Abstract
Context
Decreased calcitriol production due to impaired renal function may be a significant risk factor for falls in normal aging population.
Objective
The objective of the study was to examine the association between creatinine clearance (CrCl) and the incidence of falls and fallers in groups treated with placebo, calcitriol, estrogen therapy (ET)/estrogen + progestin therapy (HT), and calcitriol + ET/HT.
Design
This was a 3-yr, double-blind, placebo-controlled study designed to test the efficacy of calcitriol and ET/HT on bone loss and falls with analysis by intention to treat and post hoc.
Setting
The study was conducted at an academic outpatient center.
Participants
Four hundred eighty-nine normal elderly women aged 65–77 yr; 415 women completed the study.
Intervention
Subjects were randomized to placebo, calcitriol 0.25 μg twice a day, ET daily (conjugated equine estrogens 0.625 mg), HT (conjugated equine estrogen 0.625 mg + medroxyprogesterone acetate 2.5 mg) and calcitriol + ET/HT.
Main Outcome Measures
Cumulative number of falls and fallers were compared between groups with 24-h urine CrCl less than 60 and 60 ml/min or greater.
Results
Calcitriol treatment decreased the number of fallers and falls. Low CrCl less than 60 ml/min was a predictor of the number of falls per person but not fallers in the placebo group (P = 0.007). In the low CrCl group (<60 ml/min), the rate of falls decreased on calcitriol by 53% [95% confidence interval (CI) −71% to −22%; P = 0.003], calcitriol + ET/HT by 61% (95% CI −76% to −37%; P = 0.001), and ET/HT by 25% (95% CI: −55% to +24%; not significant). Calcitriol reduced the rate of falls by 30% (95% CI −49% to −4%; P = 0.027) in the CrCl 60 ml/min or greater group.
Conclusion
Calcitriol treatment decreases falls in all subjects but especially in elderly women with decreased renal function (<60 ml/min) and frequent fallers.
Falls in elderly people represent a serious public health problem: approximately 30% over the age of 65 yr fall each year, and this proportion increases to more than 50% by the age of 80 yr (1). Falls frequently lead to physical injury, fractures, deterioration in physical function, and nursing home placement (2, 3).
We and others have recently shown that treatment with calcitriol [1,25-dihydroxyvitamin D, 1,25(OH)2D] (4, 5) or the vitamin D analog, alphacalcidol (6) significantly reduces the risk of falls (4–6) and the number of fallers (6) in elderly men and women. The biological actions of vitamin D are generally mediated through its conversion in the kidney to its hormonal form, 1,25(OH)2D. A creatinine clearance (CrCl) of less than 65 ml/min has been recently reported as a significant independent risk factor for increased incidence of falls and increased risk of vertebral, hip, and radial fractures in elderly men and women treated for osteoporosis (7, 8). CrCl less than 65 ml/min was also associated with significantly lower serum 1,25(OH)2D levels (8). Treatment with alphacalcidol significantly reduced the number of falls and fallers in the low CrCl (<65 ml/min) group (9). Cross-sectional (10) and vitamin D supplementation studies (11–13) have demonstrated a positive association between higher serum 1,25(OH)2D levels and improved muscle strength and body sway, and alphacalcidol treatment has been reported to improve muscle strength and physical performance in vitamin D-deficient older women (14). Thus, the effect of calcitriol and alphacalcidol, which is a prodrug for calcitriol, can be attributed to an increase in muscle strength, improved physical performance, and balance.
According to Kidney Disease Outcomes Quality Initiative guidelines of the U.S. Kidney Foundation, CrCl less than 60 ml/min per 1.73m2 is classified as stage 3 chronic kidney disease (15), and the guidelines suggest intensive monitoring of patients with decreased CrCl (15, 16) because of a decline in serum 1,25(OH)2D and an increase in serum PTH. In an osteoporosis intervention trial primarily designed to test the efficacy of conjugated estrogens alone [estrogen therapy (ET)] or with medroxyprogesterone acetate [estrogen + progestin therapy (HT)] and calcitriol given alone or in combination on bone loss, we had the opportunity to collect prospectively data on the incidence of falls. In this post hoc analysis of subgroups, we examined the association between low CrCl less than 60 ml/min and the incidence of falls and fallers in a placebo group and compared it with calcitriol, ET/HT, and combination treatment groups.
Subjects and Methods
Experimental subjects
Four hundred eighty-nine elderly women aged 65–77 yr were enrolled into a prospective, double-blind, randomized, placebo-controlled clinical trial Sites Testing Osteoporosis Prevention and Intervention Treatments (STOP IT) to test the efficacy of conjugated equine estrogens and calcitriol given individually or in combination on bone loss (4). Women were excluded if they had severe chronic illness (chronic liver disease, severe chronic obstructive pulmonary disease, severe rheumatoid arthritis, or serious heart failure); had serum creatinine greater than 1.5 mg/dl; had primary hyperparathyroidism or active renal stone disease; or were on certain medications, such as bisphosphonates, anticonvulsants, estrogen, fluoride, or thiazide diuretics in the previous 6 months. The volunteers recruited for the study were free living, ambulatory, and willing to give written informed consent.
The women were randomized to one of four groups; ET/HT; conjugated equine estrogens 0.625 mg/d (Premarin) [medroxyprogesterone acetate 2.5 mg/d (Provera) was added if the women had a uterus]; calcitriol (Rocaltrol) 0.25 μg twice daily; and the combination of both or placebo. One woman who developed Paget’s disease was excluded from the analyses. Four hundred fifteen women came for the final study visit at 3 yr, and 337 were adherent to medication–defined as taking 80% over 3 yr. The analysis was performed on 415 women who came for the final study visit. The distribution of subjects into different treatment groups has been given in an earlier publication (4). The Creighton University Human Institutional Review Board approved the trial and all patients signed an informed consent form.
Falls
Falls data were collected prospectively by a nonvalidated interview-administered questionnaire on the incidence of falls every 6 months for the entire study period of 36 months. Falls were defined as unintentionally coming to rest on the ground, floor, or other lower level. It was not possible to accurately ascertain the exact date of the fall at the time of the scheduled visit.
Dietary intake and alcohol and smoking history
Dietary intake data were collected using 7-d food dairies. The average daily calcium, vitamin D, and caffeine intakes were calculated by a dietitian, using the Food Processor II plus nutrition and diet analysis system (version 5.1; Esha Research, Salem, OR). Alcohol and smoking history were assessed via a questionnaire. Smoking status was classified as current smokers and nonsmokers. The alcohol intake was stratified into drinkers and nondrinkers.
Baseline medication use and comorbidities
Information about current use of medications and comorbid conditions were obtained by a questionnaire. Medication use by the subjects was categorized into three groups as use of 0, one, or more than one medication. The comorbid conditions included hypertension, thyroid conditions, cancer, osteoporosis, scoliosis, diabetes, kidney disease, arthritis, chronic gastrointestinal disease, allergies, hip fracture, pulmonary disease, heart conditions, etc. and were grouped as existence of none to two, three to four, five to six, or seven mor more conditions.
Biochemical analysis
Fasting blood and 24-h urine samples were obtained at baseline and end of the study. All samples were stored frozen at −70 C until analysis. Total calcium and creatinine in urine samples and serum creatinine were determined by automated procedures (chemistry analyzer; Nova Nucleus, Waltham, MA). Measured 24-h creatinine clearance was used to stratify the groups into less than 60 (low group) and 60 ml/min or greater.
Serum 25 hydroxyvitamin D (25OHD) was assayed by a competitive protein binding assay (17) as described before (18). The competitive protein binding assay method was found to be highly correlated with HPLC (19). Serum 1,25(OH)2D was measured by a nonequilibrium radioreceptor assay (Incstar Corp., Stillwater, MN) (20, 21) as described before (18). Serum PTH was measured with the Allegro immunoradiometric assay (Nichols Institute, San Juan Capistrano, CA) (22). The limit of detection and the interassay variation, respectively, were 12.5 mmol/ liter (5 ng/ml) and 5% for serum 25OHD, 12 pmol/liter and 10% for serum 1,25(OH)2D, and 1 ng/liter and 3.5% for serum PTH assays. Urine collagen cross-links were measured by ELISA (Osteomark International, Seattle, WA) as N-telopeptides, a specific marker for bone type I collagen [expressed as nanomoles bone collagen equivalents per millimoles of creatinine].
Calcium absorption test
Calcium absorption was measured in a fasting state at the beginning and end of the study by oral administration of 18.5 × 104Bq (5 μCi) 45Ca (Amersham, Arlington Heights, IL) in 100 mg CaCl2 carrier given in a total volume of 250 ml distilled water (23) and described before (18). Calcium absorption was expressed as percentage of the actual dose per liter of blood and corrected for body weight.
Physical activity and physical performance measures
Physical Activity Scale for the Elderly (PASE) was calculated based on questionnaire items assessing various domains of physical activity, including walking, sitting activities, light sport and recreational activities, moderate sport and recreational activities, strenuous sport and recreational activities, and exercise and light housework (24). Grip strength was measured on the right hand by a handheld Jamar dynamometer (Jackson, MI). The average of three trials was recorded. Walking speed was assessed as the time taken to walk a 15-ft walkway at the subject’s normal pace and then as quickly as possible (25). Timed rising test involved the time taken by the subject to rise from a chair three times as quickly as possible (25).
Statistical analysis
All analyses were done by the SAS statistical package (version 9.1; SAS Inc., Cary, NC). The presented results are from intent-to-treat analyses. Baseline patient characteristics, biochemical variables, and physical performance measures were compared according to CrCl subgroups with Student t tests. Medication use, comorbid conditions, smoking status, and alcohol intake were compared between the two CrCl groups by χ2 tests. Poisson regression was used to examine the predictors of the cumulative number of falls at 6, 12, 24, and 36 months. Fallers, classified as no falls vs. one or more falls in 36 months, was examined with logistic regression. Treatment group and CrCl subgroups were considered as predictors in the models. Models were adjusted for significant confounders selected with backward selection from the full model at each time point. Potential confounders adjusted for in the CrCl models were age, baseline serum 25OHD, weight, height, baseline calcium absorption, number of comorbidities (none to two, three to four, five to six, and seven or more), number of medications (none, one, more than one), smoking status (smokers vs. nonsmokers), alcohol intake, and PASE score. Significant confounders that entered the models were number of comorbidities, smoking status, and PASE score. Although serum 1,25(OH)2D was significant in the backward regression analysis, we did not include it as a confounding variable because our hypothesis is that reduced 1,25(OH)2D production is the main cause of the falls. Unadjusted means and SES are given for the cumulative number of falls per person at 36 months in each group, presented with P values from the Poisson regression models. Data on the adjusted cumulative rate of falls per person at 36 months, referred to hereafter as rate of falls, are presented along with 95% confidence intervals (CIs). P < 0.05 was considered statistically significant.
Results
Of the 489 women randomized to the treatment, 415 completed the final study visit. Three hundred thirty-seven were adherent to treatment, 78% on placebo, 70% on calcitriol, 65% on HT/ET, and 62% on HT/ET + calcitriol. In the adherent group, medication compliance calculated at 36 months for the groups was 92% on placebo, 93% on calcitriol, 92% on conjugated estrogens, and 94% on medroxyprogesterone. In the less than 60/60 ml/min or greater CrCl subgroups, compliance, respectively, was 93/94% in placebo; 92/92% on calcitriol; 95/92% on the combination, and 90/93% in the ET/HT groups.
The results presented below are based on intent-to-treat analyses of 415 subjects who completed a final visit; the results from the complier analyses in 337 subjects are similar (data not given).
Baseline characteristics and biochemical variables of study population in CrCl subgroups (Table 1)
TABLE 1.
Baseline characteristics, biochemical indices, and physical performance measures according to CrCl
| Parameter | CrCl< 60 ml/min | CrCl ≥ 60 ml/min | P |
|---|---|---|---|
| n | 106 | 309 | |
| Mean measured CrCl (ml/min) | 50.9 ± 0.79 | 80.5 ± 0.88 | <0.001 |
| Age (yr) | 72.0 ± 0.34 | 71.1 ± 0.20 | 0.024 |
| Height (cm) | 157.7 ± 0.67 | 160.0 ± 0.36 | 0.002 |
| Weight (kg) | 64.9 ± 1.28 | 69.9 ± 0.71 | 0.001 |
| Dietary calcium intake (mg/d) | 747.1 ± 27.0 | 751.3 ± 18.2 | 0.898 |
| Dietary vitamin D intake (μg/d) | 3.40 ± 0.19 | 3.54 ± 0.12 | 0.522 |
| No. of comorbidities | |||
| 0–2 | 16 (15%) | 49 (16%) | 0.9 |
| 3–4 | 40 (38%) | 127 (41%) | |
| 5–6 | 31 (29%) | 84 (27%) | |
| ≥7 | 19 (18%) | 49 (16%) | |
| No. of medications | |||
| 0 | 34 (32%) | 127 (41%) | 0.043 |
| 1 | 30 (28%) | 99 (32%) | |
| >1 | 42 (40%) | 83 (27%) | |
| Serum 25OHD (ng/ml) | 32.7 ± 1.05 | 31.3 ± 0.58 | 0.249 |
| Serum 1,25(OH)2D (pg/ml) | 33.1 ± 0.76 | 35.3 ± 0.46 | 0.012 |
| 1,25(OH)2D to 25OHD ratio | 1.14 ± 0.05 | 1.26 ± 0.03 | 0.064 |
| Serum PTH (pg/ml) | 38.5 ± 1.79 | 36.6 ± 0.74 | 0.309 |
| Calcium absorption (actual dose per liter, weight corrected) | 23.6 ± 0.72 | 27.3 ± 0.42 | <0.001 |
| 24-h urine calcium (mg) | 117.5 ± 5.81 | 154.7 ± 3.75 | <0.001 |
| 24-h urine creatinine (mg) | 715.5 ± 14.4 | 949.9 ± 10.1 | <0.001 |
| 24-h urine calcium/creatinine | 0.167 ± 0.008 | 0.167 ± 0.004 | 0.969 |
| 24-h urine N-telopeptides/creatinine (nmol BCE/mmol Cr) | 51.0 ± 2.57 | 51.3 ± 1.52 | 0.93 |
| PASE score | 107.1 ± 4.76 | 119.9 ± 3.37 | 0.03 |
| Timed rise (sec) | 8.94 ± 0.38 | 8.51 ± 0.16 | 0.31 |
| Timed walk normal speed (sec) | 5.02 ± 0.10 | 4.77 ± 0.06 | 0.04 |
| Timed walk fast (sec) | 3.59 ± 0.08 | 3.36 ± 0.04 | 0.01 |
| Grip strength (kg) | 23.6 ± 0.43 | 25.7 ± 0.24 | <0.001 |
The values are means ± SEM. The characteristics, biochemical variables, and physical performance measures were compared by Student’s t test. Categorical variables were compared by χ2 test. BCE, bone collagen equivalents.
Women in the low CrCl group were significantly different for the following measurements: they were older (P = 0.014), smaller (P = 0.002), and lighter (P = 0.001) and had lower calcium absorption (P < 0.001), 24-h urine calcium (P < 0.001), creatinine (P < 0.001), serum 1,25(OH)2D (P = 0.012) and serum 1,25(OH)2D to 25OHD ratio (P = 0.06). The high CrCl group took more medications more than one (P = 0.043). The mean and median serum 25OHD levels in the less than 60 ml/min CrCl group were 32.7 and 32.1 ng/ml, respectively, whereas in the 60 ml/min or greater CrCl group, the corresponding values were 32.7 and 30.8 ng/ml, respectively. Six percent of the population had serum 25OHD levels less than 15 ng/ml. The baseline characteristics and biochemical measures (Table 1) were also compared in the two CrCl subgroups in the four treatment groups. The results were similar to that seen in the two CrCl subgroups combined (data not given).
Study participants who dropped out of the study (n = 151), compared with compliers, had lower CrCl (67.8 ± 1.5 vs. 73.6 ± 1.06 ml/min, P = 0.002) and lower serum 1, 25(OH)2D (32.9 ± 0.67 vs. 35.1 ± 0.43 pg/ml, P = 0.005) only.
In the total group of 415 women, baseline serum 1,25(OH)2D decreased with decreasing urine CrCl (r = 0.116, P = 0.018) (Fig. 1) and increasing serum creatinine (r = −0.148, P = 0.003).
Fig. 1.
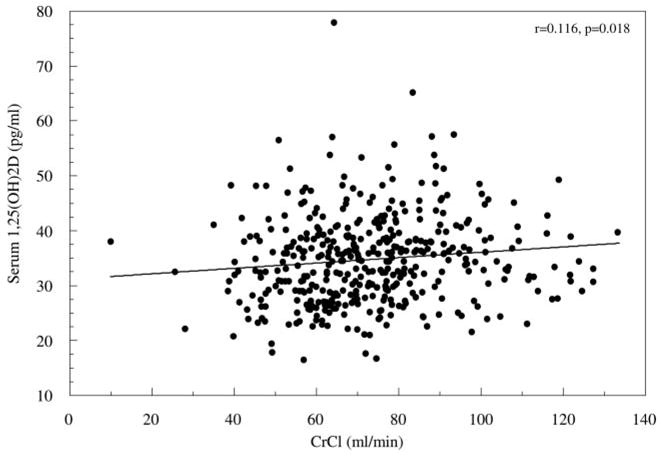
Correlation graph between measured CrCl and serum 1,25(OH)2D3.
The biochemical data from Table 1 at the end of 36 months was compared in the CrCl subgroups in the placebo group. Results were similar to that seen at baseline; statistical differences were not seen, probably due to insufficient numbers. At the end of 36 months, repeat 24-h urine CrCl decreased less than 60 ml/min in 28% of those initially above 60 ml/min. Serum 1,25(OH)2D was not measured at the end of the study.
Physical performance measures (Table 1)
At baseline, women in the low CrCl group had lower physical activity score (PASE) (P = 0.03), were significantly (P < 0.05) slower in the timed walk test, and were weaker in grip strength (P < 0.001). Baseline physical performance measures, compared between the two CrCl subgroups in the four treatment groups (data not given), showed similar trends to that seen in Table 1.
Fallers
Of 415 subjects who completed the final 36-month study visit, the percentage of subjects in each treatment group having a fall at any time during the study was 64% on placebo, 49.5% on calcitriol, 58% on calcitriol + ET/HT, and 57% on ET/HT alone (Table 2). At 36 months, calcitriol treatment reduced fallers by 46% (odds ratio 0.54, 95% CI 0.31–0.94; P = 0.03). Other treatment groups had no effect on fallers.
TABLE 2.
Distribution of falls, cumulative number of falls per person, and fallers in the treatment groups according to CrCl status
| Placebo
|
Calcitriol
|
ET/HT + calcitriol
|
ET/HT
|
|||||
|---|---|---|---|---|---|---|---|---|
| CrCl < 60 ml/min | CrCl ≥ 60 ml/min | CrCl < 60 ml/min | CrCl ≥ 60 ml/min | CrCl < 60 ml/min | CrCl ≥ 60 ml/min | CrCl < 60 ml/min | CrCl ≥ 60 ml/min | |
| n | 28 | 84 | 26 | 75 | 34 | 68 | 18 | 82 |
| Age (yr) | 72.6 ± 0.70 | 70.5 ± 0.40a | 72.18 ± 0.75 | 71.20± .38 | 71.49 ± 0.57 | 71.3 ± 0.43 | 71.7 ± 0.80 | 71.35 ± 0.41 |
| Mean CrCl (mg/dl) | 49.95 ± 2.01 | 83.8 ± 1.81a | 50.0 ± 1.68 | 78.8 ± 1.53a | 51.1 ± 1.06 | 81.1 ± 1.96a | 53.1 ± 1.43 | 78.3 ± 1.69a |
| Total falls (n) | 50 | 97 | 25 | 64 | 35 | 78 | 25 | 97 |
| Cumulative falls/person | 1.79 | 1.15b | 0.96 | 0.85 | 1.03 | 1.15 | 1.39 | 1.18 |
| Subjects | Subjects | Subjects | Subjects | Subjects | Subjects | Subjects | Subjects | |
| No. of falls | ||||||||
| 0 | 10 | 30 | 13 | 38 | 17 | 26 | 7 | 36 |
| 1 | 6 | 30 | 5 | 20 | 9 | 23 | 3 | 23 |
| 2 | 6 | 12 | 5 | 9 | 5 | 11 | 3 | 9 |
| 3 | 2 | 8 | 2 | 6 | 2 | 5 | 4 | 7 |
| ≥4 | 4 | 4 | 1 | 2 | 1 | 3 | 1 | 6 |
| No. of fallers | 18 | 54 | 13 | 37 | 17 | 42 | 11 | 46 |
The data on age and mean CrCl are presented as mean ± SEM. The comparisons between the two CrCl groups in respective treatment groups were done by Student’s t test.
P < 0.05, compared with CrCl less than 60 ml/min group in respective treatment groups.
P = 0.007, compared with CrCl less than 60 ml/min of placebo group.
Cumulative rate of falls
Comparing treatment groups with placebo in 415 subjects, the adjusted rate of falls was reduced by 36% (95% CI −51 to −17%; P = 0.0009) in the calcitriol group and reduced by 25% (95% CI −42 to −3%; P = 0.03) in the calcitriol + ET/HT group; no significant reduction was seen in the ET/HT group (Table 3). In the calcitriol treatment group, the number of falls per person at 6 months was 0.26 and averaged 0.14 for the rest of the study period, whereas the placebo group averaged 0.23 at 6-month intervals during the 3 yr.
TABLE 3.
Reduction in rate of falls according to CrCl in the treatment groups, compared to placeboa
| Treatment group | Irrespective of CrCl, % (95% CI) | P | CrCl < 60 ml/min, % (95% CI) | P | CrCl ≥ 60 ml/min, % (95% CI) | P |
|---|---|---|---|---|---|---|
| Calcitriol | −36 (−51 to −17) | 0.0009 | −53 (−71 to −22) | 0.003 | −30 (−49 to −4) | 0.027 |
| Calcitriol + ET/HT | −25 (−42 to −3) | 0.03 | −61 (−76 to −37) | 0.0002 | −4 (−29 to +30) | 0.77 |
| ET/HT | −12 (−31 to +23) | 0.32 | −25 (−55 to +24) | 0.26 | −4 (−28 to +28) | 0.77 |
Adjusted for significant covariates, number of comorbid conditions, smoking status, and PASE score.
Effect of CrCl (CrCl < 60 ml/min vs. CrCl ≥ 60 ml/min) on fallers (Table 2)
Measured 24-h urine CrCl was not a significant predictor of fallers in any of the treatment groups. There were no significant differences in fallers in the CrCl subgroups in the placebo or treatment groups (Table 2).
Effect of CrCl (CrCl < 60 ml/min vs. CrCl ≥ 60 ml/min) on falls (Table 2)
Measured 24-h urine CrCl was a significant predictor of the number of falls per person in the placebo group (P = 0.007) at 36 months from Poisson regression model. At the end of 36 months, comparing the two CrCl groups (< 60 vs. ≥ 60 ml/min), the mean ± SE number of falls per person at 36 months in the placebo group was 1.79 ± 0.44, compared with 1.15 ± 0.14, respectively (Fig. 2A) and was 63% higher (95% CI 14–133%; P = 0.007) in the CrCl less than 60 ml/min group.
Fig. 2.
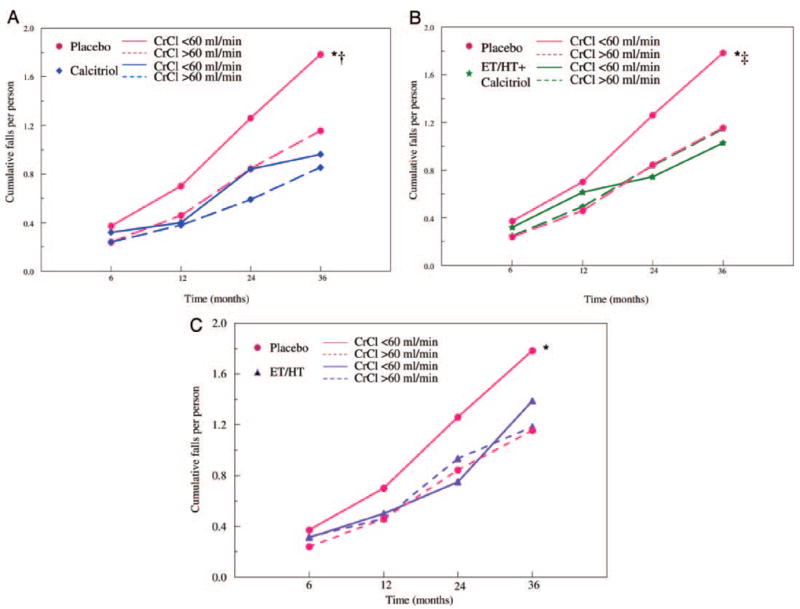
Cumulative number of falls per person in the placebo and calcitriol groups (A); placebo and ET/HT + calcitriol groups (B); and placebo and ET/ HT groups (C) over the treatment period in women with CrCl less than 60 and more than 60 ml/min. The data were adjusted for significant covariates: number of comorbid conditions, smoking status, and PASE score. *, P < 0.05, compared with placebo CrCl greater than 60 ml/min; †, P < 0.05, compared with calcitriol CrCl less than 60 ml/min; ‡, P < 0.05, compared with calcitriol + ET/HT CrCl less than 60 ml/min.
The mean number of falls per person between the CrCl subgroups was not different in the treatment groups, 0.96 ± 0.23 vs. 0.85 ± 0.13 (P = 0.35) in the calcitriol group (Fig. 2A), 1.03 ± 0.31 vs. 1.15 ± 0.18 (P = 0.36) in the calcitriol + ET/HT group (Fig. 2B), and 1.39 ± 0.32 vs. 1.18 ± 0.17 (P = 0.47) in the ET/HT group (Fig. 2C).
Effect of treatment, compared with placebo, on the rate of falls in the CrCl subgroups (Table 3)
In the CrCl less than 60 ml/min groups, calcitriol, compared with placebo, reduced the rate of falls by 53% (P = 0.0033); the effect of calcitriol treatment was seen at 12 months and was significant at 36 months. Calcitriol + ET/HT significantly reduced the rate of falls by 61% (P = 0.0002). There was a nonsignificant 25% reduction (P = 0.26) with ET/HT treatment alone.
In the 60 ml/min or greater CrCl groups, calcitriol, compared with placebo, reduced the rate of falls by 30% (P = 0.027); there was no effect of calcitriol + ET/HT or ET/HT alone.
Although calcitriol treatment significantly reduced the rate of falls in both less than 60 ml/min (P = 0.003) and 60 ml/min or greater (P = 0.03) CrCl groups, the effect was more significant in the low CrCl group.
Adverse events
The adverse events related to estrogen and calcitriol treatment has been described in detail earlier (4). Hypercalcemia associated with calcitriol treatment was mild and infrequent over the treatment period. A single episode of hypercalcuria (>300 mg/d) occurred in 7% of subjects on placebo, 6% on ET/HT, 18% on ET/HT + calcitriol, and 29% on calcitriol treatment and was probably related to perturbations of dietary calcium intake. Persistent hypercalcuria was uncommon and the calcitriol dose was reduced in two subjects.
Discussion
In this study of normal elderly women, calcitriol treatment significantly reduced the number of fallers by 46%, but no association was found between CrCl and the number of fallers. However, a 24-h CrCl less than 60ml/min was associated with an increase in the number of falls per person. In the low CrCl group, calcitriol and calcitriol + ET/HT were both effective in significantly reducing the rate of falls by 50–60%, and the effect was apparent by 12 months.
The results of the present study are in agreement with the observations by Dukas et al. (7–9) in a slightly older population, mean age 75 yr, from Switzerland. In community-dwelling elderly men and women, Dukas et al. (7, 8) reported that CrCl less than 65 ml/min defined by the Cockroft-Gault formula is an independent risk factor for increased frequency of falls and fractures, which they attributed to a reduction in serum 1,25(OH)2D levels. They also reported that alphacalcidol, which is a prodrug for calcitriol, reduced the number of falls and fallers in the low CrCl group (9). However, one study reported that long-term vitamin D supplementation, 700 IU/d reduced the number of fallers in ambulatory women irrespective of CrCl (26).
A decrease in the muscle strength with aging is associated with an increased incidence of falls in the elderly (27). In the present study, characteristics of women in the low CrCl group with the higher fall rate were lower physical activity, decreased muscle strength, lower physical performance, lower serum 1,25(OH)2D, and decreased conversion of serum 25OHD to 1,25(OH)2D. An important finding was that the receptors for 1,25(OH)2D in muscle decrease with aging (28) and if combined with lower serum 1,25(OH)2D could be responsible for decreased muscle strength and the increase in the rate of falls in elderly women. A smaller but significant reduction in the rate of falls with calcitriol treatment was seen also in women with CrCl 60 ml/min or greater, possibly through up-regulation of 1,25(OH)2D receptors in muscle.
It is possible that 25OHD, which is the substrate for 1,25(OH)2D, is not high enough for elderly subjects in whom there is impaired conversion of 25OHD to 1,25(OH)2D in the kidney (29). There is evidence suggesting that serum 25OHD levels higher than 30–32 ng/ml are needed to reduce serum PTH in the elderly (30). Although serum levels of 1,25(OH)2D are usually tightly regulated, we did observe some substrate dependency of 1,25(OH)2D production in a seasonal study of serum 25OHD (18). PTH has been reported to be an independent risk factor for falls in an Australian elderly population (31), although that was not seen in this study or in a study from Boston (26).
Is it possible that simple vitamin D therapy can have the same effect as the D hormone-calcitriol in reducing falls? Studies on the effect of vitamin D supplementation on risk of falling/falls are inconsistent. Vitamin D, 400 IU/d, was effective in reducing fallers in one study (32) but not in another (33). Vitamin D, 800 IU/d, reduced the risk of falls or fallers in four studies (11, 12, 26, 34) but was ineffective in four others (35–38). There are several possible explanations for the inconsistent data; most of these studies had high numbers of subjects with hypovitaminosis D (<20 ng/ml) or vitamin D insufficiency (<15 ng/ml), and the vitamin D dose might not have increased serum 25OHD high enough. Also, compliance to treatment is often low; in a study of vitamin D supplementation, 1000–1500 IU/d, given to 625 older men and women, the rate of falls but not the number of fallers decreased by 27%; however, when the data were reanalyzed in a compliant group, both falls and fallers decreased more significantly on vitamin D (39). In this present study, an increased rate of falls were observed in women with mean serum 25OHD of 30 ng/ml so that hypovitaminosis D, i.e. serum 25OHD less than 20 ng/ml, was not a contributing cause. It is possible that vitamin D therapy could be effective in reducing falls in patients with low serum 25OHD and normal CrCl who have the ability to convert to 1,25(OH)2D; however, the ability to convert decreases with age, particularly after the age of 75 yr (29).
The present study has several limitations. Falls were a secondary outcome in the study, although number of events and the long observations period compensates in part. The fall incidence data were based on participants’ own report. The study population is comprised mostly of healthy Caucasian women between the ages of 65 and 77 yr, and the observations and estimates of CrCl cannot be generalized to men and women of other races, especially those with low body weight.
In conclusion, the results of the present study show that low CrCl (<60 ml/min) is a risk factor for falls in elderly women. Decreased conversion of 25OHD to the active hormonal form of vitamin D-calcitriol by the aging kidney associated with decreased physical performance and an age related decrease in calcitriol receptors in muscle are explanations for increased rate of falls. Calcitriol treatment reduces the incidence of falls; the mechanism by which calcitriol affects the fall incidence is not yet clear. An up-regulation of calcitriol receptors in muscle by calcitriol treatment is a strong possibility, but also there could be an improvement in balance through its action on central or peripheral nervous system. Whether simple vitamin D supplementation in subjects with impaired renal function is as effective as calcitriol or vitamin D analogs in preventing falls is not known, but because of relative costs of medication, a comparative study is needed.
Not many therapies reduce falls in the elderly. Interventions that are proposed to be beneficial in fall prevention are a program of muscle strengthening and improved balance (pooled risk ratio 0.8, 95% CI 0.66–0.98; 20% reduction), multidisciplinary intervention (pooled risk ratio 0.73, 95% CI 0.63–0.85; 27% reduction), withdrawal of psychotropic medications (relative hazard ratio 0.34, 95% CI 0.16–0.74; 66% reduction), exercise intervention (risk ratio 0.51, 95% CI 0.36–0.73; 49% reduction), etc. (40). The data from this study suggest that targeting women with decreased renal function for treatment with calcitriol also could be an effective strategy (50–60% reduction).
Acknowledgments
We thank Karen A. Rafferty for her help in food dairy data collection and analysis. We also thank Kurt E. Balhorn for some laboratory analysis and Jeff Detter for data management.
Abbreviations
- CI
Confidence interval
- CrCl
creatinine clearance
- ET
estrogen therapy
- HT
estrogen + progestin therapy
- 25OHD
25 hydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- PASE
Physical Activity Scale for the Elderly
Footnotes
This work was presented as an abstract at the 27th Annual Meeting of the American Society for Bone and Mineral Research, Nashville, Tennessee, September 23–27, 2005.
Disclosure Summary: J.C.G. is a consultant for Wyeth Research and Pfizer; he has received research grants from Wyeth Research and Pfizer, equity (shares) in Pfizer, and lecture fees from Wyeth Research and Pfizer. P.B.R. and L.M.S. have nothing to disclose.
This work was supported by National Institute of Aging Grants UO1-AG10373 and RO1-AG10358 and Wyeth Pharmaceuticals, Hoffmann-La Roche Inc., and Pfizer (Pharmacia & Upjohn).
References
- 1.Bergland A, Wyller TB. Risk factors for serious fall related injury in elderly women living at home. Inj Prev. 2004;10:308–313. doi: 10.1136/ip.2003.004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 3.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 1993;41:1226–1234. doi: 10.1111/j.1532-5415.1993.tb07307.x. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86:3618–3628. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher JC. The effects of calcitriol on falls and fractures and physical performance tests. J Steroid Biochem Mol Biol. 2004;89–90:497–501. doi: 10.1016/j.jsbmb.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Dukas L, Bischoff HA, Lindpaintner LS, Schacht E, Birkner-Binder D, Damm TN, Thalmann B, Stahelin HB. Alfacalcidol reduces the number of fallers in a community-dwelling elderly population with a minimum calcium intake of more than 500 mg daily. J Am Geriatr Soc. 2004;52:230–236. doi: 10.1111/j.1532-5415.2004.52060.x. [DOI] [PubMed] [Google Scholar]
- 7.Dukas L, Schacht E, Stahelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005;16:1683–1690. doi: 10.1007/s00198-005-1903-7. [DOI] [PubMed] [Google Scholar]
- 8.Dukas LC, Schacht E, Mazor Z, Stahelin HB. A new significant and independent risk factor for falls in elderly men and women: a low creatinine clearance of less than 65 ml/min. Osteoporos Int. 2005;16:332–338. doi: 10.1007/s00198-004-1690-6. [DOI] [PubMed] [Google Scholar]
- 9.Dukas L, Schacht E, Mazor Z, Stahelin HB. Treatment with alfacalcidol in elderly people significantly decreases the high risk of falls associated with a low creatinine clearance of <65 ml/min. Osteoporos Int. 2005;16:198–203. doi: 10.1007/s00198-004-1671-9. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig-Chiello P, Tyndall A, Theiler R. Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80:54–58. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 11.Bischoff HA, Stahelin HB, Dick W, Akos R, Knecht M, Salis C, Nebiker M, Theiler R, Pfeifer M, Begerow B, Lew RA, Conzelmann M. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 12.Pfeifer M, Begerow B, Minne HW, Abrams C, Nachtigall D, Hansen C. Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J Bone Miner Res. 2000;15:1113–1118. doi: 10.1359/jbmr.2000.15.6.1113. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 14.Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA. Muscle strength, functional mobility and vitamin D in older women. Aging (Milano) 2000;12:455–460. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation. K/DOQI practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 42:S7–S28. [PubMed] [Google Scholar]
- 16.Klawansky S, Komaroff E, Cavanaugh PF, Jr, Mitchell DY, Gordon MJ, Connelly JE, Ross SD. Relationship between age, renal function and bone mineral density in the U.S. population. Osteoporos Int. 2003;14:570–576. doi: 10.1007/s00198-003-1435-y. [DOI] [PubMed] [Google Scholar]
- 17.Haddad JG, Chyu KJ. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971;33:992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 18.Rapuri PB, Kinyamu HK, Gallagher JC, Haynatzka V. Seasonal changes in calciotropic hormones, bone markers, and bone mineral density in elderly women. J Clin Endocrinol Metab. 2002;87:2024–2032. doi: 10.1210/jcem.87.5.8475. [DOI] [PubMed] [Google Scholar]
- 19.Rapuri PB, Gallagher JC. Effect of Vitamin D supplement use on serum concentrations of total 25OHD levels in elderly women. J Steroid Biochem Mol Biol. 2004;89–90:601–604. doi: 10.1016/j.jsbmb.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Hollis BW. Assay of circulating 1,25-dihydroxyvitamin D involving a novel single-cartridge extraction and purification procedure. Clin Chem. 1986;32:2060–2063. [PubMed] [Google Scholar]
- 21.Reinhardt TA, Horst RL, Orf JW, Hollis BW. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984;58:91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts JT, Jr, Segre GV. Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem. 1987;33:1364–1367. [PubMed] [Google Scholar]
- 23.Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF. Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest. 1979;64:729–736. doi: 10.1172/JCI109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 25.Judge JO, Lindsey C, Underwood M, Winsemius D. Balance improvements in older women: effects of exercise training. Phys Ther. 1993;73:254–262. doi: 10.1093/ptj/73.4.254. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166:424–430. doi: 10.1001/archinte.166.4.424. [DOI] [PubMed] [Google Scholar]
- 27.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 29.Kinyamu HK, Gallagher JC, Petranick KM, Ryschon KL. Effect of parathyroid hormone (hPTH[1–34]) infusion on serum 1,25-dihydroxyvitamin D and parathyroid hormone in normal women. J Bone Miner Res. 1996;11:1400–1405. doi: 10.1002/jbmr.5650111005. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher JC, Kinyamu HK, Fowler SE, Dawson-Hughes B, Dalsky GP, Sherman SS. Calciotropic hormones and bone markers in the elderly. J Bone Miner Res. 1998;13:475–482. doi: 10.1359/jbmr.1998.13.3.475. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, Zochling J, Sitoh YY, Lau TC, Schwarz J, Seibel MJ. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab. 2004;89:1572–1576. doi: 10.1210/jc.2003-031782. [DOI] [PubMed] [Google Scholar]
- 32.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents severe falls in elderly community-dwelling women: a pragmatic population-based 3-year intervention study. Aging Clin Exp Res. 2005;17:125–132. doi: 10.1007/BF03324585. [DOI] [PubMed] [Google Scholar]
- 33.Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–1136. doi: 10.1093/oxfordjournals.aje.a008690. [DOI] [PubMed] [Google Scholar]
- 34.Harwood RH, Sahota O, Gaynor K, Masud T, Hosking DJ. A randomised, controlled comparison of different calcium and vitamin D supplementation regimens in elderly women after hip fracture: the Nottingham Neck of Femur (NONOF) study. Age Ageing. 2004;33:45–51. doi: 10.1093/ageing/afh002. [DOI] [PubMed] [Google Scholar]
- 35.Chapuy MC, Pamphile R, Paris E, Kempf C, Schlichting M, Arnaud S, Garnero P, Meunier PJ. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13:257–264. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 36.Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 37.Porthouse J, Cockayne S, King C, Saxon L, Steele E, Aspray T, Baverstock M, Birks Y, Dumville J, Francis R, Iglesias C, Puffer S, Sutcliffe A, Watt I, Torgerson DJ. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330:1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, Thomas J, Lowndes C, Hopper JL, Wark JD. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 40.Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;4:CD000340. doi: 10.1002/14651858.CD000340. (Review) [DOI] [PubMed] [Google Scholar]


