Abstract
Radiation pneumonitis is an unpredictable complication of radiotherapy for lung cancer and a condition which can cause significant morbidity. The ability to identify patients at a high risk of developing pneumonitis is critical, since it will enable the individualization of the treatment plan. Because the cytotoxic effect of radiation is propagated through ROS and ROS-driven oxidative stress, the role of anti-oxidant defense systems in radiation pneumonitis was investigated. Using the pneumonitis-sensitive C3H/HeN mice as a model, we demonstrated that the anti-oxidant response of the lung correlated well with that of RBC. We then proceeded to test whether differences of RBC anti-oxidant response would predict the pneumonitis development in patients. SOD, GPX, CAT activities and glutathione in RBC were measured at baseline and then weekly for 6 weeks of treatment in fifteen eligible patients receiving concurrent chemo-radiotherapy for unresectable stage III NSCLC. Striking differences were found in the anti-oxidant activities of RBC with respect to the pneumonitis development. Those who developed pneumonitis showed higher SOD and lower GPX activities at baseline compared to those who did not (3.7 vs. 6.8 unit/mg for median SOD, 16.5 vs. 10.7 nmol/min/mg for median GPX). The functional imbalance of SOD and GPX was displayed consistently throughout the treatment period. The sensitivity and specificity of pneumonitis prediction were further increased when the GPX/SOD ratio was analyzed (pre-treatment P = 0.0046). Our results provide a strong rationale to monitor SOD and GPX activities of RBC to identify patients who are at risk of developing pneumonitis, and to implement a strategy of increasing the GPX/SOD ratio in order to lower the risk.
Keywords: Radiotherapy, Lung cancer, Radiation pneumonitis, RBC, Anti-oxidant system, SOD activity, GPX activity
INTRODUCTION
Lung cancer is the leading cause of cancer death in both men and women in the US [1]. Non small cell lung cancer (NSCLC) accounts for more than 75% of all lung cancers. At the time of diagnosis, the majority of NSCLC patients present with locally advanced or metastatic disease that cannot be cured by surgery. Radiotherapy (RT) in combination with chemotherapy is most often the primary treatment modality for stage III patients. The goal of RT is to deliver the cytotoxic dose to the tumor, while minimizing the radiation exposure to the surrounding normal tissues. RT-induced pneumonitis is one of the most serious dose-limiting toxicities in patients receiving thoracic RT for lung cancer. Chemotherapy administered in this setting is expected to provide systemic control as well as to enhance loco-regional control via radiation sensitization. While the benefit of combination therapy has been supported by several studies [2, 3], this is unfortunately achieved at the expense of increased acute normal tissue toxicity. Studies from the Radiation Therapy Oncology Group (RTOG) have shown that concurrent chemo-radiotherapy is associated with greater treatment-related side effects including esophagitis and pneumonitis [4, 5].
Symptoms of pneumonitis do not appear until at least 1 to 3 months after the completion of treatment. Late fibrosis might also develop months to years post-therapy. A possible contribution of cytokines and chemokines to the development of radiation-induced lung injury has been suggested [6–10]. The risk of radiation pneumonitis could also be strongly affected by various endogenous (genotypic) and exogenous (environmental) factors which vary among individuals [11]. However, no generally accepted means are currently available to predict an individual’s risk of developing pneumonitis. Identifying molecular markers capable of predicting pulmonary injury before or during the early phase of treatment will facilitate the treatment modifications to minimize the extent of radiation injury as well as to offer possible early preventive intervention.
Since the cytotoxic effect of ionizing radiation is mediated through the generation of reactive oxygen species (ROS) and ROS-driven oxidative stress, it is to be expected that the anti-oxidant systems would play a major role in treatment-related toxicities. Superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT), together with glutathione (GSH), form the first-line of defense against ROS in irradiated tissues. SOD converts superoxide anion (O2•−) to H2O2, which is then detoxified to water by GPX and CAT. If not removed, H2O2 itself causes oxidative damage to biomolecules, or it can be converted to a more damaging hydroxyl radical (•OH) species. Reduced glutathione, GSH, is involved in the non-enzymatic removal of ROS and also serves as the hydrogen donor in GPX-mediated reaction. The oxidized glutathione (GSSG) is either reduced back to GSH via glutathione reductase-mediated reaction, or exported out of the cells. Therefore, the GSH equivalent to the sum of GSH + 2GSSG, serves as a measure of the total glutathione capacity of cells at any given time in response to oxidative stress. Pre-clinical studies have shown the role of the anti-oxidant mechanisms in determining the degree of oxidative damage in the irradiated tissues [12–14]. However, no clinical data is available correlating the functionality of these proximal oxidative stress systems and the susceptibility to radiation pneumonitis.
The purpose of the present study is to address the above question in stage IIIA/IIIB lung cancer patients receiving concurrent chemo-radiotherapy. Because lung tissue cannot be obtained from these patients, we used red blood cells (RBC) as a surrogate to assess the anti-oxidant capacity of the patient. This was based on the results we obtained using radiation pneumonitis-sensitive inbred mice (C3H/HeN) [15, 16] as an experimental model. We first demonstrated that oxidative stress response in the lung correlates with that in RBC in irradiated mice. Based on these pre-clinical data, serial blood samples were collected in patients receiving concurrent chemo-radiation therapy. Here we report the results of assessing the functionality of the RBC anti-oxidant systems in predicting the risk of developing radiation pneumonitis in these patients.
MATERIALS AND METHODS
Animals and irradiation
Male C3H/HeN mice (5 to 6 weeks old) were used. Whole body irradiation (10 Gy) was performed by using 4 MV photon beams (MeVatron, Siemens, Germany). Irradiation dose was calculated at the mid depth of mice in the field size of 40 cm with a dose rate of 0.2 Gy/min as previously described [12]. Mice were sacrificed and the lungs were removed at the specified time after irradiation. The tissues were washed and frozen by freeze-clamping with dry ice-cooled tongs immediately. Blood was drawn by heart puncture into tubes containing 50 μl of anticoagulant citrate dextrose solution (Baxter, Deerfield, IL). Red blood cells were separated from plasma by centrifugation (1800 g, 5 min) at 4°C and washed three times with phosphate buffered saline. Tissue and RBC samples were stored at −80°C until analyzed.
Enzyme activity and glutathione analyses
Enzyme activities were measured following previously described procedures [12, 17] with minor modifications. Total superoxide dismutase (SOD) activity was measured by monitoring the reduction of cytochrome c at 550 nm in a reaction mixture containing 50 mM potassium phosphate (pH 7.5), 0.1 mM xanthine, 0.5 munit/ml xanthine oxidase, 0.1 mM EDTA and 10 μM cytochrome c. One unit of SOD represents the enzyme activity that causes a 50% inhibition in the reduction of cytochrome c. Aliquots of samples were taken to determine MnSOD activity by the addition of 5 mM KCN to inhibit CuZnSOD activity [18]. CuZnSOD activity was evaluated by subtracting MnSOD activity from the total SOD activity. Glutathione peroxidase (GPX) activity was measured by monitoring the oxidation of NADPH at 340 nm in a reaction mixture containing 50 mM potassium phosphate (pH 7.4), 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 unit/ml glutathione reductase, and 1 mM GSH. The reaction was started by the addition of 0.25 mM H2O2. Catalase (CAT) activity was measure by monitoring the removal of H2O2 at 240 nm in a reaction mixture containing 50 mM potassium phosphate (pH 7.0) and 10 mM H2O2. The reduced (GSH) and oxidized (GSSG) glutathione were measured by using HPLC as previously described [13, 17]. Total glutathione contents were expressed as the GSH equivalent to the sum of GSH + 2GSSG.
Patient eligibility
Patients with surgically unresectable stage IIIA/IIIB non-small cell lung cancer (NSCLC) were recruited. Blood samples from patients undergoing concurrent chemo-radiotherapy on Institutional Review Board (IRB) approved clinical protocols were used for this study. Informed consents were obtained from all patients before registration. To minimize potential confounding factors, only those patients receiving concurrent definitive radiotherapy and paclitaxel-based chemotherapy were included. Patients were excluded if they had received inductive radiotherapy, chemotherapy or prior thoracic radiotherapy. Patients with unfavorable Eastern Cooperative Oncology Group (ECOG) performance status (2 or greater) or chronic obstructive pulmonary disease (COPD) were also excluded. Staging evaluations included history, physical examination, chest X-ray, and CT with intravenous contrast, including the chest and upper abdomen through the liver and adrenal glands. A total of fifteen eligible patients recruited between September 2003 and April 2005 were analyzed in this study.
Treatment, blood sampling, and clinical assessment
Thoracic radiotherapy was delivered in 2 Gy daily fractions, 5 fractions a week, over a total treatment time of 6 weeks. Blood samples were collected from patients at baseline and then weekly during the 6 week period of treatment. The samples were centrifuged at 4°C for 10 min at 1800 g within 1 h upon collection. The cells were washed three times with phosphate-buffered saline, aliquoted, and stored at −80°C until analyzed. At the time of completion of treatment, serial RBC samples from each patient were analyzed together. Radiation pneumonitis was diagnosed in accordance with the RTOG/EORTC guidelines at one and three months post-therapy [19]. It is outlined as follows: grade 0 is no change from baseline; grade 1 is defined as mild symptoms with slight radiographic appearances; grade 2 is defined as moderate symptoms with low grade fever and patchy radiographic appearances; grade 3 is defined as severe symptoms with dense radiographic changes; grade 4 is defined as severe respiratory insufficiencies requiring assisted ventilation. The physicians grading pneumonitis (G.Y. and N.R.) were blinded to the anti-oxidant data.
Analysis of sensitivity and specificity of the test variables
To evaluate the predictive value of each test variable, the discriminant analysis was performed [20] by using the computer package SAS (version 8.2). The posterior probability was calculated using the generalized square distance and the multivariate normal distribution with the prior probability of 0.5 for each variable. To evaluate the classification criterion, error-rate estimates were obtained based on the “leave-one-out” cross-validation [21]. This “leave-one-out” procedure was repeated for every observation and the number of misclassified observations was counted for the error-rate estimate. Sensitivity was calculated as a proportion of patients with pneumonitis who were correctly classified by the cross-validation. Specificity was calculated as a proportion of patients without pneumonitis who were correctly classified by the cross-validation.
Western blot analysis
Protein extracts from the patients’ RBC were analyzed in duplicate by SDS-polyacrylamide gel electrophoresis. Protein concentrations were measured by the method of Lowry et al. [22]. One gel was stained with Coomassie blue, destained and dried for record keeping. A second gel was transferred to a nitrocellulose membrane in a Trans-Blot apparatus (BioRad, Hercules, CA) for Western blot analysis. The following monoclonal antibodies were used: anti-SOD (Santa Cruz, CA), anti-GPX (Lab Frontier, Korea), anti-CAT (Lab Frontier, Korea), and anti-GAPDH (Lab Frontier, Korea) antibodies. Immunoreactive proteins were detected with secondary antibody and visualized by using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, NJ).
RESULT
Comparison of the anti-oxidant defense systems of lung tissue and of RBC in radiation pneumonitis-sensitive C3H/HeN mice
Inbred C3H/HeN mice manifest pulmonary injury patterns similar to those of humans when irradiated [15, 16]. The animals were exposed to either sham irradiation or a single fraction of 10 Gy whole body irradiation, and sacrificed 2 h, 6 h, and 24 h after irradiation. First, SOD (total, MnSOD, and CuZnSOD), GPX, and CAT activities were measured in the irradiated lung. As shown in the left panels of Figure 1, MnSOD and CuZnSOD (panel A), GPX (panel B), and CAT (panel C) activities were increased as early as 2 h after irradiation, with a concomitant decrease in total glutathione contents (panel D). The right panels of Figure 1 show the patterns of SOD, GPX, CAT, and glutathione changes of the RBC in response to irradiation. Since the mature RBC is devoid of mitochondria, the SOD activity of RBC is primarily that of CuZnSOD. It is evident that changes in the lung and RBC closely resembled each other with the exception of CAT. In general, SOD and GPX activities as well as glutathione levels were higher in lung than in RBC. The levels of CAT activities were similar between the lung and the RBC. However, the CAT activities of RBC did not change in response to radiation during the course of the experiment.
Figure 1. Changes of SOD, GPX, CAT activities and glutahione content of C3H/HeN mice after whole body irradiation.
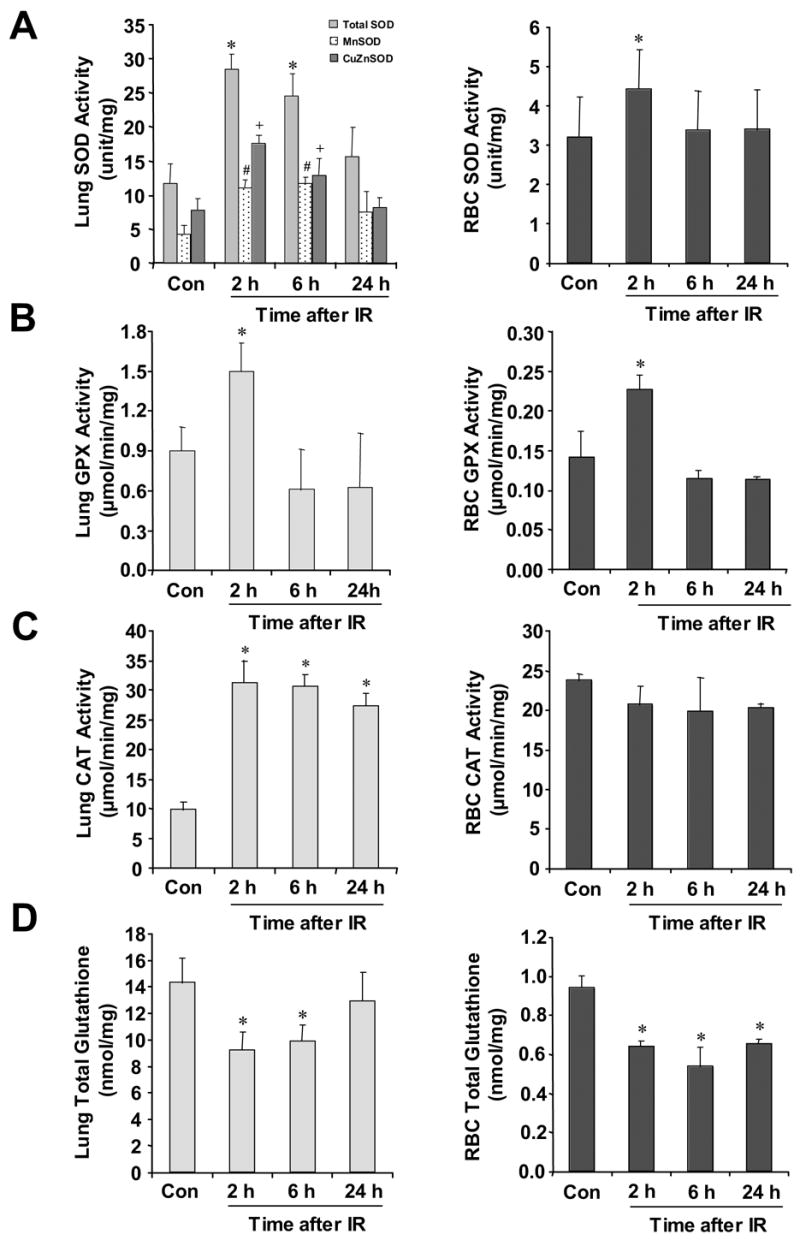
Mice were irradiated at a single fraction of 10 Gy. SOD (total, MnSOD, and CuZnSOD) (panel A), GPX (panel B) and CAT (panel C) activities of lung (left panel) were determined at 2 h, 6 h, and 24 h after irradiation. SOD (panel A), GPX (panel B), and CAT (panel C) activities of RBC (right panel) were also determined at the same time points. Total glutathione contents (panel D) of the lung tissue and RBC are expressed as the GSH equivalent to the sum of GSH + 2GSSG. Results are expressed as means ± standard deviations (n=10). Significance of the differences was determined by using the Student’s t-test. *P< 0.05, #P< 0.05, or +P< 0.05 vs. un-irradiated control, Con.
Analysis of the anti-oxidant activities in RBC of the patients before and during therapy
To test whether differences in the anti-oxidant mechanisms of the RBC might be correlated with radiation pneumonitis susceptibility, non-small cell lung cancer (NSCLC) patients receiving concurrent radiotherapy and chemotherapy had serial blood collections before and during treatment. A total of fifteen eligible patients were treatment naive, and had stage III disease with good performance status (0, 1) and normal organ function. The characteristics of these fifteen patients are summarized in Table 1. Based on the RTOG/EORTC criteria, eight patients out of fifteen experienced the symptoms of pneumonitis within 3 months after completion of treatment.
Table 1.
Patient characteristics
| Characteristic | Number of Patients | % |
|---|---|---|
| Number of patients | 15 | 100 |
| Age, years | ||
| Mean | 61 | |
| Range | 44–74 | |
| Sex | ||
| Male | 5 | 33.3 |
| Female | 10 | 66.7 |
| ECOG performance status | ||
| 0 | 7 | 46.7 |
| 1 | 8 | 53.3 |
| AJCC Clinical stage | ||
| IIIA | 3 | 25.0 |
| IIIB | 12 | 75.0 |
| Radiation | ||
| Conformal 3D | 8 | 53.3 |
| IMRT | 7 | 46.7 |
| Chemotherapy | ||
| Yes | 15 | 100.0 |
| Concurrent | 15 | 100.0 |
| Inductive | 0 | 0.0 |
| Post-operative | 0 | 0.0 |
| No | 0 | 0.0 |
| Pneumonitis | ||
| Yes | 8 | 53.3 |
| Grade 1 | 1 | 6.7 |
| Grade 2 | 3 | 20.0 |
| Grade 3 | 3 | 20.0 |
| Grade 4 | 1 | 6.7 |
| No | 7 | 46.7 |
| Histology | ||
| Adenocarcinoma | 6 | 40.0 |
| Squamous carcinoma | 6 | 40.0 |
| Poorly differentiated | 3 | 20.0 |
IMRT: Intensity-Modulated Radiation Therapy.
When their anti-oxidant systems were analyzed in relation to the presence or absence of pneumonitis, striking differences were revealed. SOD activities were higher in the pneumonitis group (Figure 2A), while the reverse was true with GPX (Figure 2B). As shown in Table 2, pre-treatment median and ranges of SOD activities were 3.7 (3.4–5.0) in the group without pneumonitis, vs. 6.8 (6.4–7.1) in the group with pneumonitis (P = 0.0192). For GPX, pre-treatment activity levels for patients without pneumonitis and those with pneumonitis were 16.5 (14.7–20.6) and 10.7 (10.2–14.8), respectively (P = 0.0379). These differences remained through each week of treatment. The week 2, 4 and 6 values of SOD activities, and the week 6 value of GPX barely missed statistical significance. Because high levels of SOD and low levels of GPX activities were associated with pneumonitis development, we next examined the ratio of GPX/SOD activities between the two groups. The results were even more striking (pre-treatment P = 0.0046). As shown in Figure 3A, a significant difference between the two groups can be easily visualized. Because the difference in SOD and GPX activities between the two groups was detected from the beginning, we tested whether this was also reflected in the steady state protein levels of these enzymes. As shown in the Western blot data of Figure 3B, the amount of SOD, GPX or CAT proteins present in RBC, however, did not differ significantly among patients with or without pneumonitis.
Figure 2. Comparison of SOD and GPX activities of the lung cancer patients without pneumonitis vs. with pneumonitis.


SOD (panel A) and GPX (panel B) activities of RBC were plotted separately for patients without pneumonitis and with pneumonitis. The boxes indicate the 25th and the 75th percentile (the lower and upper edge, respectively), and the lines within the boxes represents the median. Median numeric values and number of patients evaluated at each time point are shown in Table 2. The points at the ends of the whiskers are the greatest or the smallest points that are not outliers. Outliers (circles) are plotted individually with a patient serial number.
Table 2.
Comparison of the SOD and GPX activities between patients with and without pneumonitis
| Variable | Time | Without pneumonitis | With pneumonitis | P-value* |
|---|---|---|---|---|
| Median SOD activity (range) from n evaluable patients | ||||
| SOD | Pre | 3.7 (3.4–5.0) n=7 | 6.8 (6.4–7.1) n=7 | 0.0192 |
| Week 1 | 4.1 (3.7–4.5) n=7 | 7.3 (6.4–7.7) n=8 | 0.0140 | |
| Week 2 | 3.5 (3.3–5.5) n=7 | 7.0 (6.1–7.4) n=8 | 0.0541 | |
| Week 3 | 4.3 (3.9–4.8) n=7 | 6.0 (5.5–6.8) n=8 | 0.0277 | |
| Week 4 | 3.6 (3.5–4.7) n=7 | 5.9 (5.1–6.3) n=8 | 0.0721 | |
| Week 5 | 4.2 (3.8–5.2) n=7 | 6.8 (6.2–7.3) n=7 | 0.0128 | |
| Week 6 | 3.5 (3.4–4.2) n=5 | 6.4 (5.9–7.5) n=6 | 0.0519 | |
|
| ||||
| Median GPX activity (range) from n evaluable patients | ||||
| GPX | Pre | 16.5 (14.7–20.6) n=7 | 10.7 (10.2–14.8) n=7 | 0.0379 |
| Week 1 | 19.4 (15.2–22.5) n=7 | 11.9 (10.3–14.1) n=8 | 0.0059 | |
| Week 2 | 18.4 (16.7–22.5) n=7 | 11.2 ( 9.9–13.7) n=8 | 0.0022 | |
| Week 3 | 19.1 (15.1–22.5) n=7 | 11.4 (10.2–11.5) n=8 | 0.0093 | |
| Week 4 | 18.1 (15.1–24.7) n=7 | 11.6 (10.9–12.1) n=8 | 0.0264 | |
| Week 5 | 18.2 (16.3–22.3) n=7 | 11.8 (11.5–12.3) n=7 | 0.0175 | |
| Week 6 | 23.0 (14.8–23.8) n=5 | 13.2 (12.0–13.4) n=6 | 0.1255 | |
P-values were obtained by Wilcoxon two-sample test.
Figure 3. Distribution of the GPX/SOD ratio of the lung cancer patients.


The ratio of GPX/SOD activity is plotted before (pre) and during the treatment (panel A). Open circle, patients without pneumonitis; closed triangle, patients with pneumonitis. The amounts of SOD, GPX, and CAT proteins were probed by Western blot analysis (panel B). Equal amounts of proteins (50 μg for SOD, 100 μg for GPX and 80 μg for CAT) from pre-treatment RBC samples were analyzed. A total of 30 μg was used for GAPDH Western blot as a loading control.
Glutathione is a major small anti-oxidant molecule. The level of glutathione has been shown to influence the acute radiation effect in animals and humans [12, 23]. We did not find a statistically significant difference in the dynamics of GSH or GSSG changes. Also, we did not find any significant difference in CAT activity between the two groups (data not shown). The treatment per se did not cause any appreciable changes of SOD or GPX activities. Since the patients received 2 Gy fractionated radiotherapy for 5 consecutive days in a week, and the blood samples were drawn once a week at the end of the week’s treatment, it is possible that the short term temporal changes of their activities, if any, could be passed undetected, unlike in the animal experiments in which the activities were monitored within 24 h after a single 10 Gy whole body irradiation.
Analysis of sensitivity and specificity to predict the development of pneumonitis
To evaluate the classification (without pneumonitis vs. with pneumonitis) performance of SOD and GPX activities or the ratio of GPX/SOD, the discriminant analysis was performed [20]. To evaluate the classification criterion, error-rate estimates were obtained based on the “leave-one-out” cross-validation [21]. The classification capacities of each variable are shown in Table 3. Statistical significance of the variables was calculated using the likelihood ratio criterion. The smaller P-values of GPX/SOD ratio than those of SOD or GPX activities indicated a greater discriminating power of the GPX/SOD ratio than SOD or GPX activity alone. Sensitivity and specificity of the classification performance are also shown in Table 3.
Table 3.
Test performance characteristics of the SOD, GPX activities, and the relative ratio of the GPX/SOD activity
| Variable | Time | N | Sensitivity (%) | Specificity (%) | Overall error (%) | P-value* |
|---|---|---|---|---|---|---|
| SOD | Pre | 14 | 85.7 | 71.4 | 21.4 | 0.0038 |
| Week 1 | 15 | 87.5 | 85.7 | 13.4 | 0.0026 | |
| Week 2 | 15 | 75.0 | 71.4 | 26.8 | 0.0155 | |
| Week 3 | 15 | 87.5 | 85.7 | 13.4 | 0.0226 | |
| Week 4 | 15 | 87.5 | 71.4 | 20.1 | 0.1705 | |
| Week 5 | 14 | 100.0 | 71.4 | 14.3 | 0.0051 | |
| Week 6 | 11 | 83.3 | 80.0 | 18.3 | 0.0148 | |
|
| ||||||
| GPX | Pre | 14 | 71.4 | 71.4 | 28.6 | 0.0360 |
| Week 1 | 15 | 87.5 | 71.4 | 20.5 | 0.0028 | |
| Week 2 | 15 | 87.5 | 71.4 | 20.5 | 0.0028 | |
| Week 3 | 15 | 87.5 | 85.7 | 13.4 | 0.0025 | |
| Week 4 | 15 | 87.5 | 57.1 | 27.7 | 0.0284 | |
| Week 5 | 14 | 85.7 | 71.4 | 21.4 | 0.0136 | |
| Week 6 | 11 | 83.3 | 60.0 | 28.3 | 0.0507 | |
|
| ||||||
| GPX/SOD | Pre | 14 | 85.7 | 71.4 | 21.4 | 0.0046 |
| Week 1 | 15 | 100.0 | 100.0 | 0.0 | 0.0009 | |
| Week 2 | 15 | 100.0 | 71.4 | 14.3 | 0.0058 | |
| Week 3 | 15 | 87.5 | 85.7 | 13.4 | 0.0018 | |
| Week 4 | 15 | 87.5 | 71.4 | 20.5 | 0.0233 | |
| Week 5 | 14 | 85.7 | 71.4 | 21.4 | 0.0058 | |
| Week 6 | 11 | 83.3 | 80.0 | 18.3 | 0.0098 | |
P-values were obtained by F-statistics based on the Wilk’s Lambda.
Since the differences in SOD and GPX activities predict the risk of pneumonitis development before the start of treatment, we tested whether the repeated measurements of activities of these enzymes during treatment improve the predictive value. To this end, the frequency of higher SOD levels compared with the median pre-treatment value up to a specified week was analyzed using the logistic regression. Similarly, the frequency of the lower GPX levels than the median pre-treatment value was analyzed in a univariate manner. A model fit statistic called Akaike information criterion (AIC) was examined where the smaller value indicates the better model fitting [24]. The AIC of SOD was found to decrease at week 1 (from pre-treatment value of 18.7 to 15.8), but increased slightly at week 2 (16.4) and week 3 (17.4). In the case of GPX, the AIC was decreased gradually until week 3, but the degree of AIC decrease was smaller after the week 2 (pre-treatment, 21.4; week 1, 19.1; week 2, 16.4; week 3, 15.8). When the GPX/SOD ratio was tested, a quasi-complete separation of the data points occurred at week 1, i.e., the incidence of pneumonitis was completely separated by the GPX/SOD ratio with an exception of a single GPX/SOD value. Therefore, surveillance up to the week 1 or 2 following the start of treatment may be sufficient to predict pneumonitis.
DISCUSSION
Radiation pneumonitis is a serious and potentially lethal treatment-related complication for lung cancer. Recent progress in combining radiosensitizers with radiotherapy for the management of lung cancer has shown very encouraging results in several Phase II clinical trials. The promising results are, however, offset by a high incidence of pneumonitis [25, 26]. The occurrence of pneumonitis is unpredictable. Therefore, reliable biochemical or cellular markers in identifying individuals at a high risk of developing radiation pneumonitis are most desirable for early treatment modifications in order to avoid serious complications. These biomarkers may also allow the selection of patients who may be able to tolerate higher doses of radiation.
Although both laboratory and clinical studies have suggested the involvement of genetic and environmental factors in contributing to the risk of pneumonitis, there are few data to support or refute this hypothesis. This is the first report in humans, showing that high SOD and low GPX activities in RBC are good predictors of the risk of pneumonitis. When SOD activity is high, the conversion of superoxide anion (O2•−) to hydrogen peroxide (H2O2) is facilitated. High SOD activity in conjunction with low GPX activity will lead to increased levels of H2O2 and H2O2-derived reactive species such as hydroxyl radical (•OH). We propose that the imbalance of SOD and GPX activities may play a role in initiating and propagating oxidative damage, and thus predisposing to radiation pneumonitis. Considering the important role of immune cells in radiation pneumonitis, it is also plausible that the peroxide signaling may increase the recruitment of these cells to the site of irradiation. In addition to serving as a predictive marker for treatment-related toxicity, the implementation of a strategy aimed at increasing the ratio of GPX/SOD activities may reduce the risk of radiation pneumonitis.
In our series of lung cancer patients, the anti-oxidant system of those who developed pneumonitis is clearly compromised when compared to those who did not. The significance of the imbalanced GPX/SOD function of RBC in predicting radiation pneumonitis risk, however, will need to be further verified using a larger sample size. Various characteristics of the radiotherapy dose-volume histograms (DVHs) and patients’ baseline pulmonary function will also need to be characterized in future studies, although the patients with COPD were excluded in the current study and the DVHs were equally distributed among fifteen patients. Whether increasing the GPX/SOD ratio will cause the tumors to be spared of the effect of treatment is another important issue that warrants future investigation. Our results suggest that the use of anti-oxidant supplements during therapy should be carefully evaluated in the context of the anti-oxidant defense mechanisms as a whole. Translation of pre-clinical studies to clinical testing is often challenging because of the different animal models, treatment protocols, and end-point analyzed. Despite these limitations, the use of appropriate animal models and specimens would be helpful in understanding the molecular mechanisms of radiation pneumonitis and in developing novel intervention strategies.
Cytokine production in the lung following irradiation has been documented in many studies over the last 10 years. Inflammatory cytokines have been implicated in the development and perpetuation of post-irradiation injury in various tissues, including the lung [27]. Serum levels of cytokines, in particular, interleukin-6 (IL-6) and transforming growth factor-β (TGF-β), are inconclusive in predicting radiation pneumonitis [28–32]. To what extent such changes are a direct result of radiation as opposed to a consequence of up-regulation of other cytokines and chemokines remains to be established [33, 34]. Although not presented in the current study, we analyzed the plasma levels of IL-6 and TGF-β both before and during treatment. We were not able to find a meaningful correlation between the changes of these cytokines and the risk of pneumonitis. We plan to expand our study to test whether the predictive value of the anti-oxidant activities in RBC can be improved in combination with plasma cytokine measurement.
Although pneumonitis has been recognized as a distinct clinical complication following thoracic irradiation, there is no diagnostic test to assess the risk of pneumonitis. We provide the first evidence in clinical setting that high SOD and low GPX activities in RBC predict susceptibility of lung cancer patients to radiation pneumonitis. Because the serious incidences of pneumonitis have been observed after all treatment modalities, including radiotherapy, chemotherapy, and even some of the more recently developed molecular targeted therapies, the use of patients’ RBC to gauge differences in the functionality of anti-oxidant defense mechanisms may have broader clinical implications.
Acknowledgments
Grant support: NIH grants CA109480 and Cancer Center Support Grant CA16056. We thank Dr. Christine Ambrosone for the critical review and suggestions of the article.
The abbreviations used are
- RT
radiotherapy
- SOD
superoxide dismutase
- GPX
glutathione peroxidase
- CAT
catalase
- RBC
red blood cell
- ROS
reactive oxygen species
- NSCLC
non-small lung cancer
- ECOG
Eastern Cooperative Oncology Group
- AJCC
American Joint Committee on Cancer
- RTOG
Radiation Therapy Oncology Group
- EORTC
European Organization for Research and Treatment of Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Dillman RO, Herndon J, Seagren SL, Eaton WL, JrGreen MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88(17):1210–1215. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 4.Byhardt RW, Scott C, Sause WT, Emami B, Komaki R, Fisher B, Lee JS, Lawton C. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1998;42(3):469–478. doi: 10.1016/s0360-3016(98)00251-x. [DOI] [PubMed] [Google Scholar]
- 5.Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, Ryu JK, Bosch W, Emami B. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(2):318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145(6):762–767. [PubMed] [Google Scholar]
- 7.Johnston CJ, Wright TW, Rubin P, Finkelstein JN. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and -sensitive mice after thoracic irradiation. Exp Lung Res. 1998;24(3):321–337. doi: 10.3109/01902149809041538. [DOI] [PubMed] [Google Scholar]
- 8.Franko AJ, Sharplin J, Ghahary A, Barcellos-Hoff MH. Immunohistochemical localization of transforming growth factor beta and tumor necrosis factor alpha in the lungs of fibrosis-prone and “non-fibrosing” mice during the latent period and early phase after irradiation. Radiat Res. 1997;147(2):245–256. [PubMed] [Google Scholar]
- 9.Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proc Natl Acad Sci U S A. 1997;94(12):6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallahan DE, Geng L, Shyr Y. Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst. 2002;94(10):733–741. doi: 10.1093/jnci/94.10.733. [DOI] [PubMed] [Google Scholar]
- 11.Roach M, Gandara DR, Yuo HS, Swift PS, Kroll S, Shrieve DC, Wara WM, Margolis L, Phillips TL. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13(10):2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 12.Park EM, Park JS, Hwang TS, Kim WC, Park YM. Role of oxidative stress in the radiation-induced lung pathogenesis in mice. J Biol Mol Biochem. 2001;34(6):544–550. [Google Scholar]
- 13.Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell Physiol. 2000;183(1):100–107. doi: 10.1002/(SICI)1097-4652(200004)183:1<100::AID-JCP12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic Biol Med. 2003;34(6):689–695. doi: 10.1016/s0891-5849(02)01372-2. [DOI] [PubMed] [Google Scholar]
- 15.Franko AJ, Sharplin J, Ward WF, Hinz JM. The genetic basis of strain-dependent differences in the early phase of radiation injury in mouse lung. Radiat Res. 1991;126(3):349–356. [PubMed] [Google Scholar]
- 16.Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. Compartmental responses after thoracic irradiation of mice: strain differences. Int J Radiat Oncol Biol Phys. 2005;62(3):862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Park EM, Park YM, Gwak YS. Oxidative damage in tissues of rats exposed to cigarette smoke. Free Radic Biol Med. 1998;25(1):79–86. doi: 10.1016/s0891-5849(98)00041-0. [DOI] [PubMed] [Google Scholar]
- 18.Spitz DR, Oberley LW. An assay for superoxide dismutase in mammalian tissue homogenates. Anal Biochem. 1989;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 19.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 20.Rao CR. Linear Statistical Inference and its Applications. New York: John Wiley; 1973. [Google Scholar]
- 21.Lachenbruch PA, Mickey MA. Estimation of error rates in discriminant analysis. Technometrics. 1968;10:1–10. [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 23.Bhattathiri VN, Sreelekha TT, Sebastian P, Remani P, Chandini R, Vijayakumar T, Nair MK. Influence of plasma GSH level on acute radiation mucositis of the oral cavity. Int J Radiat Oncol Biol Phys. 1994;29(2):383–386. doi: 10.1016/0360-3016(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 24.Agresti A. Categorical Data Analysis. New York: John Wiley; 1990. [Google Scholar]
- 25.Antonia SJ, Wagner H, Williams C, Alberts M, Hubbell D, Robinson L, Hilstro J, Ruckdeschel JC. Concurrent paclitaxel/cisplatin with thoracic radiation in patients with stage IIIA/B non-small cell carcinoma of the lung. Semin Oncol. 1995;22(4 Suppl 9):34–37. [PubMed] [Google Scholar]
- 26.Reckzeh B, Merte H, Pfluger KH, Pfab R, Wolf M, Havemann K. Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancer. J Clin Oncol. 1996;14(4):1071–1076. doi: 10.1200/JCO.1996.14.4.1071. [DOI] [PubMed] [Google Scholar]
- 27.Fu XL, Huang H, Bentel G, Clough R, Jirtle RL, Kong FM, Marks LB, Anscher MS. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys. 2001;50(4):899–908. doi: 10.1016/s0360-3016(01)01524-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Rubin P, Williams J, Hernady E, Smudzin T, Okunieff P. Circulating IL-6 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2001;49(3):641–648. doi: 10.1016/s0360-3016(00)01445-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Williams J, Ding I, Hernady E, Liu W, Smudzin T, Finkelstein JN, Rubin P, Okunieff P. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002;12(1 Suppl 1):26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- 30.Vujaskovic Z, Groen HJ. TGF-beta, radiation-induced pulmonary injury and lung cancer. Int J Radiat Biol. 2000;76(4):511–516. doi: 10.1080/095530000138510. [DOI] [PubMed] [Google Scholar]
- 31.Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, Jirtle RL. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1998;41(5):1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 32.Novakova-Jiresova A, Van Gameren MM, Coppes RP, Kampinga HH, Groen HJ. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol. 2004;71(2):183–189. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Barthelemy-Brichant N, Bosquee L, Cataldo D, Corhay JL, Gustin M, Seidel L, Thiry A, Ghaye B, Nizet M, Albert A, Deneufbourg JM, Bartsch P, Nusgens B. Increased IL-6 and TGF-beta1 concentrations in bronchoalveolar lavage fluid associated with thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58(3):758–767. doi: 10.1016/S0360-3016(03)01614-6. [DOI] [PubMed] [Google Scholar]
- 34.Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation-induced pulmonary fibrosis: examination of chemokine and chemokine receptor families. Radiat Res. 2002;157(3):256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]


