Abstract
NTAL (non-T cell activation linker)/LAB (linker for activation of B cells), now officially termed LAT2 (linker for activation of T cells 2) is a 25-30 kD transmembrane adaptor protein (TRAP) associated with glycolipid-enriched membrane fractions (GEMs; lipid raft) in specific cell types of hematopoietic lineage. Tyrosine phosphorylation of NTAL/LAB/LAT2 is induced by FcεRI aggregation and Kit dimerization in mast cells, FcγRI aggregation in monocytes, and BCR aggregation in B cells. NTAL/LAB/LAT2 is also expressed in resting NK cells but, unlike the related TRAP, LAT, not in resting T cells. As demonstrated in monocytes and B cells, phosphorylated NTAL/LAB/LAT2 recruits signaling molecules such as Grb2, Gab1 and c-Cbl into receptor-signaling complexes. Although gene knock out and knock down studies have indicated that NTAL/LAB/LAT2 may function as both a positive and negative regulator of mast cell activation, its precise role in the activation of these and other hematopoietic cells remains enigmatic.
Keywords: NTAL, LAB, LAT2, Transmembrane adaptor protein, Mast cells
Introduction
NTAL (non-T cell activation linker) was originally identified in 2002 in the laboratory of Vaclav Horejsi (ASCR, Prague, Czech Republic) following sequencing of a previously unidentified tyrosine phosphorylated protein of 30 kD found in the glycolipid-enriched membrane (GEM or lipid rafts) fractions isolated from the THP-1 myeloid cell line (Brdicka et al., 2002). The molecule was subsequently also described and given the name LAB (linker for activation of B cells) by the group of Weiguo Zhang in 2003 (Duke University, North Carolina, USA) following human genome database search for LAT (linker for activation of T cells) homologs in B cells and other cell types (Janssen et al., 2003). The molecule has now been given the official name LAT2 by the Human Genome Organization Nomenclature Committee based on the structural similarity of this molecule to LAT (Gilfillan & Iwaki, 2006). To avoid confusion we will use the NTAL/LAB/LAT2 designation for this molecule in this article.
Structure
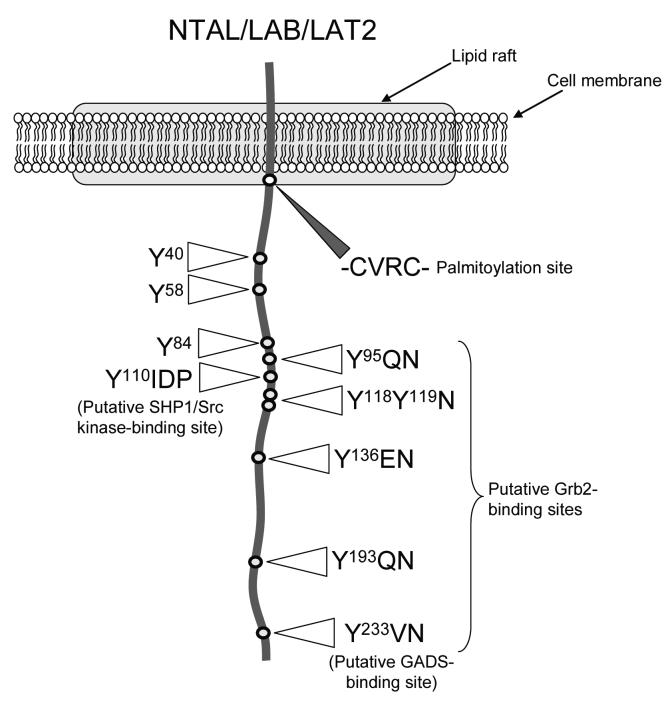
The human NTAL/LAB/LAT2 gene is located on chromosome 7 (7q11.23) and is identical to the wbscr5 gene, which is part of a gene locus deleted in Williams-Beuren syndrome (Brdicka et al., 2002; Gilfillan & Iwaki, 2006; Janssen et al., 2003). This gene consists of 11 exons and encodes a 243 amino acid protein with a molecular weight of approximately 30 kD (Brdicka et al., 2002; Gilfillan & Iwaki, 2006; Janssen et al., 2003). Both longer and shorter alternatively spliced isoforms, however, have been reported at the cDNA level (Gilfillan & Iwaki, 2006). The murine form is a protein of 203 amino acids with a molecular weight of approximately 25 kD (Brdicka et al., 2002). Human NTAL/LAB/LAT2 has a short 4 amino acid extracellular domain, a single 18 amino acid transmembrane span and a 221 amino acid cytosolic domain (Brdicka et al., 2002; Gilfillan & Iwaki, 2006; Janssen et al., 2003). The cytosolic juxta-membrane region of NTAL/LAB/LAT2 has a -CVRC- palmitoylation site (Figure 1) which results in this molecule being targeted to reside in the GEMs/lipid rafts (Brdicka et al., 2002; Janssen et al., 2003). Contained within the cytosolic domain of NTAL/LAB/LAT2 are 10 tyrosines which are potential targets for tyrosine kinases. Six of these tyrosines are found within 5 YXN motifs (one of these motifs is Y118Y119N) which are recognized as putative binding sites for the cytosolic adaptor molecule Grb2 following the phosphorylation of these tyrosines (Brdicka et al., 2002; Koonpaew et al., 2004). One of the YXN motifs (Y233VN, human NTAL/LAB/LAT2 sequence) in LAT has been recognized as a binding site for the Grb2-related cytosolic adaptor molecule, GADS (Gilfillan & Tkaczyk, 2006), however, as yet, phosphorylated NTAL/LAB/LAT2 has not been demonstrated to bind this molecule.
Figure 1.
Representation of the structure and location of human NTAL/LAB/LAT2. The striped triangle designates the position of the juxtamembrane palmitoylation site, and the open triangles designate the position of the potential tyrosine phosphorylation sites.
In addition to these potential binding sites, Y110 is part of a YIDP sequence also found in Kit which, in this latter molecule, is recognized as a putative Src kinase/SHP-1 binding site (Linnekin, 1999). Again, whether such interactions occur with NTAL/LAB/LAT2 is currently unknown. The remaining 3 tyrosines do not appear to be part of recognized binding motifs. NTAL/LAB/LAT2 however does posses 3 RXXK motifs which, although not yet demonstrated for NTAL/LAB/LAT2, may permit constitutive binding of SH3 domain-containing signaling molecules such as Src kinases (Gilfillan & Tkaczyk, 2006). Unlike LAT, NTAL/LAB/LAT2 does not possess a direct binding site for the signaling molecule, phospholipase C (PLC)γ1 (Janssen et al., 2004) and, furthermore, does not possess a direct binding site for phosphoinositide 3-kinase (PI3K) (Gilfillan & Tkaczyk, 2006).
Expression and turnover
NTAL/LAB/LAT2 is primarily expressed in spleen and hematopoietic cells, such as B cells, mast cells, NK cells, and monocytes, but not resting T cells (Brdicka et al., 2002; Janssen et al., 2003). As described in the previous section, NTAL/LAB/LAT2 was identified as a product of wbscr5 gene on chromosome 7q11.23. It has not yet been determined whether the expression of NTAL/LAB/LAT2 is inducible. The expression of this gene has, however, been reported to be downregulated in human acute myeloid leukemia subtype M2 (Fliegauf et al., 2004). At present, it is unclear how NTAL/LAB/LAT2 is involved in leukemogenesis. The turnover rate, and its regulation, for NTAL/LAB/LAT2 are unknown. However, it has been reported to be ubiquitinated following B cell receptor (BCR) stimulation in Ramos B cell line (Brdicka et al., 2002), which suggests that it may be targeted to the proteasome for degradation by a receptor-dependent mechanism.
Biological Function
NTAL/LAB/LAT2 possesses no inherent catalytic activity. Rather, it acts as an adaptor/scaffolding molecule, the function of which is to recruit and tether critical signaling molecules into the receptor-signaling complex (signalosome). This property is dependent on the phosphorylation of specific tyrosine residues contained within its cytosolic tail as detailed above. Transfection studies conducted in 293T cells reveal that Src family kinases, such as Lyn and Lck, ZAP-70, and the related kinase, Syk, can all phosphorylate NTAL/LAB/LAT2 directly (Brdicka et al., 2002). The use of Lyn−/− mouse bone marrow-derived mast cells (BMMCs) and selective tyrosine kinase inhibitors have demonstrated that both Lyn and Syk are required for NTAL/LAB/LAT2 phosphorylation in mast cells following antigen-dependent FcεRI aggregation (Tkaczyk et al., 2004). Studies conducted in human mast cells suggest that the receptor tyrosine kinase, Kit, can also directly phosphorylate NTAL/LAB/LAT2 (Tkaczyk et al., 2004). Unpublished observations from our laboratory have also demonstrated that Lyn, Syk, and Kit selectively phosphorylate different tyrosine residues within NTAL/LAB/LAT2.
The phosphorylation of NTAL/LAB/LAT2 following antigen-mediated BCR aggregation in B cells (Brdicka et al., 2002), FcγRI (high affinity receptor for IgG) aggregation in monocytes (Brdicka et al., 2002), FcεRI (high affinity receptor for IgE) in mast cells (Brdicka et al., 2002; Tkaczyk et al., 2004; Volna et al., 2004; Zhu et al., 2004) and SCF (stem cell factor)-mediated Kit dimerization in mast cells, suggest a role for NTAL/LAB/LAT2 in the function of these cell types. Despite the generation of NTAL/LAB/LAT2-deficent mice (Volna et al., 2004; Zhu et al., 2004) and the use of siRNA/shRNA gene knock down approaches (Janssen et al., 2003; Tkaczyk et al., 2004), the precise role that NTAL/LAB/LAT2 plays in the function of hematopoietic cells, however, remains unclear.
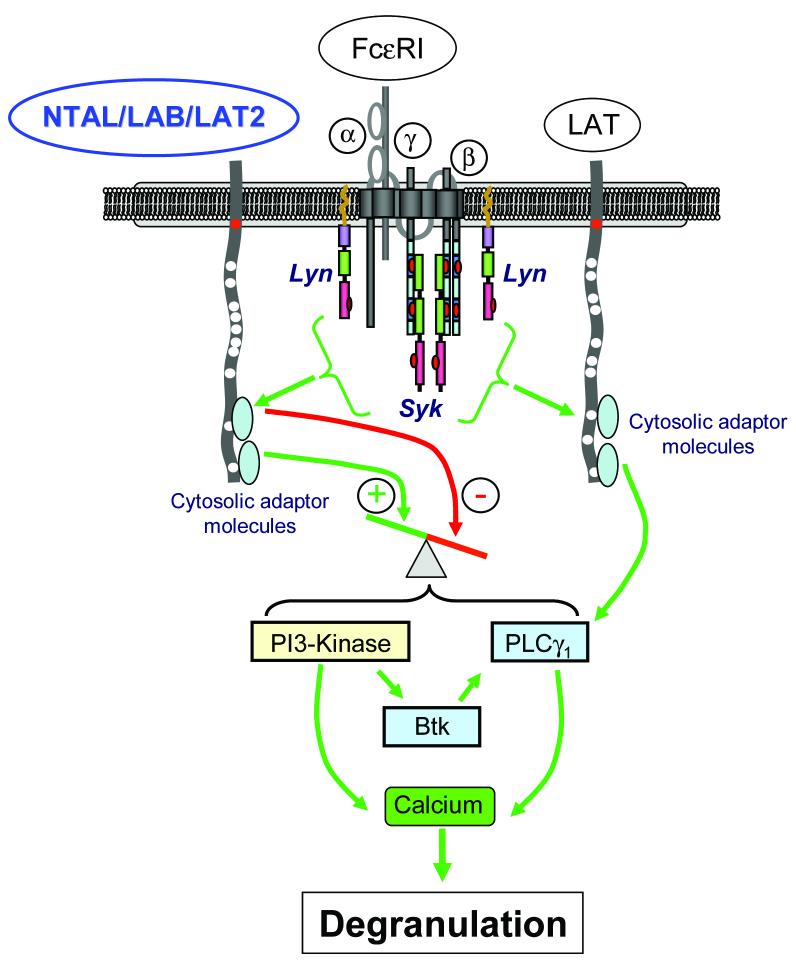
BMMCs derived from NTAL/LAB/LAT2-deficient mice display enhanced degranulation and cytokine production following FcεRI aggregation compared to wild type responses, which suggests that NTAL/LAB/LAT2 may be a negative regulator of antigen-dependent mast cell activation (Volna et al., 2004; Zhu et al., 2004). This conclusion was supported by the enhanced passive cutaneous anaphylactic responses observed in the NTAL/LAB/LAT2−/− mice (Volna et al., 2004). In contrast, NTAL/LAB/LAT2−/− / LAT−/− double knock out BMMCs displayed a greater defect in antigen-mediated degranulation than that observed in both the LAT−/− and NTAL/LAB/LAT2−/− single knock out BMMCs (Volna et al., 2004), which suggested that LAT and NTAL/LAB/LAT2 cooperate to positively regulate degranulation (Zhu et al., 2004). This latter conclusion had been previously suggested by siRNA (Tkaczyk et al., 2004) and subsequently shRNA (Jensen, Tkaczyk, & Gilfillan, unpublished observations) gene knock down studies in human mast cells which demonstrated that reduction in NTAL/LAB/LAT2 expression resulted in a significant reduction in the ability of these cells to degranulate in response to antigen. Furthermore, the ability of SCF to potentiate antigen-mediated degranulation was also ablated following NTAL/LAB/LAT2 gene knock down, suggesting a role for NTAL/LAB/LAT2 in the integration of the Kit- and FcεRI-dependent signaling pathways required for this response (Tkaczyk et al., 2004). The most likely explanation for above contradictory data is that, in mast cells, NTAL/LAB/LAT2 regulates both negative and positive pathways for receptor-mediated mast cell activation (Figure 2).
Figure 2.
Simplified representation of the potential roles of NTAL/LAB/LAT2 in FcεRI-mediated mast cell degranulation. In this figure, the positively regulated pathways are represented by green arrows, and the negative pathways by red arrows. FcεRI-aggregation leads to activation of the tyrosine kinases, Lyn and Syk, which subsequently phosphorylate LAT and NTAL/LAB/LAT2. LAT regulates degranulation by recruiting cytosolic adaptor molecules and phospholipase (PL)Cγ1 thereby increasing calcium flux in a PLCγ1-dependent manner. NTAL/LAB/LAT2 appears to regulate both negative and positive pathways for the regulation of degranulation, however, the molecules regulating these responses are currently unknown. Nevertheless, it has been proposed that the positive pathway may involve activation of PI3K. For further details and discussions of this pathway, please refer to Gilfillan & Tkaczyk (2006).
How NTAL/LAB/LAT2 may negatively and/or positively regulate mast cell activation remains unclear. It has been postulated that NTAL/LAB/LAT2 may downregulate antigen-mediated signaling in mast cells by competing with LAT for a limited pool of signaling molecules in the lipid rafts (Volna et al., 2004). This would account for the potentiation of antigen-mediated PLCγ1, PI3K and ERK activation, and enhanced calcium mobilization observed in the NTAL/LAB/LAT2−/− BMMCs (Volna et al., 2004; Zhu et al., 2004). The increase in LAT phosphorylation which was also observed in these cells (Volna et al., 2004; Zhu et al., 2004), however, may suggest that this hyper-responsive phenotype reflects aberrant signaling associated with an overcompensation of LAT-mediated responses. A positive role for NTAL/LAB/LAT2 signaling in activated mast cells may be explained by binding of associated signaling molecules to phosphorylated NTAL/LAB/LAT2. These molecules in mast cell activation have not yet been identified. However, based on observations in B cell lines and on the amino acid sequence, it is likely that NTAL/LAB/LAT2, once phosphorylated in mast cells, binds Grb2 allowing other downstream molecules to indirectly bind to NTAL/LAB/LAT2 (Brdicka et al., 2002). It has also been postulated that NTAL/LAB/LAT2 may play a role in the regulation of PI3K in activated mast cells (Gilfillan & Tkaczyk, 2006; Rivera, 2005).
As with mast cells, the precise role for NTAL/LAB/LAT2 in B cell development and activation is currently unclear. Although both LAT and NTAL/LAB/LAT2 are found in B cells, LAT is limited to early B cells whereas NTAL/LAB/LAT2 more typifies mature B cells (Wang et al., 2005). NTAL/LAB/LAT2−/− mice display little phenotypic differences from the wild type animals in terms of B cell development and sizes of thymus, spleen, and lymph nodes (Volna et al., 2004; Zhu et al., 2004). In NTAL/LAB/LAT2−/− B cell cultures, if anything, there was a slight increase in BCR-mediated cell proliferation and calcium flux (Wang et al., 2005). There were also increased levels of natural antibodies and an enhanced humoral response to a T cell-dependent antigen (Wang et al., 2005). The conclusion from this study was that NTAL/LAB/LAT2 does not play an equivalent critical role in B cells as to that played by LAT in T cells (Wang et al., 2005). In contrast to these studies, a decreased BCR-dependent calcium flux and ERK activation was observed in the A20 B cell line following siRNA-induced NTAL/LAB/LAT2 gene knock down (Janssen et al., 2003). Furthermore, it has been proposed that NTAL/LAB/LAT2 may positively contribute to B cell signaling by binding to Grb2, thus eliminating the negative regulatory signal provided by this latter cytosolic adaptor molecule (Stork et al., 2004). Thus as for mast cells, the simplest explanation for these apparently contradictory data is that, in B cells, NTAL/LAB/LAT2 may regulate both negative and positive BCR-mediated signaling pathways.
Finally, data from NTAL/LAB/LAT2−/− / LAT−/− double knock out NK cells have provided evidence that NTAL/LAB/LAT2 and/or LAT are important integrators for ITAM-dependent signaling cascades downstream of DAP12 leading to cytotoxicity (Chiesa et al., 2006).
Possible Medical Application
Although the role that NTAL/LAB/LAT2 plays in the activation of hematopoietic cells remains somewhat enigmatic, the data supporting a role for this molecule in the regulation of antigen- and/or SCF-induced mast cell activation, suggests that this, and the related TRAP, LAT, would be attractive target molecules for pharmacological intervention for the treatments of mast cell-driven disorders such as allergic asthma. Given that these molecules possess no catalytic activity, it is difficult to envisage, however, how such molecules may be targeted. One possible approach would be to target the Grb2-binding sites or, if determined to be important, the YIDP motif, with small molecule inhibitors but, due to the ubiquitous expression of Grb2, this may present problems in being able to selectively target mast cells. Perhaps the easiest way to disrupt signaling mediated by NTAL/LAB/LAT2 may be by inhibiting the kinases responsible for NTAL/LAB/LAT2 phosphorylation, namely Lyn, Syk, and Kit. Inhibitors for all three kinases have been identified and, at least Gleevec, which can inhibit Kit, has been successfully utilized in the clinic for leukemic disorders (Escribano et al., 2006). Again the question of selectivity must be raised regarding the potential effects of these agents on other cells. However, the more restricted expression of these kinases to specific cell types of hematopoietic lineage may allow for more selective targeting.
Footnotes
Work in the authors' laboratory is supported by the NIAID Intramural Program within the National Institutes of Health and a Japan Society for the Promotion of Science research fellowship for Japanese Biomedical and Behavioral Research at National Institutes of Health to S. Iwaki.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, et al. Non-T cell activation linker (NTAL): A transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa S, Mingueneau M, Fuseri N, Malissen B, Raulet DH, Malissen M, et al. Multiplicity and plasticity of natural killer cell signaling pathways. Blood. 2006;107:2364–2372. doi: 10.1182/blood-2005-08-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano L, Akin C, Castells M, Schwartz LB. Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets. 2006;5:61–77. doi: 10.2174/187152806775269303. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Stock M, Berg T, Lubbert M. Williams-Beuren syndrome critical region-5/non-T-cell activation linker: A novel target gene of AML1/ETO. Oncogene. 2004;23:9070–9081. doi: 10.1038/sj.onc.1208042. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Iwaki S. Lat2. AfCS-Nature Molecule Pages. 2006 doi:10.1038/mp.a003809.01. [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Janssen E, Zhu M, Craven B, Zhang W. Linker for activation of B cells: A functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-γ1 binding. J Immunol. 2004;172:6810–6819. doi: 10.4049/jimmunol.172.11.6810. [DOI] [PubMed] [Google Scholar]
- Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB: A new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003;4:117–123. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- Koonpaew S, Janssen E, Zhu M, Zhang W. The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J Biol Chem. 2004;279:11229–11235. doi: 10.1074/jbc.M311394200. [DOI] [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Rivera J. NTAL/LAB and LAT: A balancing act in mast-cell activation and function. Trends Immunol. 2005;26:119–122. doi: 10.1016/j.it.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stork B, Engelke M, Frey J, Horejsi V, Hamm-Baarke A, Schraven B, et al. Grb2 and the non-T cell activation linker NTAL constitute a Ca2+-regulating signal circuit in B lymphocytes. Immunity. 2004;21:681–691. doi: 10.1016/j.immuni.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- Volna P, Lebduska P, Draberova L, Simova S, Heneberg P, Boubelik M, et al. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J Exp Med. 2004;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Horvath O, Hamm-Baarke A, Richelme M, Gregoire C, Guinamard R, et al. Single and combined deletions of the NTAL/LAB and LAT adaptors minimally affect B-cell development and function. Mol Cell Biol. 2005;25:4455–4465. doi: 10.1128/MCB.25.11.4455-4465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Liu Y, Koonpaew S, Granillo O, Zhang W. Positive and negative regulation of FcεRI-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]