Abstract
Candida albicans is a commensal fungus of the normal flora yet causes opportunistic infection following trauma or surgery and during immunosuppression. C. albicans virulence factors include morphogenesis into invasive filaments, adherence to host cells, and secretion of proteases. This study evaluated the role of fungal hyphal extension in experimental C. albicans keratitis using genetically altered yeast strains. Scarified corneas of adult BALB/c mice were topically inoculated with wild-type (SC5314) or 10 transposon-induced mutant strains of C. albicans and monitored for 4 days post inoculation. In vitro growth kinetics and the yeast strains’ ability to bud into pseudohyphae or hyphae were also compared. The wild-type human isolate had a high degree of virulence in the murine cornea, and four fungal strains deficient in genes regulating adherence or encoding membrane proteins did not significantly differ from the parental strain (P > 0.3). Five yeast strains deficient in genes involved in filamentation resulted in fully or partially attenuated keratomycosis (P < 0.0001). The overall growth kinetics of wild-type and mutant strains were similar in rich media (P > 0.9), but mutants with deficient morphogenesis had reduced filamentation in vitro. Phenotypic switching from yeasts to filamentous forms facilitates the establishment and progression of experimental corneal disease by C. albicans.
Keywords: Candida albicans, keratitis, keratomycosis, microbial pathogenesis, ocular infection
1. Introduction
Candida albicans is a commensal fungus of the normal conjunctival flora [1] and an occasional contaminant of contact lenses [2] and eye-banked donor corneas [3]. C. albicans and other fungal eye infections are most commonly acquired following trauma or surgery, during hospitalization, and with immunosuppression [4]. Research into the pathogenesis of fungal eye infection is a pressing need to develop innovative approaches to prevent and to control disease.
Unlike highly specialized pathogens that express a single major virulence factor (e.g., Clostridium tetani), Candida albicans possesses a multitude of activities that contribute to its pathogenesis. Adherence, germination, enzyme production, and morphogenesis (the ability of C. albicans to change from its yeast form to hyphal forms) may contribute to the virulence of fungal infections. Phenotypic switching between yeasts and pseudohyphae and hyphae may be a potentially important step in pathogenesis [5]. Rapid morphogenic interconversion and hyphal extension have been associated with increased fungal invasiveness [6]. While the role of fungal morphogenesis in producing human disease is unsettled [7], opportunities are arising for probing the virulence pathways involved in fungal keratitis.
Fungal virulence is “the relative capacity of a microbe to cause damage to a host [8].” Ocular pathogenicity of C. albicans begins with an adverse interaction between fungi and host tissues [9]. Adherence and enzyme production contribute to the virulence of fungal infections, but phenotypic switching from yeasts to pseudohyphae and hyphae is a key step in the pathogenesis of candidiasis [5]. We used a traumatic keratomycosis mouse model to determine if genes involved in morphogenesis, adhesion, or produce membrane proteins affect the virulence of C. albicans during corneal infection.
2. Results
2.1 In Vitro Growth
Growth kinetics were similar (P < 0.05) for all C. albicans yeast strains (Figure 1), although suv3−/− grew slightly slower than the other strains. Mutant strains filamented in YPD agar at 25°C or 37°C at rates similar to controls, except the sla2−/− mutant filamented 3 days later (Table 2). No differences were seen between mutant and wild-type strains grown on SLAD media (Table 2). Filamentation induced with fetal bovine serum and grown at 30°C produced wrinkled colonies for control strains within 4 days (Table 2). Mutant strains produced similar results as controls, except mds3−/− produced wrinkled colonies within 6 days while sla2−/− and suv3−/− took up to 8 days.
Figure 1.
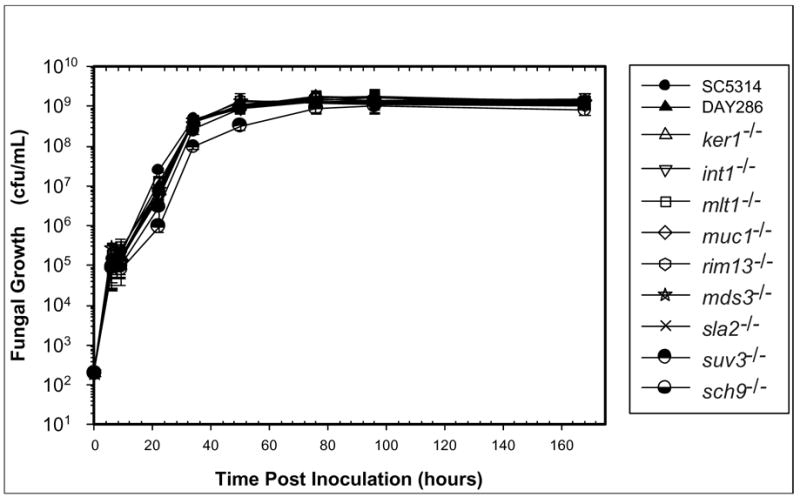
In vitro growth kinetics. Each Candida albicans yeast strain was inoculated into YPD liquid media. Mock-inoculated YPD liquid media served as the negative control. The optical density was measured for the cultures at 6, 9, 22, 34, 50, 76, 96, and 168 hours PI. Two independent experiments with triplicate samples were performed and the means were calculated and reported. SC5314 is the wild-type C. albicans strain and DAY286 is the mutant control.
TABLE 2.
Filamentation Assays
| Embedded Growth | YPD with 10% serum | SLAD | M199 pH 4 | M199 pH 8 | ||
|---|---|---|---|---|---|---|
| Strain | YPD at 25°C | YPD at 37°C | ||||
| SC5314 | +++ | +++ | +++ | + | + | +++ |
| DAY286 | +++ | +++ | +++ | + | + | +++ |
| ker1−/− | +++ | +++ | +++ | + | + | +++ |
| int1−/− | +++ | +++ | +++ | + | + | +++ |
| muc1−/− | +++ | +++ | +++ | + | + | +++ |
| mlt1−/− | +++ | +++ | +++ | + | + | +++ |
| rim13−/− | +++ | +++ | +++ | + | + | − |
| mds3−/− | +++ | +++ | ++ | + | + | − |
| sla2−/− | + | + | + | + | − | − |
| sch9−/− | +++ | +++ | +++ | + | − | + |
| suv3−/− | +++ | +++ | + | + | − | + |
filamentation observed after 3 to 4 days
filamentation observed after 5 to 6 days
filamentation observed after 7 days or longer
no filamentation observed within 14 days
On M199 media buffered at pH 4, SC5314, DAY286, mds3−/−, and rim13−/− produced filaments at 5 days; int1−/−, ker1−/−, muc1−/−, and mlt1−/− produced filaments by 8 days; and sla2−/−, suv3−/−, and sch9−/− did not produce filaments (Table 2). When grown at pH 8, all five morphogenesis mutants (mds3−/−, rim13−/−, sla2−/−, sch9−/−, and suv3−/−) had no or reduced filamentation (Figure 2).
Figure 2.
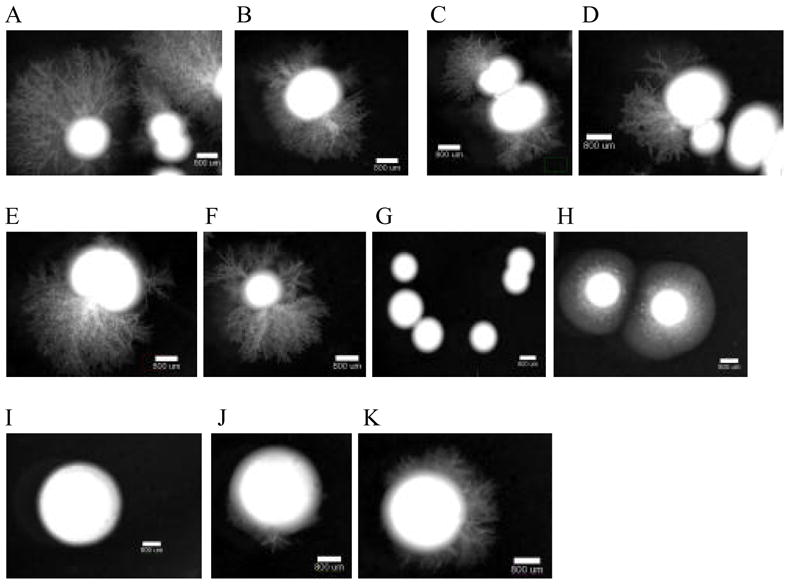
Filamentation assay on M199 pH 8. Each strain was grown overnight at 30°C in YPD liquid media. The cultures were diluted to a concentration of 10 cfu/μL, where 5 μL was added to medium 199 (M199) containing Earle’s salts and glutamine but lacking sodium bicarbonate (Gibco BRL) and buffered with 155 mM Tris-HCl at pH 8 + 1.5% agar. Pictures were taken at 14 day PI (A) SC5314, (B) DAY286, (C) ker1−/− (D) int1−/−, (E) muc1−/−, (F) mlt1−/−, (G) rim13−/−, (H) mds3−/−, (I) sla2−/−, (J) sch9−/−, and (K) suv3−/−. A and B are controls, C to F are mutants involved in adhesion or encode membrane proteins, and G to K are morphogenesis mutants.
2.2 In Vivo Virulence
Wild-type and parental control strains produced moderate to severe disease in the murine cornea. The severity of keratitis with the four mutants with genes deficient in membrane proteins or involved in adhesion did not differ significantly (P > 0.3) from control strains (Figure 3). Mutant strains lacking genes that regulate morphogenesis produced less disease severity in the mice than the control strains. The sla2−/− strain produced a moderate infection at 1 day PI but resolved after 4 days. After 2 days PI, this strain had significantly less severe keratitis compared to controls (P < 0.0001). The mds3−/− strain produced a mild infection that resolved by the 4 day follow-up and was less severe than control strains (P < 0.0001). The rim13−/−, sch9−/−, and suv3−/− strains had completely attenuated virulence in the mouse keratitis model (Figure 4).
Figure 3.
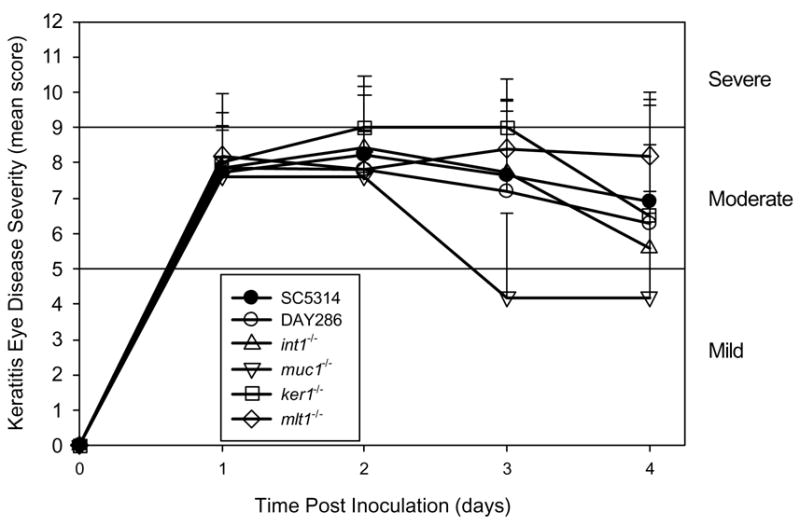
Mean keratitis scores for mutants in genes involved in adhesion or membrane proteins. Error bars represent standard deviation. There is no statistical difference in any mutant strain score at any time point when compared to the wild-type yeast strain, SC5314.
Figure 4.
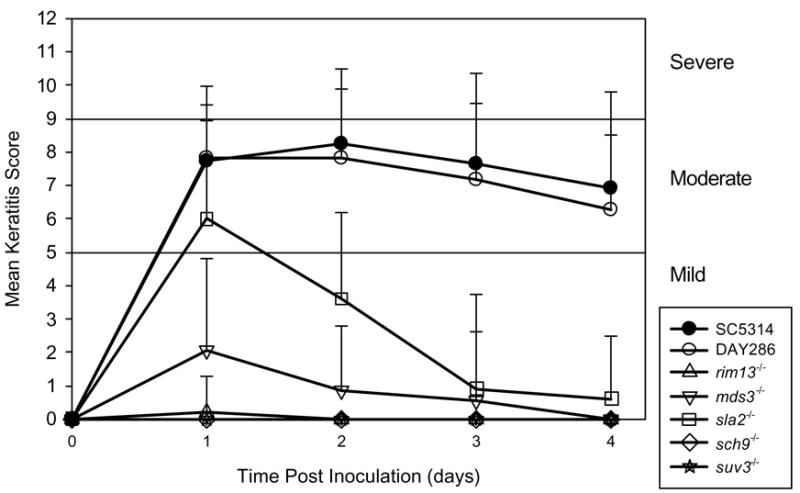
Mean keratitis scores for the morphogenesis mutants. Error bars represent standard deviation. All mutants had partially or fully attenuated virulence in the mouse keratitis model.
3. Discussion
Virulence factors have been identified for Candida albicans infections of the blood, lung, or kidney, but few studies have examined the molecular pathogenesis of fungal ocular infection. The purpose of this study was to determine if C. albicans virulence factors identified in other tissues also contribute to fungal infection in the cornea. The mutants used in this paper were selected from a transposon-induced mutagenesis library described previously [10]. A literature search of the mutants characterized from the library was performed and those that were previously hypothesized as virulence factors were selected for use in our mouse keratitis model. These mutants were grouped into categories of genes involved in morphogenesis, adhesion, or produced membrane proteins.
Our results confirm that virulence factors differ depending on the site infected, and suggest that morphogenesis is important for C. albicans corneal infection. Adhesion appeared to have a minor role in experimental keratitis, but the scarification process in the mouse model disrupts the corneal epithelial barrier and may bypass the role of fungal adhesion mechanisms.
Filamentation has a key role in C. albicans corneal infection. Morphogenic conversion and hyphal extension that contribute to fungal invasiveness are genetically regulated [11]. All mutants used in this study that were deficient in genes regulating morphogenesis led to attenuated virulence in the mouse keratitis model. Morphogenesis genes involved in keratitis were rim13, mds3, sla2, sch9, and suv3. Complete attenuation was seen with the rim13−/−, sch9−/−, and suv3−/− mutants, and mds3−/− mutant produced mild disease that resolved by 4 days. Keratitis scores for sla2−/− mutant produced a moderate disease similar to wild-type at 1 day PI, yet cleared by 4 days. The mechanism by which the mouse resolves the disease with this mutant is not understood, but we propose that sla2 plays a later role in invasion that would delay resolution.
Both rim13 and mds3 are known to be involved in the pathogenesis of candidiasis. Rim13p is a calpain-like protease that likely mediates activation of the transcription factor Rim101p via C-terminal cleavage [12, 13]. The RIM101 pathway is required for alkaline pH-induced hyphal formation [14] and RIM101p contributes to virulence in mouse models [13, 15, 16]. Our results extend these finding to corneal infection. Mds3p is also required for growth and hyphal formation at alkaline pH, although it appears to be independent of the RIM101 pathway [10, 15]. Mds3 is involved in virulence in a mouse systemic model [10] as well as the ocular model.
The results reported here are the first in vivo studies to implicate sla2, sch9, and suv3 as C. albicans virulence factors. Sla2p is an actin-binding protein required for alkaline pH-induced hyphal formation [17, 18]. Sch9p is a serine threonine protein kinase required for embedded hyphal growth [15]. Suv3p is an ATP-dependent RNA helicase involved in mitochondrial RNA catabolism, a protein required for embedded hyphal growth, and growth under oxygen-limited conditions [15].
Genes mutated in the strains used in this study that are involved in adhesion or that encode membrane proteins were int1, muc1, ker1, and mlt1. These mutants produced similar keratitis scores as wild-type, although muc1−/− began to resolve the disease after 2 days PI. Our findings indicate that adhesion does not play a major role in C. albicans virulence of the traumatized cornea. Int1p is an integrin-like protein [19] that mediates intestinal colonization [20], systemic infection [21], and is involved in cell adhesion [22]. In the ocular model, however, int1 mutant induced similar keratitis scores as wild-type controls. Muc1p is a cell surface glycoprotein involved in a filamentous growth transduction pathway in S. cerevisiae [15] and is hypothesized to be a virulence factor [23]. Ker1p is an alkaline induced protein of the plasma membrane, and shown to attenuate virulence in the mouse kidneys and lungs [24]. Finally, Mlt1p is a vacuolar membrane transporter of the ABC (ATP-binding cassette) family and is required for virulence in a mouse peritonitis model [25]. Even though int1, ker1, and mlt1 are virulence factors in systemic C. albicans infection, none of these mutants affected the severity of experimental corneal infection.
Growth mutant strains were similar to wild-type yeasts, indicating no obvious growth advantage or disadvantage that would influence virulence in the mouse keratitis studies. The suv3−/− mutant did have a slightly slower growth pattern than the rest of the strains; however, this decrease was not significant. We also noticed that the five mutants involved in hyphal morphogenesis had a reduced ability or were unable to produce filaments when grown on M199 pH 8 agar. Alkaline-induced morphogenesis within or around fungal cells may affect virulence in C. albicans corneal infection.
Candidiasis models are being developed for infection of several tissues and organs, but few studies have focused on understanding virulence factors of C. albicans corneal infection [26]. Genes that contribute to C. albicans infection of the cornea differ from previously identified virulence factors of other infection models, indicating the need for tissue-specific studies.
4. Materials and Methods
4.1 Strains and growth conditions
Yeast strains used for this study are listed in Table 1. SC5314 is a wild-type, highly virulent C. albicans strain isolated from a human patient [27]. The C. albicans mutants come from a library of fungal mutants derived from SC5314 by transposon-induced mutagenesis [10]. All yeast strains were routinely grown in YPD (1% yeast extract, 2% peptone-tryptone, 2% dextrose) media at 30°C. Yeast were harvested during exponential growth and suspended in sterile phosphate-buffered saline (PBS).
TABLE 1.
C. albicans Strains Evaluated in Mouse Keratitis Model
| C. albicans Strain | Possible Role in Pathogenesis | Reference |
|---|---|---|
| SC5314 | Wild-type strain that produces severe experimental keratomycosis | Gillum et al. [27] |
| DAY286 | Mutant reference strain that provides positive control | Davis et al.[10] |
| ker1−/− | Lysine/glutamate-rich plasma-membrane protein | Galán et al.[24] |
| int1−/− | Membrane protein involved in filamentation and epithelial adherence | Bendel et al. [21] |
| muc1−/− | Hyphal cell-wall protein | Li & Palacek[23] |
| mlt1−/− | ATP-dependent transporter protein | Theiss et al.[32] |
| rim13−/− | Protease involved in filamentation induced by the RIM101 pathway | Noble et al. [15] |
| mds3−/− | Protein involved in pH-dependent filamentation | Noble et al. [15] |
| sla2−/− | Protein that contributes to INT1-induced filamentation | Asleson et al.[17] |
| sch9−/− | Protein kinase involved in filamentation and chlamydospores formation | Nobile et al.[15] |
| suv3−/− | RNA helicase involved in chlamydospores formation | Nobile et al.[15] |
4.2 In vitro growth kinetics
Each strain was grown overnight in 5 mL YPD liquid media. The concentration was determined using a spectrophotometer to measure the optimal density (OD) at a wavelength of 600 nm and a conversion factor of one OD600 unit being equivalent to 3 × 107 colony-forming units (cfu)/mL [28]. After suspension of 1000 cfu of each strain in 5 mL YPD liquid media and incubation at 27°C with continuous shaking for 7 days, concentrations were measured at 6, 9, 22, 34, 50, 76, 96, and 168 hours post inoculation (PI). Two independent experiments with triplicate samples were performed.
4.3 Filamentation assays
Yeast strains were grown overnight at 30°C in YPD liquid media then diluted to a concentration of 10 cfu/μL. Five μL were added to growth media that contained 1.5% agar. Embedded growth in YPD agar was examined at 25°C or 37°C as described previously [29]. Growth on medium 199 (M199) containing Earle’s salts and glutamine but lacking sodium bicarbonate and buffered with 155 mM Tris-HCl was observed at pH 4 and pH 8 [30]. Growth on YPD agar supplemented with 10% fetal bovine serum and synthetic low ammonium dextrose (SLAD) media (0.17% yeast nitrogen base without amino acids or ammonium sulfate, 50 μM ammonium sulfate, and 2% dextrose) [31] was also monitored at 37°C. Colonies were examined for filamentation daily for 14 days.
4.4 Virulence studies in a mouse model
Immunocompetent adult female BALB/c mice 6 to 8 weeks old were anesthetized, and their corneas superficially scarified [28]. A 5 μL inoculum containing 1.0 × 105 cfu of each C. albicans yeast strain was applied to the scarified cornea. Scarified mouse eyes inoculated with sterile PBS served as the negative control. A minimum of 5 mice were used per group. Animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and our institutional policies. Mice were monitored daily for 4 days PI and scored with the aid of a dissecting microscope [28]. A grade of 0 to 4 was assigned to each of three criteria (area of opacity, density of opacity, and surface regularity) to yield a total score of 0 to 12. A total score <5 was categorized as mild eye disease, from 5 to 9 was considered moderate, and > 9 was severe disease. Score results were evaluated for statistical significance by the Kruskal-Wallis one-way analysis of variance test.
Figure 5.
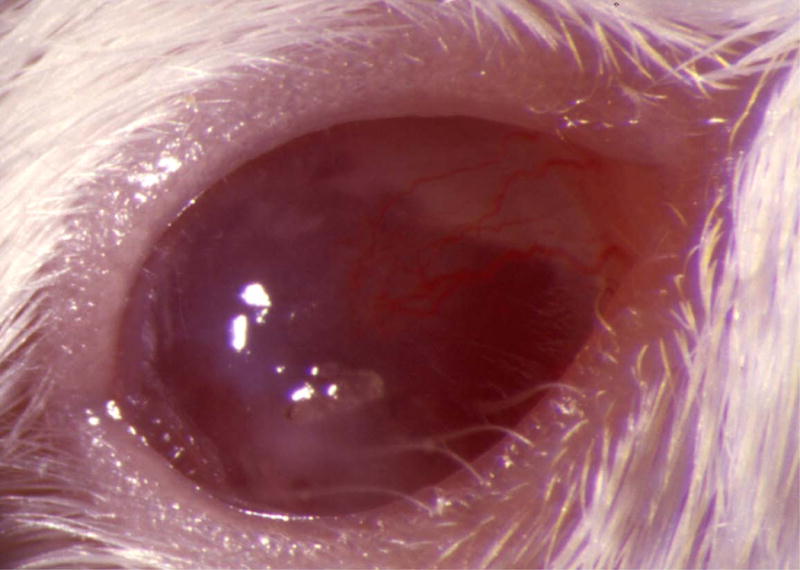
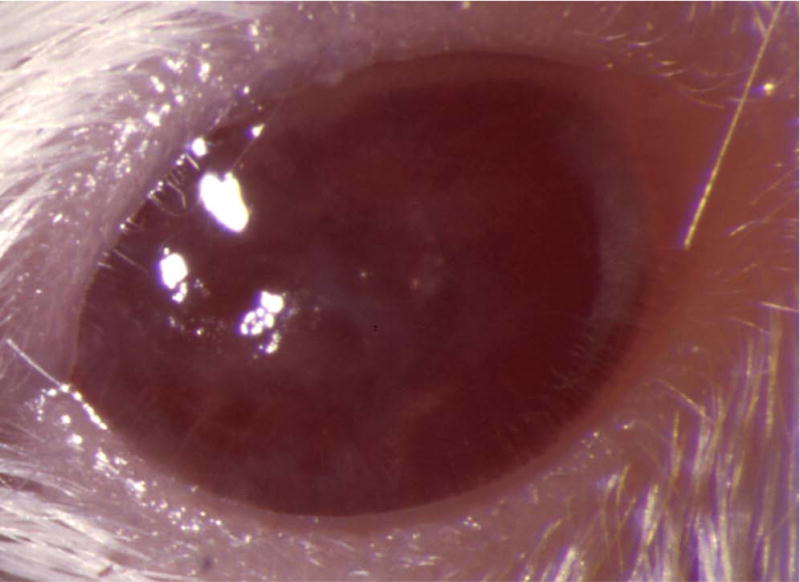
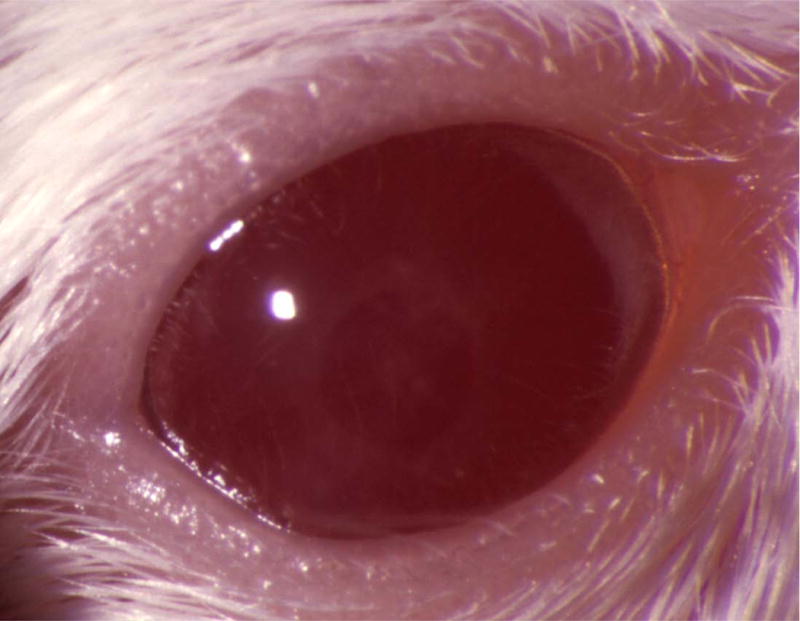
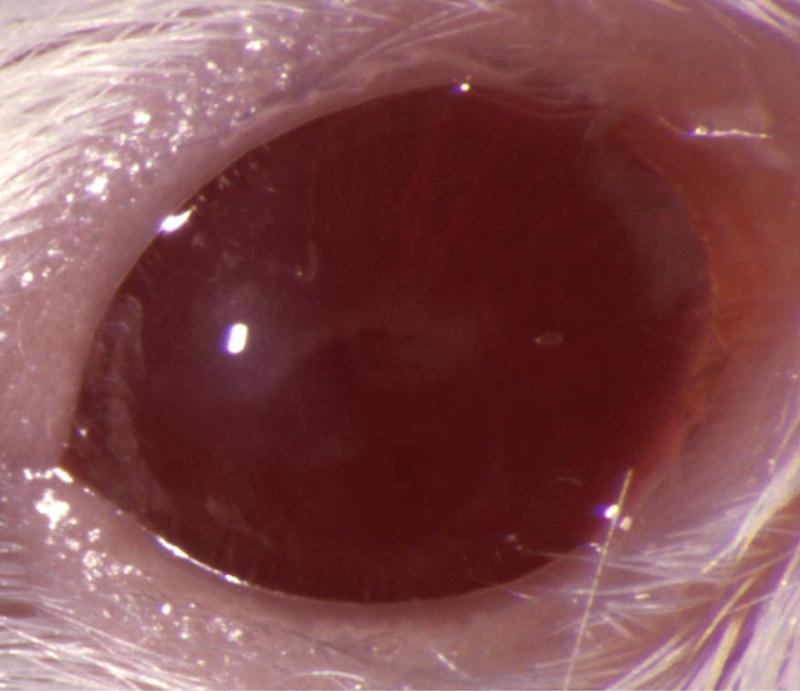
Mouse corneas inoculated with C. albicans. Mouse eyes were scarified and inoculated with 105 cfu. Pictures were taken at 1 day PI using a slit-lamp biomicroscope and camera. (A) SC5314 (wild-type), (B) DAY286, (C) rim13−/−, and (D) sterile PBS.
Acknowledgments
A. P. Mitchell, Ph.D., Columbia University, and M. C. Lorenz, Ph.D., University of Texas Houston, provided the Tn7 mutants. This work was supported by a T32 institutional research training grant (EY07001) and a P30 core grant (EY02520) from the National Eye Institute, the Research to Prevent Blindness, Inc., the Lions Eye Bank Foundation, and the Sid W. Richardson Foundation.
Abbreviations
- YPD
yeast peptone dextrose
- PBS
phosphate-buffered saline
- OD
optical density
- cfu
colony-forming units
- PI
post inoculation
- M199
medium 199
- SLAD
synthetic low ammonium dextrose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu T, Mitchell B, Carothers T, Coats D, Brady-McCreery K, Paysse E, et al. Molecular analysis of the pediatric ocular surface for fungi. Curr Eye Res. 2003;26:33–6. doi: 10.1076/ceyr.26.1.33.14253. [DOI] [PubMed] [Google Scholar]
- 2.Driebe WT., Jr Present status of contact lens-induced corneal infections. Ophthalmol Clin North Am. 2003;16:485–94. doi: 10.1016/s0896-1549(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 3.Kercher L, Wardwell SA, Wilhelmus KR, Mitchell BM. Molecular screening of donor corneas for fungi before excision. Invest Ophthalmol Vis Sci. 2001;42:2578–83. [PubMed] [Google Scholar]
- 4.Thomas PA. Fungal infections of the cornea. Eye. 2003;17:852–62. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 5.Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–24. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Whiteway M, Oberholzer U. Candida morphogenesis and host-pathogen interactions. Curr Opin Microbiol. 2004;7:350–7. doi: 10.1016/j.mib.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis. 2001;184:337–44. doi: 10.1086/322044. [DOI] [PubMed] [Google Scholar]
- 9.Soll DR. Candida commensalism and virulence: the evolution of phenotypic plasticity. Acta Trop. 2002;81:101–10. doi: 10.1016/s0001-706x(01)00200-5. [DOI] [PubMed] [Google Scholar]
- 10.Davis DA, Bruno VM, Loza L, Filler SG, Mitchell AP. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics. 2002;162:1573–81. doi: 10.1093/genetics/162.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15:456–67. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Martin SJ, Bruno VM, Mitchell AP, Davis DA. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot Cell. 2004;3:741–51. doi: 10.1128/EC.3.3.741-751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr Genet. 2003;44:1–7. doi: 10.1007/s00294-003-0415-2. [DOI] [PubMed] [Google Scholar]
- 14.Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–8. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nobile CJ, Bruno VM, Richard ML, Davis DA, Mitchell AP. Genetic control of chlamydospore formation in Candida albicans. Microbiology. 2003;149:3629–37. doi: 10.1099/mic.0.26640-0. [DOI] [PubMed] [Google Scholar]
- 16.Davis D, Edwards JE, Jr, Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun. 2000;68:5953–9. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asleson CM, Bensen ES, Gale CA, Melms AS, Kurischko C, Berman J. Candida albicans INT1-induced filamentation in Saccharomyces cerevisiae depends on Sla2p. Mol Cell Biol. 2001;21:1272–84. doi: 10.1128/MCB.21.4.1272-1284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melms AS, Gausmann U, Swoboda RK, Dominguez A, Kurischko C. Sequence analysis of SLA2 of the dimorphic yeasts Candida albicans and Yarrowia lipolytica. Yeast. 1999;15:1519–28. doi: 10.1002/(SICI)1097-0061(199910)15:14<1519::AID-YEA475>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Gale C, Finkel D, Tao N, Meinke M, McClellan M, Olson J, et al. Cloning and expression of a gene encoding an integrin-like protein in Candida albicans. Proc Natl Acad Sci USA. 1996;93:357–61. doi: 10.1073/pnas.93.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendel CM, Kinneberg KM, Jechorek RP, Erlandsen SL, Sahar DE, Wells CL. The Candida albicans INT1 gene facilitates cecal colonization in endotoxin-treated mice. Shock. 2000;13:453–8. doi: 10.1097/00024382-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Bendel CM, Kinneberg KM, Jechorek RP, Gale CA, Erlandsen SL, Hostetter MK, et al. Systemic infection following intravenous inoculation of mice with Candida albicans int1 mutant strains. Mol Genet Metab. 1999;67:343–51. doi: 10.1006/mgme.1999.2875. [DOI] [PubMed] [Google Scholar]
- 22.Gale CA, Bendel CM, McClellan M, Hauser M, Becker JM, Berman J, et al. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–8. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Palecek SP. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell. 2003;2:1266–73. doi: 10.1128/EC.2.6.1266-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galán A, Casanova M, Murgui A, MacCallum DM, Odds FC, Gow NA, et al. The Candida albicans pH-regulated KER1 gene encodes a lysine/glutamic-acid-rich plasma-membrane protein that is involved in cell aggregation. Microbiology. 2004;150:2641–51. doi: 10.1099/mic.0.26339-0. [DOI] [PubMed] [Google Scholar]
- 25.Theiss S, Kretschmar M, Nichterlein T, Hof H, Agabian N, Hacker J, et al. Functional analysis of a vacuolar ABC transporter in wild-type Candida albicans reveals its involvement in virulence. Mol Microbiol. 2002;43:571–84. doi: 10.1046/j.1365-2958.2002.02769.x. [DOI] [PubMed] [Google Scholar]
- 26.Braun BR, Head WS, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics. 2000;156:31–44. doi: 10.1093/genetics/156.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–82. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 28.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44:210–6. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 29.Brown DH, Jr, Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol. 1999;34:651–62. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- 30.Bensen ES, Martin SJ, Li M, Berman J, Davis DA. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiol. 2004;54:1335–51. doi: 10.1111/j.1365-2958.2004.04350.x. [DOI] [PubMed] [Google Scholar]
- 31.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–90. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 32.Theiss S, Kohler GA, Kretschmar M, Nichterlein T, Hacker J. New molecular methods to study gene functions in Candida infections. Mycoses. 2002;45:345–50. doi: 10.1046/j.1439-0507.2002.00792.x. [DOI] [PubMed] [Google Scholar]


