way ANOVA P = 0.001, old AL was significantly greater than young AL (P < 0.001), old CR (P < 0.05) and young CR (P < 0.05).].
Figure 1.
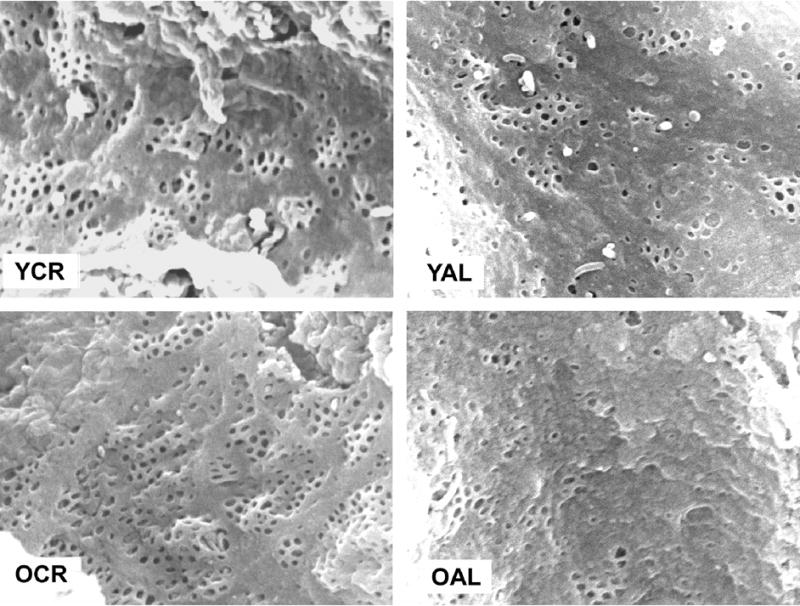
Scanning electron micrographs of livers from young CR (YCR), young AL (YAL), old CR (OCR) and old AL (OAL) rats. Original magnification × 25,000. Scale bar represents 1μm.
Figure 2.
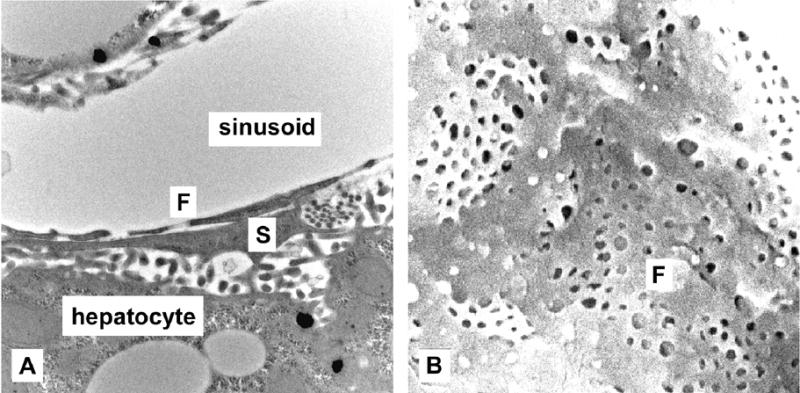
Transmission electron micrograph (A, original magnification × 20,000) and scanning electron micrograph (B, original magnification × 25,000) showing fenestrations [F] overlaying a stellate cell [S] in a young AL rat. This was more frequent in the AL rats, particularly the old AL rats.
The results of the immunohistochemistry are shown in Table 3 and representative slides are shown in Figure 3. Intense peri-sinusoidal staining for von Willebrands factor, collagen IV and Sirius red was seen most frequently in old AL rats. Caveolin-1 expression was found to be intense in the peri-sinusoidal regions in the young AL and young CR rats but was reduced in the old AL rats. Caloric restriction reduced the age-related changes in the expression of collagen IV and caveolin-1 (Figure 4).
Table 3.
Immunohistochemistry for collagen IV, caveolin-1, von Willebrand’s factor and Sirius red. The results are presented as the percentage of livers with intense perisinusoidal staining.
| Stain | Young AL (n=5) | Young CR (n=5) | Old AL (n=6) | Old CR (n=4) |
|---|---|---|---|---|
| Collagen IV | 20% | 0% | 66% | 0% |
| Caveolin-1 | 80% | 80% | 33% | 50% |
| Von Willebrand’s factor | 0% | 0% | 66% | 75% |
| Sirius Red | 0% | 0% | 33% | 0% |
Figure 3.
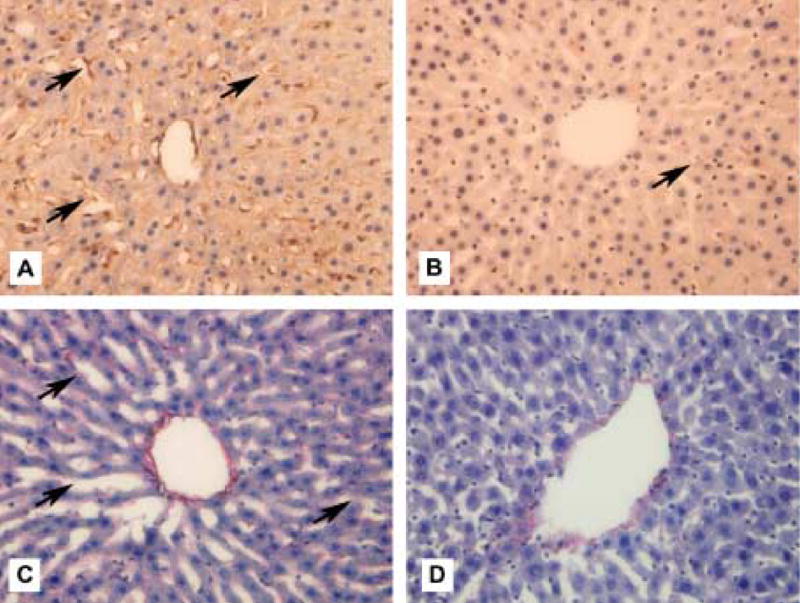
Immunohistochemistry of collagen IV and staining for Sirius red in old AL and CR rat livers showing central vein and surrounding sinusoids and hepatocytes. [A] Collagen IV in old AL rat liver showing peri-sinusoidal staining (→). [B] Collagen IV staining in old CR rat liver showing less peri-sinusoidal expression. [C] Sirius red in old AL rat liver showing perisinusoidal staining. [D] Sirius red staining in old CR rat liver showing less peri-sinusoidal staining. Original magnification × 400.
Figure 4.
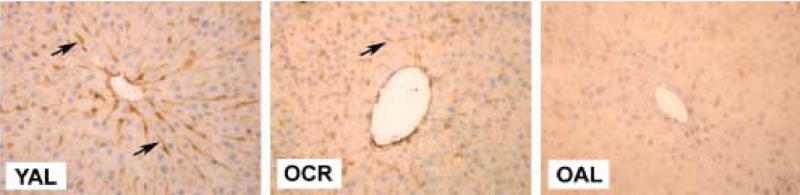
The effects of and CR on the expression of caveolin-1. There is intense perisinusoidal staining (→) in the YAL rat liver, which is partly maintained in the OCR rat liver [B] but markedly diminished in the OAL. Original magnification × 400.
DISCUSSION
These results confirm the presence of significant age-related changes in the ultrastructure and immunohistochemistry of the liver sinusoidal endothelium reported in rats (Le Couteur et al, 2001), non-human primates (Cogger et al, 2003), mice (Warren et al, 2005) and humans (McLean et al, 2003).
CR rats had a higher porosity at 24 months of age compared to AL rats. The age-related increase in the liver sinusoidal endothelial thickness and extracellular matrix of the Space of Disse were also ameliorated by CR. CR had a dramatic effect on the liver sinusoidal endothelial cell, which indicates that pseudocapillarization is an integral part of the aging process. Furthermore, it provides one mechanism for the effects of CR on lipids and vascular disease. One of the most important functions of fenestrations appears to be related to the transfer of chylomicrons remnants across the endothelium for subsequent hepatic metabolism (Fraser et al, 1995). This transfer of lipoproteins is impaired in old age (Hilmer et al, 2005). Preservation of fenestrations by CR will presumptively be associated with improved hepatic clearance of chylomicron remnants and hence, less risk of developing systemic vascular disease.
We were also interested in the possibility that caveolin-1 might be mechanistically involved in age-related defenestration. Caveolin-1 is a key structural protein of caveolae and plays a role in the aging process (Cho et al, 2003). Liver fenestrations may possibly be a form of fused caveolae and caveolin-1 has been identified lining the walls of fenestrations (Ogi et al, 2003). Consistent with this report, we found intense peri-sinusoidal expression of caveolin-1 in young healthy livers. There was a decrease in expression of caveolin-1 with age, which correlates with the loss of fenestrations with age in the sinusoid. These associations support the conclusion that caveolae are related structurally to fenestrations and that changes in the expression of caveolin-1 are important in the aging process. CR attenuated the age-related reduction in peri-sinusoidal expression of caveolin-1
In conclusion, aging is associated with changes in liver sinusoidal endothelial cell. CR prevented the age-related changes in endothelial thickness, fenestration porosity and peri-sinusoidal collagen IV and caveolin-1 expression. The reversibility of age-related pseudocapillarization by CR shows that it is a plausible therapeutic target for the prevention of age-related dyslipidaemia.
Acknowledgments
This work was supported by grants form the Australian National Health and Medical Research Council (Australia) the Ageing and Alzheimer’s Research Foundation (University of Sydney, Australia) and the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. We acknowledge the support of the Pathology Department of the Concord RG Hospital especially Ms Sue Brammah and Dr Sun Yung Kwun.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, Park SC. Senescent phenotype can be reversed by reduction of caveolin status. J Biol Chem. 2003;278:27789–95. doi: 10.1074/jbc.M208105200. [DOI] [PubMed] [Google Scholar]
- 2.Cogger VC, Mross PE, Hosie MJ, Ansselin AD, McLean AJ, Le Couteur DG. The effect of acute oxidative stress on the ultrastructure of the perfused rat liver. Pharmacology & Toxicology. 2001;89:306–311. doi: 10.1034/j.1600-0773.2001.d01-165.x. [DOI] [PubMed] [Google Scholar]
- 3.Cogger VC, Muller M, Fraser R, McLean AJ, Khan J, Le Couteur DG. The effects of oxidative stress on the liver sieve. Journal of Hepatology. 2004;41:370–376. doi: 10.1016/j.jhep.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Experimental Gerontology. 2003;38:1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Everitt A, Roth GS, Le Couteur DG, Hilmer SN. Calorie restriction versus drug therapy to delay the onset of aging diseases and extend life. Age. 2005;27:1–10. doi: 10.1007/s11357-005-3284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–874. [PubMed] [Google Scholar]
- 7.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilmer SN, Cogger VC, Fraser R, McLean AJ, Sullivan D, Le Couteur DG. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 9.Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 10.Le Couteur DG, Cogger VC, Markus AMA, Harvey PJ, Yin ZL, Ansselin AD, McLean AJ. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 11.Le Couteur DG, Fraser R, Cogger VC, McLean AJ. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002;359:1612–1615. doi: 10.1016/S0140-6736(02)08524-0. [DOI] [PubMed] [Google Scholar]
- 12.Le Couteur DG, Fraser R, Hilmer S, Rivory LP, McLean AJ. The hepatic sinusoid in aging and cirrhosis - Effects on hepatic substrate disposition and drug clearance. Clinical Pharmacokinetics. 2005;44:187–200. doi: 10.2165/00003088-200544020-00004. [DOI] [PubMed] [Google Scholar]
- 13.McCuskey RS. Sinusoidal endothelial cells as an early target for hepatic toxicants. Clin Hemorheol Microcirc. 2006;34:5–10. [PubMed] [Google Scholar]
- 14.McLean AJ, Cogger VC, Chong GC, Warren A, Markus AMA, Dahlstrom JE, Le Couteur DG. Age-related pseudocapillarization of the human liver. Journal of Pathology. 2003;200:112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 15.Ogi M, Yokomori H, Oda M, Yoshimura K, Nomura M, Ohshima S, Akita M, Toda K, Ishii H. Distribution and localization of caveolin-1 in sinusoidal cells in rat liver. Med Electron Microsc. 2003;36:33–40. doi: 10.1007/s007950300004. [DOI] [PubMed] [Google Scholar]
- 16.Schmucker DL. Age-related changes in liver structure and function: implications for disease? Experimental Gerontology. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Warren A, Bertolino P, Cogger VC, McLean AJ, Fraser R, Le Couteur DG. Hepatic pseudocapillarization in aged mice. Exp Gerontol. 2005;40:807–12. doi: 10.1016/j.exger.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–59. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]


