Abstract
Accommodation may indirectly influence visually guided eye growth by affecting the retinal defocus signal used to guide growth. Specifically, increased lags of accommodation associated with low stimulus-response (S-R) function slopes will impose increased hyperopic blur on the retina and may induce axial elongation and myopia. The purpose of this study was (1) to measure accommodation in awake, free viewing marmosets and (2) compare accommodation behavior in marmosets before and after inducing different amounts of myopia with binocular spectacle lenses. In untreated marmosets, the average accommodation S-R slope approached one, but showed considerable inter-individual variability (mean ±SD: 0.964 ±0.249 for monocular viewing; 0.895 ±0.235 for binocular viewing; monocular and binocular measures not significantly different). The monocular S-R slopes were significantly reduced following a period of lens rearing that produced axial myopia (change in slope = −0.30 ±0.30, p<0.01) and the reduction in slope was proportional to the amount of myopia induced (p<0.01). The S-R slopes measured either under monocular or binocular conditions before induction of myopia were not well correlated with the degree of myopia induced (monocular: r=−0.240, p=0.453; binocular: r=−0.060, p=0.824). These results support the hypothesis that the reduction in S-R slope in myopes is a consequence of the myopia induced. The alternative hypothesis – that low S-R slope increases susceptibility to the development of myopia – is not supported by the weak correlation between the pre-manipulation S-R slopes and the magnitude of the myopic shift.
Keywords: Accommodation, Callithrix jacchus, Emmetropization, Marmoset, Myopia
1. Introduction
The nature of the relationship between accommodation and the development of myopia is an old and controversial subject. Historically, two types of evidence have indirectly suggested that accommodation plays a role in the development of myopia: (1) Positive correlations between the occurrence of myopia and the amount of nearwork, increases in reading activity, and level of education are well known and suggest a possible role for accommodation (see for example Angle & Wissmann, 1980, Curtin, 1985, Goldschmidt, 1968, Richler & Bear, 1980, Sato, 1993, Sperduto, Seigel, Roberts & Rowland, 1983, Zylbermann, Landau & Berson, 1993), although recent attempts at systematically correlating the degree of myopia with the amount of reading reported do not support this hypothesis (Mutti, Mitchell, Moeschberger, Jones & Zadnik, 2002, Saw, Chua, Hong, Wu, Chan, Chia, Stone & Tan, 2002). (2) The effectiveness of the non-specific muscarinic antagonist atropine at reducing the progression of myopia (e.g. Bedrossian, 1971, Brodstein, Brodstein, Olson, Hunt & Williams, 1984, Chou, Shih, Ho & Lin, 1997, Chua, Balakrishnan, Chan, Tong, Ling, Quah & Tan, 2006, Shih, Chen, Chou, Ho, Lin & Hung, 1999) has suggested a causal relationship between the ciliary muscle activity responsible for the accommodation response and myopia. However, how atropine and other muscarinic antagonists actually reduce myopia progression does not necessarily involve only the ciliary muscles, and remains open to speculation (McBrien, Moghaddam & Reeder, 1993, Schwahn & Schaeffel, 1994, Stone, Lin & Laties, 1991).
Studies using animal models have shown that eye growth and the development of refractive state can be visually controlled (for reviews see Norton, 1999, Smith III, 1998, Wallman & Winawer, 2004, Wildsoet, 1997), and have been used to test directly the role of accommodation in emmetropization and the development of myopia. For example, it has been shown that animals in which the accommodation response is blocked either surgically or pharmacologically are, for the most part, still capable of regulating their eye growth and refractive state to compensate for lens-imposed defocus (Schaeffel, Troilo, Wallman & Howland, 1990, Schwahn & Schaeffel, 1994, Wildsoet, 2003, Wildsoet, Howland, Falconer & Dick, 1993). In addition, the fact that growth in local regions of the eye can be independently modulated by spectacle lenses that cover only part of the visual field (Diether & Schaeffel, 1997) argues strongly that factors other than accommodation must be involved.
Nevertheless, it seems quite plausible that accommodation is likely to be indirectly involved in the etiology of myopia because of the effects it has on retinal defocus (Charman, 1999, Flitcroft, 1998), which is the likely link between nearwork and myopia. Indeed, the finding that hyperopic defocus imposed on animal eyes by spectacle lenses causes myopia as a compensatory response has led to increasing acceptance of the possibility that hyperopic retinal defocus experienced during nearwork tasks like reading may stimulate increased eye growth and the development of myopia, particularly if the accommodative response is insufficient. In support of this hypothesis, accommodative insufficiency has been observed both during late-onset myopia (Jiang & Morse, 1999) and during the development of myopia in children (Abbott, Schmid & Strang, 1998, Gwiazda, Bauer, Thorn & Held, 1995, Gwiazda, Thorn, Bauer & Held, 1993, Gwiazda, Hyman, Norton, Hussein, Marsh-Tootle, Manny, Wang & Everett, 2004, Nakatsuka, Hasebe, Nonaka & Ohtsuki, 2005, but see Rosenfield, Desai & Portello, 2002).
Differences in accommodative function may also explain why some extensive readers become myopic and others do not. Accommodation has been studied in a number of different ways (e.g. accommodative amplitude, lag, facility, S-R slopes, and open-loop accommodation), compared in emmetropes, hyperopes, and myopes, and in general found accommodative function to be reduced in myopes (for reviews see Allen & O'Leary, 2006, Gwiazda & Marran, 2000, Rosenfield, 1998), yet it remains unclear how it might be involved in the development of refractive state.
It is unclear whether the reductions in accommodation accuracy observed in developing myopia are a cause or an effect of the refractive error. Several studies have reported increases in accommodative lag in the period preceding the onset of myopia (Drobe & de Saint-Andre, 1995, Goss, 1991, Gwiazda, Thorn & Held, 2005), but a recent study by Mutti et al. (2006) reported that elevated accommodative lags typically occurred following the onset of myopia, suggesting that reduced accommodation is a consequence of the refractive change. In this study, we address the question of cause and effect between accommodation behavior and the development of myopia by examining accommodative performance in awake, free-viewing, marmoset monkeys before and after induction of experimental myopia.
2. Methods
2.1. Animals and Experimental Manipulations
Twenty common marmosets (Callithrix jacchus) were used in this study of accommodation; eight additional marmosets were used to calibrate the instrumentation used to measure accommodation. All marmosets were bred and housed in family groups in our animal facility. Artificial lighting was provided using daylight-balanced fluorescent lamps (Durotest Vita-Light, Philadelphia, PA) on a 12 hour light/12 hour dark diurnal cycle. Temperature was maintained at 75±2°F with 45±5% humidity. Food and water were provided ad libitum within the animal's home cage and consisted of a formulated dry pellet (Mazuri New World Diet 5MA5; PMI Feeds, Richmond, IN) with regularly varied supplements of fresh fruit and protein. All marmosets in our facility were given regular access through a flexible 4-meter long tubular run to a remote activity cage containing large branches for climbing and a variety of toys for enrichment purposes. The home cages contained a nest box, perches, and branches for climbing. All animal care and use in this study conforms to USDA standards and the ARVO statement for the use of animals in ophthalmic and vision research.
Accommodation stimulus-response (S-R) functions were examined in six untreated marmosets and in 14 marmosets treated binocularly with equal power spectacle lenses to affect eye growth and refraction (Graham & Judge, 1999, Hung, Crawford & Smith, 1995, Schaeffel, Glasser & Howland, 1988). Of the lens-treated marmosets six had accommodation S-R slopes measured only after the experimental manipulation and eight had slopes measured both before and after the experimental manipulation. Spectacle lenses were created by mounting PMMA contact lenses (12 mm diameter with 8 mm base curve) in nylon washer frames that were attached by stainless steel wires to a pedestal mounted on the cranium (technique based on earlier designs by Graham & Judge, 1999, Siegwart & Norton, 1994, Troilo & Nickla, 2005). The nylon washers were contoured to fit over the bridge of the nose and the wires were adjusted so that the lenses fit close to, but not on, the face and prevented the marmoset from looking around the lens frame. Although animals wore identical lenses over each eye, 12 of the 14 lens-treated marmosets wore negative lenses (either −3, −5, −7, or −10 D), one animal wore +10 D lenses, and one wore plano lenses. Table 1 lists the conditions, ages, and refractive states at the time when accommodation S-R functions were measured. Experimentally induced changes in refractive state were measured by retinoscopy and Hartinger refractometry under cycloplegia, and reported as the average of the spherical equivalents from both measures. Axial length changes were measured with A-scan ultrasonography and reported as changes in vitreous chamber depth. For details see Troilo and Nickla (2005).
Table 1.
Marmosets, Conditions, and Ages Measured
All ages are given in days. Onset refers to the age at the beginning of the lens rearing manipulation. Duration indicates the number of days the manipulation took place. Age at measure refers to the age at which a S-R function was completed. IR Refs indicate the videorefractor measure used to calculate stimulus demand and response for those functions (see text for complete description).
| Subject | Eye | Condition | Age at onset (d) |
Duration (d) |
Age at first measure |
IR Ref at first measure |
Age at second measure |
IR Ref at second measure |
|---|---|---|---|---|---|---|---|---|
| Untreated | ||||||||
| K3 | Right | Untreated | - | - | 104 | 3.53 | - | - |
| Left | Untreated | 3.63 | ||||||
| M3 | Right | Untreated | - | - | 165 | 3.85 | - | - |
| Left | Untreated | 4.04 | ||||||
| P3 | Right | Untreated | - | - | 176 | 4.08 | - | - |
| Left | Untreated | 4.10 | ||||||
| S3 | Right | Untreated | - | - | 170 | 2.60 | - | - |
| Left | Untreated | 1.74 | ||||||
| U3 | Right | Untreated | - | - | 73 | 2.19 | - | - |
| Left | Untreated | 1.71 | ||||||
| W3 | Right | Untreated | - | - | 81 | 4.00 | - | - |
| Left | Untreated | - | ||||||
| Treated - one measure of accommodation after manipulation | ||||||||
| H2 | Right | −10 D | 40 | 43 | 127 | −0.86 | - | - |
| Left | −10 D | −1.26 | ||||||
| H3 | Right | −3 D | 34 | 53 | 122 | −1.71 | - | - |
| Left | −3 D | −2.00 | ||||||
| I2 | Right | +10 D | 40 | 44 | 90 | 0.30 | - | - |
| Left | +10 D | 0.92 | ||||||
| J3 | Right | −5 D | 32 | 32 | 85 | −4.39 | - | - |
| Left | −5 D | −0.76 | ||||||
| W2 | Right | −10 D | 29 | 59 | 107 | −4.23 | - | - |
| Left | −10 D | −2.91 | ||||||
| Z2 | Right | Plano | 42 | 51 | 106 | −4.77 | - | - |
| Left | Plano | −3.37 | ||||||
| Treated - pre- and post-manipulations measures of accommodation | ||||||||
| E5 | Right | −7 D | 110 | 50 | 85 | 1.19 | 184 | −2.02 |
| Left | −7 D | 1.35 | −1.64 | |||||
| F5 | Right | −7 D | 110 | 50 | 101 | 3.05 | 190 | −1.97 |
| Left | −7 D | 3.12 | −2.07 | |||||
| L3 | Right | −5 D | 112 | 11 | 104 | 3.29 | 174 | 3.56 |
| Left | −5 D | 3.45 | 3.38 | |||||
| P4 | Right | −7 D | 111 | 22 | 95 | −0.28 | 138 | −3.4 |
| Left | −7 D | −0.35 | −2.67 | |||||
| Q3 | Right | −5 D | 125 | 42 | 121 | 3.9 | 175 | 2.82 |
| Left | −5 D | 3.83 | 3.13 | |||||
| R3 | Right | −5 D | 125 | 42 | 121 | 3.77 | 175 | 1.46 |
| Left | −5 D | 3.74 | 2.66 | |||||
| U4 | Right | −7 D | 131 | 22 | 114 | −1.57 | 222 | −3.85 |
| Left | −7 D | −0.83 | −3.91 | |||||
| X3 | Right | −10 D | 123 | 42 | 89 | 3.74 | 179 | −0.87 |
| Left | −10 D | 3.48 | 0.51 | |||||
2.2. Measurement of Accommodation
Accommodation S-R functions were measured in awake, free-viewing marmosets. Accommodation and eye position were measured using an infrared videorefractor (PowerRefractor, MultiChannel Systems, Germany), which records refractive state along the vertical meridian, pupil diameter and eye position at 25 Hz (Schaeffel, Wilhelm & Zrenner, 1993).
The videorefractor was calibrated on a separate group of 8 marmosets using methods described for humans (Schaeffel et al., 1993) and for small animals (Schaeffel, Hagel, Eikermann & Collett, 1994). A series of trial lenses ranging from −12 to +12 D were placed 2-3 mm in front of the cyclopleged and anesthetized animals and the slope of the fundus reflex induced with each lens was measured using the videorefractor. The effective refractive state was calculated from the lens power and the cycloplegic refraction of each eye and plotted against the brightness profile of the fundus reflex measured as the change in pixel intensity across the pupil vertically. A linear regression was fit to the data plotted in this way (refractive state = −0.874+2.394·slope of pixel intensity, r=0.874) and served as a calibration function to determine refractive state from the measured slope of fundus illuminance.
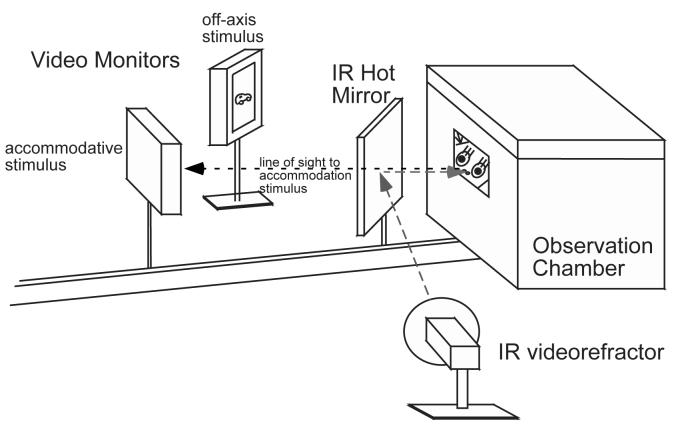
Accommodation measures were made on marmosets while they viewed stimuli presented on a video monitor (6.35 × 4.45 cm) located at different distances varying from 0.5 to 0.1 meter directly in front of a window in a black Plexiglas observation chamber (Figure 1). A second monitor was located approximately 30 degrees off-axis from the line of sight to the accommodative stimulus monitor. The stimulus was displayed on the two monitors in an alternating fashion to maintain the animal's attention. Because of their curious nature, the marmosets tended to approach the window to observe the stimuli. The observation window was constructed in such a way that refractions could be made either under monocular or binocular conditions. When the marmoset was looking through the window, eye position was monitored using the infrared videorefractor to track the first Purkinje image relative to the pupil center. Accommodation data were collected when the marmosets were observed to look from the off-axis monitor to the accommodative stimulus as the stimuli were switched. Data were collected continuously as long as the animal held its position of gaze and averaged to comprise one data point on the S-R plot. The data for a single S-R function were collected over 1-5 measurement sessions. Complete accommodation data sets (S-R functions for both eyes under monocular and binocular conditions) for an animal were collected over an average period of 12 days (range: 1-20 days).
Figure 1.
A schematic of the set-up for measuring accommodation in marmosets. Marmosets viewed video stimuli at varying distances from a window in an observation chamber. The video stimuli were alternately presented on two video monitors to determine when the marmoset was attending to the accommodative stimulus (see text for details). An infrared videorefractor (PowerRefractor) was used to measure accommodation to targets at different distances. The videorefractor was aligned with the accommodative stimulus and the window in the observation chamber using an infrared hot mirror.
Refractive errors were uncorrected during accommodation testing so accommodative demands and responses were adjusted for the refractive state of the eye as determined by earlier cycloplegic refraction. Accommodative demand was calculated from the dioptric value of the visual stimulus (reciprocal of the stimulus distance) plus the subject's most recent cycloplegic refraction measured with the IR videorefractor (always within 2 wks of the accommodation measurements). Accommodative response was calculated as the refraction measured by the IR videorefractor minus the subject's most recent cycloplegic refraction.
2.3. Analysis of Accommodation S-R Functions
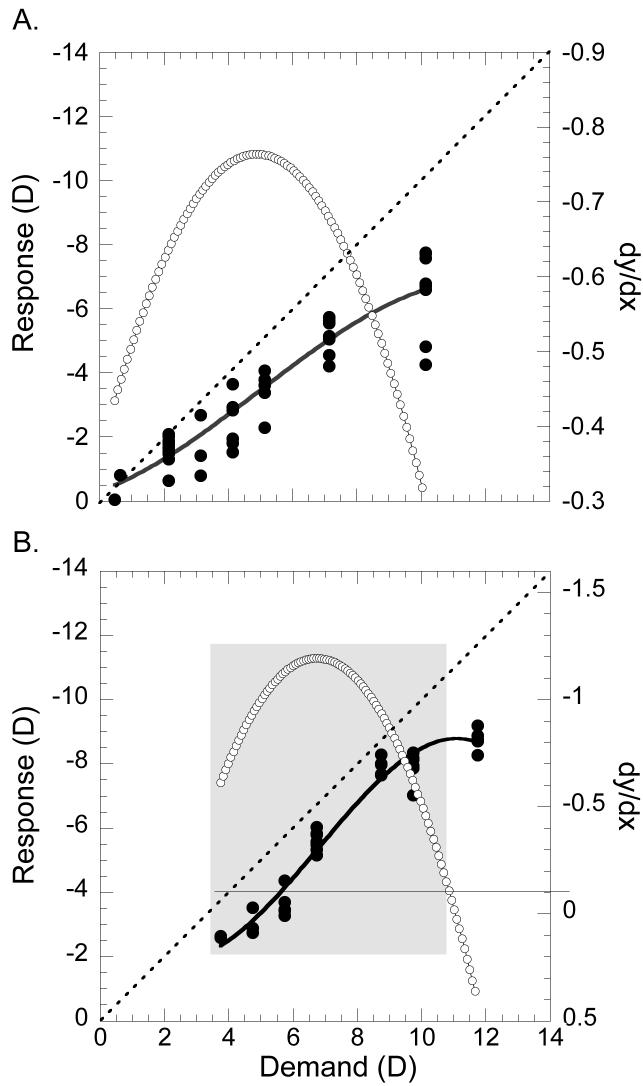
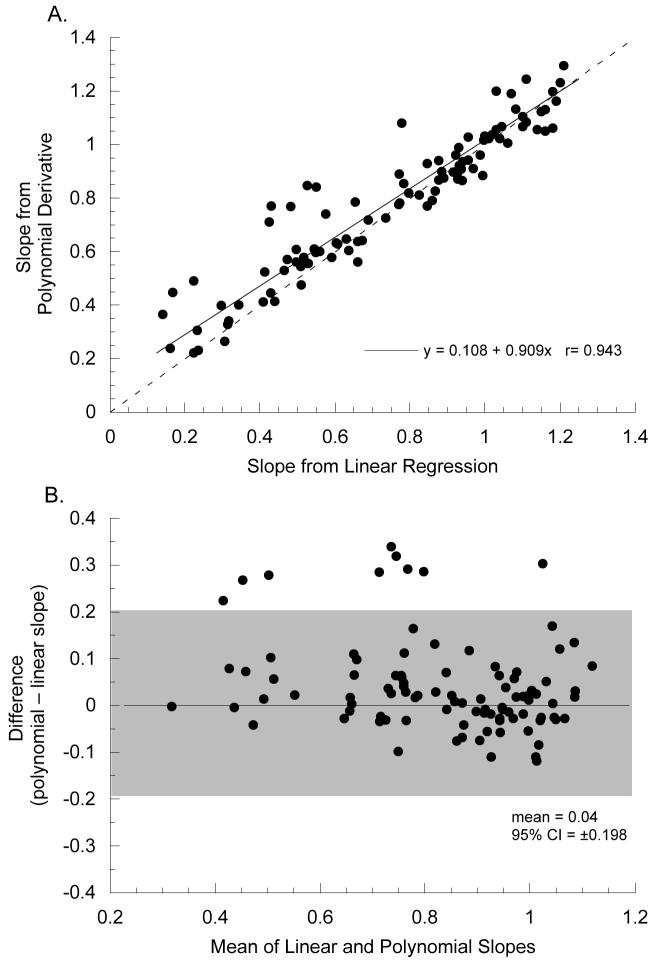
Closed-loop accommodative behavior was estimated from the mean slope of the S-R function (Flitcroft, 1991, Toates, 1970, Toates, 1972). Because of the sigmoid nature of the S-R function and the way accommodative demand was calculated for our subjects (see above), some of our S-R data sets included data that were collected near demands of zero (optical infinity) or at the limits of the accommodative response where the response saturates. In these data sets the responses at those demands do not change with changing demand making simple linear regression inappropriate for measuring the S-R slope. Rather than subjectively restricting the data sets to estimates of the linear portion of such functions we devised an objective means of measuring accommodation S-R slopes over the response range to changing accommodative demands. Accommodation S-R data were first fit with third-order polynomials and then the first derivative (dy/dx) of the polynomial was calculated. The incremental slopes derived across the polynomial function were averaged to give the mean slope of the S-R function. Only those slopes with values less than −0.1 were included in the averaging, thereby ignoring the flat parts of the function within 10% of zero-change in slope (see Figure 2). Because this analysis takes into account any flat region in the S-R function, but is approximately equivalent to a linear regression for more linear S-R functions, we applied it to all of our subjects. Model II reduced major axis (orthogonal) regression was used to compare the accommodation S-R slopes calculated from polynomial derivatives with the slopes from linear regression fits (Figure 3). Model II regression was used because both variables are measurement variables, and there is no assumption of a causal relationship (Sokol & Rohlf, 1981). The two variables are significantly correlated (r=0.943, z-test, p<0.01) but the method of polynomial slope derivation gives slightly, but significantly, steeper slopes than linear regressions (Figure 3: mean difference ±SE, 0.04±0.01, paired t-test, p<0.01) because it omits the flat parts of saturating functions.
Figure 2.
Examples of S-R functions illustrating the procedure to objectively determine the average S-R slope (dy/dx) across the range of changing responses using the first derivative of a polynomial. The diagonal dashed line has a slope = 1 and is shown for reference. Black circles show measures of the subject's refraction response (left y-axis) for a given accommodative demand (x-axis). A 3rd order polynomial is fit to the data and is indicated by a solid line. White circles give the values of the first derivative (right y-axis) taken from the polynomial function. The polynomial derivative was used to remove flat regions from the function before calculating the average slope. (A) An example of a S-R function in which the function is nearly linear. A linear regression fit to the data (r2=0.936) gives a slope of 0.66. The average change in response for a given change in accommodative demand derived from the polynomial fit to the data (r=0.938) has a slope of 0.63 in this example. (B) An example of a saturating S-R function from a different animal. A linear regression fit to these data (r=0.951) gives a slope of 0.859. The polynomial fit (r=0.977) shows response saturation indicated by flat region on the right end of the function. By accepting only the data corresponding to derivatives <−0.1 (indicated by the horizontal line extending from the right y-axis) the flat portion of the function is ignored and only those derivatives corresponding to the data highlighted within the grey box are used to determine the accommodation S-R slope. The S-R slope of the function calculated in this way yields a value of 0.905.
Figure 3.
Comparison of methods to estimate accommodation S-R slopes. (A) Slopes of linear regressions fit to the S-R data are plotted on the x-axis. Estimates from averaging the first derivative of 3rd order polynomial fits are plotted on the y-axis (see Figure 2 and text for a complete explanation). The solid lines gives the Model II reduced major axis regression and the dashed line has a slope=1. (B) Bland-Altman plot (Bland & Altman, 1986) showing the 95% confidence interval (shaded area) for the difference between the accommodation S-R slopes measured from polynomial derivatives or linear regressions. The method of polynomial slope derivation omits flat regions in the accommodation S-R function due to sub-threshold responses at low demands or response saturation at high demands and so tends to give steeper slopes than simple linear in data sets exhibiting those characteristics.
In those marmosets in which accommodation was assessed both before and after visual manipulations of eye growth and refraction were performed, comparison of the accommodative functions as described above was further restricted to only the regions of the functions with overlapping accommodative demands.
2. 4. Data and Statistical Analysis
Statistical analyses were performed on data from both eyes of each individual subject or, if indicated, on data from only the right eyes using Statview (SAS, Carey, NC) and KaleidaGraph data analysis and graphing software (Synergy Software, Reading, PA). A Kolmogorov-Smirnov test for normality was performed on the data (n=97) shown in Figure 3. The results show that the data from either the linear (Chi-square=2.062; p=0.713) or polynomial (Chi-square=1.320; p>0.999) measures of S-R slope are not significantly different from an ideal normal distribution. We confirmed this, using graphical analyses (quantile-normal probability plots) of various transforms to determine whether any provided a more normal distribution and found that none did. The r values from the normal probability plots of the linear (0.979) and polynomial (0.988) derived data were closer to unity than any of eight other common transforms.
As indicated throughout the Results section, means with standard deviations are used for descriptive data and means with standard errors are used for comparison of group data. Paired t-tests were used to test changes in the same eyes before and after lens rearing and to compare data from the two of eyes of individuals. Unpaired t-tests were used to examine differences between data sets from different marmosets. Pearson correlation coefficients (r) were used to describe various associations between groups of data and the statistical significance of these correlations was tested using the Fisher z transform. ANOVA was used for analysis of simple linear regressions models for hypothesis testing, and the coefficient of determination (r2) was reported in those cases.
3. Results
Complete data showing the accommodation S-R functions from both eyes of all subjects are presented graphically (Figures 4, 7, and 8). As a measure of the reliability of the S-R functions, the 95% CI for the slopes of linear regression functions fitted to the data was calculated for each subject (mean 95% CI = 0.204 ±0.065). Because the linear fit method does not take into account any non-linearities in the data, the 95% confidence interval will be even less for the third-order polynomials, which provide better fits to the nonlinear data sets. The S-R slopes calculated from the polynomial derivatives are presented in Tables 2 and 3.
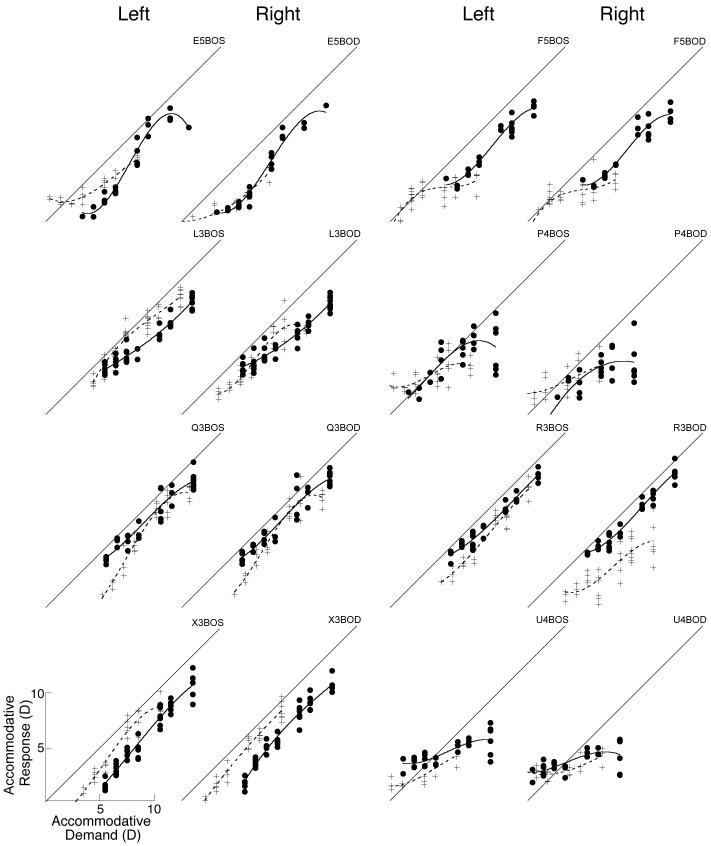
Figure 4.
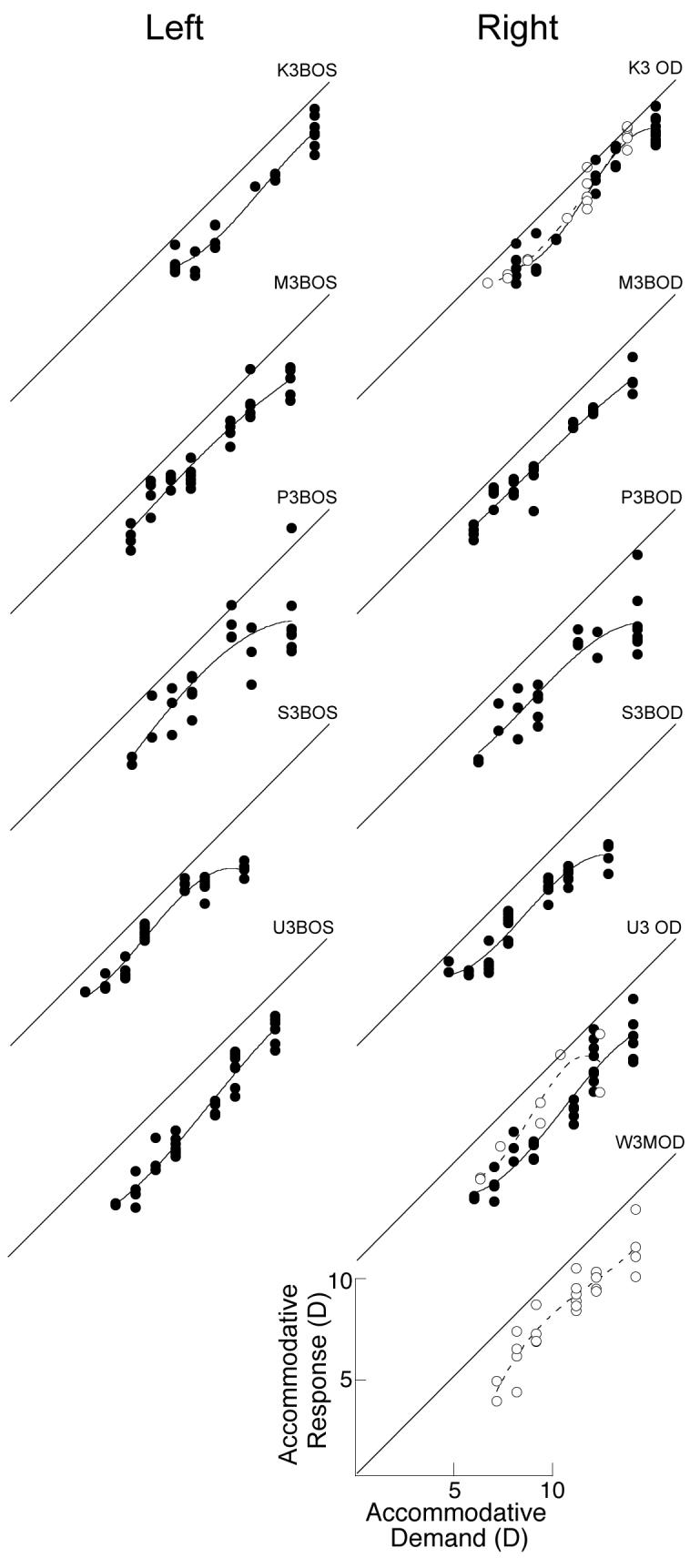
Accommodation S-R functions of the individual eyes of six untreated marmosets. Data are fit with 3rd order polynomials. Black circles fit with solid lines are data collected during binocular viewing. White circles with dashed lines are data collected during monocular viewing. Data from right eyes are shown in the right hand column and data from left eyes are shown in the left hand column. Diagonal lines indicate S-R slopes of 1 and are shown for reference. The total range of stimulus and response values is 0 to 16 D for each graph.
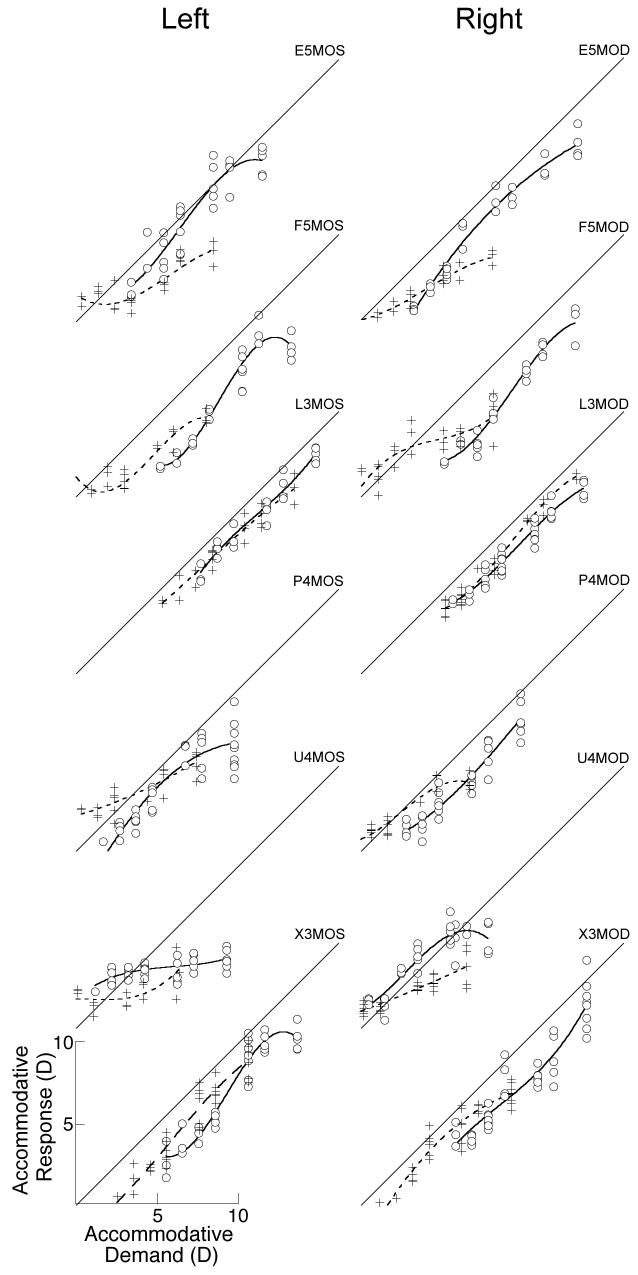
Figure 7.
Accommodation S-R functions from both eyes of six marmosets measured during monocular viewing before and after induced changes in refractive state. Data from right eyes are shown in the right hand column and data from left eyes are shown in the left hand column. Data are fit with 3rd order polynomials. White circles fit with solid lines are data collected before visual manipulations, crosses fit with dashed lines show data collected after the manipulation. Diagonal lines indicate S-R slopes of 1 and are shown for reference. The total range of stimulus and response values is 0 to 16 D for each graph.
Figure 8.
Accommodation S-R functions from eight marmosets measured during binocular viewing before and after induced changes in refractive state. Data collected before visual manipulations are shown as black circles fit with solid lines, all other details are same as in Figure 7.
Table 2.
Slopes of Accommodation S-R Functions in Untreated Marmosets
Subjects shown in this table were either never treated with visual manipulations (untreated) or were measured before rearing with spectacle lenses to produce changes in eye growth and refractive state (pre-treatment). Additional information on these marmosets is found in Tables 1 and 3, and the S-R functions are shown in Figures 5, 8, and 9.
| Subject | Eye | Monocular S-R Slopes |
Binocular S-R Slopes |
|---|---|---|---|
| Untreated | |||
| K3 | Right | 1.056 | 1.055 |
| Left | - | 0.960 | |
| M3 | Right | - | 0.922 |
| Left | - | 0.937 | |
| P3 | Right | - | 0.818 |
| Left | - | 0.855 | |
| S3 | Right | - | 0.770 |
| Left | - | 0.905 | |
| U3 | Right | 0.946 | 0.988 |
| Left | - | 1.093 | |
| W3 | Right | 1.028 | - |
| Left | - | - | |
| Pre-treatment | |||
| E5 | Right | 1.294 | 1.053 |
| Left | 1.223 | 1.318 | |
| F5 | Right | 0.899 | 0.754 |
| Left | 1.082 | 0.715 | |
| L3 | Right | 0.890 | 0.662 |
| Left | 0.989 | 0.740 | |
| P4 | Right | 0.923 | 1.016 |
| Left | 0.981 | 0.892 | |
| Q3 | Right | - | 0.928 |
| Left | - | 0.890 | |
| R3 | Right | - | 0.880 |
| Left | - | 0.897 | |
| U4 | Right | 0.785 | 0.306 |
| Left | 0.236 | 0.364 | |
| X3 | Right | 0.876 | 1.346 |
| Left | 1.255 | 1.198 | |
| Mean ±SD | 0.964±0.249 | 0.895±0.235 | |
Table 3.
Results of Experimental Manipulations
Change in refractive state and vitreous chamber (VC) depth is the difference between the post-manipulation and the pre-manipulation cycloplegic measure (post–pre). For pre- and post-manipulation measures of accommodation, the slopes given are for only the regions of overlapping demands (see text for details).
| Subject | Eye | Condition | Change in Refractive State (D) |
Change in VC Depth (mm) |
Monocular S-R Slope pre- manipulation |
Monocular S-R Slope post- manipulation |
Binocular S-R Slope pre- manipulation |
Binocular S-R Slope post- manipulation |
|---|---|---|---|---|---|---|---|---|
| Post-manipulation measures of accommodation only | ||||||||
| H2 | Right | −10 D | −8.58 | 0.898 | - | - | - | 0.63 |
| Left | −10 D | −8.87 | 0.954 | - | - | - | 0.604 | |
| H3 | Right | −3 D | −6.88 | 0.759 | - | 0.555 | - | 0.628 |
| Left | −3 D | −2.51 | 0.593 | - | - | - | 0.544 | |
| I2 | Right | +10 D | 0.88 | 0.522 | - | - | - | 0.647 |
| Left | +10 D | 0.92 | 0.584 | - | - | - | 0.781 | |
| J3 | Right | −7 D | −13.12 | 1.016 | - | 0.827 | - | 0.770 |
| Left | −7 D | −4.43 | 0.833 | - | 0.561 | - | 0.717 | |
| W2 | Right | −10 D | −8.52 | 1.069 | - | 0.609 | - | 0.600 |
| Left | −10 D | −5.09 | 0.951 | - | 0.776 | - | 0.812 | |
| Z2 | Right | Plano | −2.35 | 0.753 | - | - | - | 0.768 |
| Left | Plano | 0.38 | 0.507 | - | - | - | 0.632 | |
| Pre- and post-manipulation measures of accommodation | ||||||||
| E5 | Right | −7 D | −6.23 | 0.497 | 1.294 | 0.500 | 1.053 | 0.778 |
| Left | −7 D | −4.03 | 0.528 | 1.223 | 0.584 | 1.318 | 0.723 | |
| F5 | Right | −7 D | −2.61 | 0.716 | 0.899 | 0.452 | 0.754 | 0.307 |
| Left | −7 D | −3.95 | 0.758 | 1.082 | 0.700 | 0.715 | 0.305 | |
| L3 | Right | −5 D | 1.69 | 0.020 | 0.890 | 1.028 | 0.662 | 0.782 |
| Left | −5 D | −0.60 | 0.089 | 0.989 | 0.857 | 0.740 | 0.891 | |
| P4 | Right | −7 D | −1.24 | 0.285 | 0.923 | 0.623 | 1.016 | 0.451 |
| Left | −7 D | −3.53 | 0.295 | 0.981 | 0.488 | 0.892 | 0.436 | |
| Q3 | Right | −5 D | −2.15 | 0.197 | - | - | 0.928 | 1.244 |
| Left | −5 D | −1.54 | 0.181 | - | - | 0.890 | 1.285 | |
| R3 | Right | −5 D | −1.44 | 0.189 | - | - | 0.880 | 0.707 |
| Left | −5 D | 0.61 | 0.244 | - | - | 0.897 | 1.102 | |
| U4 | Right | −7 D | −3.02 | 0.270 | 0.785 | 0.425 | 0.306 | 0.365 |
| Left | −7 D | −3.16 | 0.295 | 0.236 | 0.508 | 0.364 | 0.538 | |
| X3 | Right | −10 D | −1.16 | 0.459 | 0.876 | 0.596 | 1.346 | 1.066 |
| Left | −10 D | −1.89 | 0.461 | 1.255 | 1.060 | 1.198 | 0.794 | |
3.1. Accommodation in Untreated Marmosets
The slopes of accommodation S-R functions measured during monocular or binocular viewing for both eyes of all untreated marmosets were not significantly different (mean±SE: monocular 0.964±0.064 vs. binocular 0.895±0.046, paired t-test, p=0.273). This group includes data from both untreated marmosets and pre-treatment measurements from our experimental group (Table 2). There were no significant differences in the accommodation S-R slopes between these untreated and pre-treatment groups (mean±SE: monocular, 1.010±0.033 vs. 0.953±0.080, unpaired t-tests, p=0.736; binocular, 0.919±0.035 vs. 0.872±0.073, unpaired t-tests, p=0.634), and the slopes measured during monocular viewing were correlated with those measured during binocular viewing (n=14, r=0.669, z-test, p<0.01). Individual S-R functions from the untreated marmosets are shown in Figure 4. The S-R functions measured pre-treatment in the experimental marmosets are shown in Figures 7 and 8.
Accommodation S-R slopes were similar in the two eyes of untreated marmosets. There were no significant differences between the accommodation S-R slopes in the right and left eyes in either monocular (mean±SE: 0.944±0.072 vs. 0.961±0.152, paired t-test, p=0.903) or binocular (0.884±0.068 vs. 0.896±0.065, paired t-test, p=0.704) measured S-R functions. The interocular S-R slopes measured under binocular conditions were significantly correlated (n=13, r=0.899, z-test, p<0.01), however the interocular slopes measured under monocular conditions were not (n=6, r=0.542, z-test, p=0.293), possibly because of the small sample tested.
3.2. Effects of visual manipulations
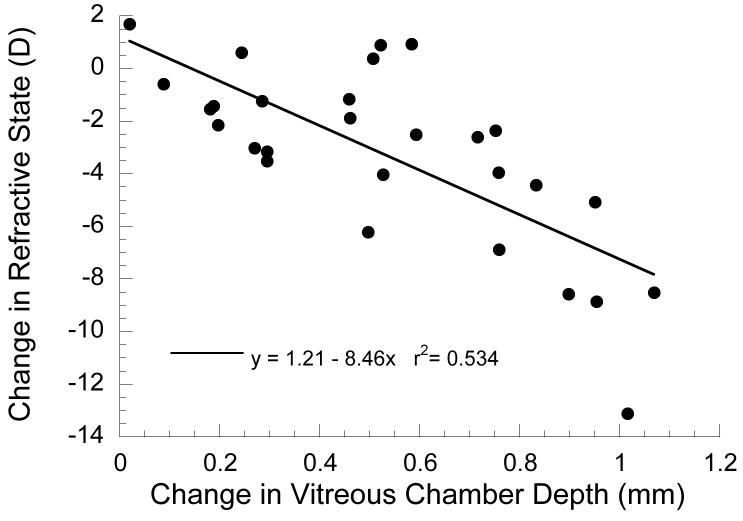
The effects of binocular lens wear on the refractive states and axial lengths (measured as vitreous chamber depth) of 14 marmosets are summarized in Table 3. Overall there were significant changes in refractive state (post-lens–pre-lens, mean±SE: −3.30±0.66 D, one-sample t-test, p<0.001) and axial length (0.53 ±0.06 mm, one-sample t-test, p<0.01). There was a wide range in the induced changes (change in vitreous chamber depth ranged from 0.02 to 1.07 mm; change in refractive state ranged from +1.67 to −13.13 D), and the change in refractive state is significantly correlated with the change in vitreous chamber depth (Figure 5: r=0.731, z-test, p<0.001).
Figure 5.
The effect of lens rearing on refractive state and vitreous chamber depth. Data are the differences between the post-manipulation measurement and the pre-manipulation measurement. The change in refractive state (y-axis) and vitreous chamber depth (x-axis) are significantly correlated (p<0.01).
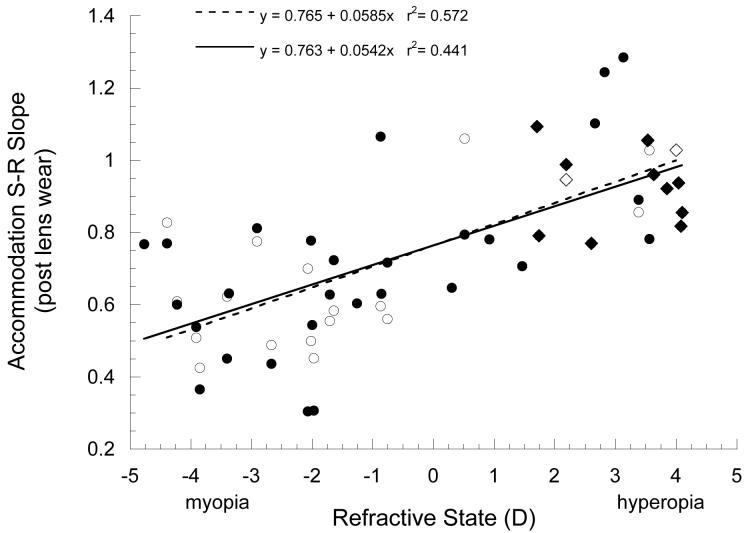
Following lens wear, the accommodation S-R slopes measured from the functions obtained during either monocular or binocular viewing were significantly correlated with refractive state whether using both eyes from each subject (binocular slopes, r=0.664, z-test, p<0.01; monocular slopes, r=0.756, z-test p<0.01), or using only the right eyes (binocular slopes, r=0.581, z-test, p<0.01; monocular slopes, r=0.803, z-test p<0.01). Over the range of refractive errors induced by spectacle lens wear, and including slopes from untreated control marmosets to increase the range for regression analysis (Figure 6), we found that the accommodation S-R slopes were inversely proportional to refractive state (binocular slopes, F=23.95, ANOVA, p<0.01; monocular slopes, F=28.45, ANOVA, p<0.01), with lower slopes associated with more myopia.
Figure 6.
Accommodation S-R slope is significantly correlated with refractive state. Black circles, fit with the solid linear regression line, show slopes measured during binocular viewing following lens treatment. White circles, fit with the dashed linear regression line, show slopes measured under monocular conditions in the same marmosets. Accommodation S-R slopes for untreated marmosets (diamonds) are shown for comparison, black symbols show S-R slopes measured under binocular conditions, white symbols show S-R slopes measured under monocular conditions.
A subset of the experimental marmosets completed lens treatment earlier (n=6, mean duration = 47.6 days, mean age at completion=88 days) than the other treated marmosets (n=8, mean duration = 38.6 days, mean age at completion=180 days). Comparison of the marmosets showing induced axial myopia in these two groups showed that the amount of axial elongation and myopia was significantly greater in the younger marmosets (mean±SE, younger vs. older: change in vitreous chamber depth, 0.83±0.06 vs. 0.38±0.05, unpaired t-test, p<0.01; change in refractive state, −6.00±1.26 vs. −2.52±0.44 D, unpaired t-test, p<0.01). There were no significant differences in S-R slope measured under monocular conditions (0.666±0.056 vs. 0.594±0.058, unpaired t-test, p=0.450), however binocular slopes were significantly lower following lens treatment in the younger group (0.670±0.028 vs. 0.897±0.081, unpaired t-test, p<0.05).
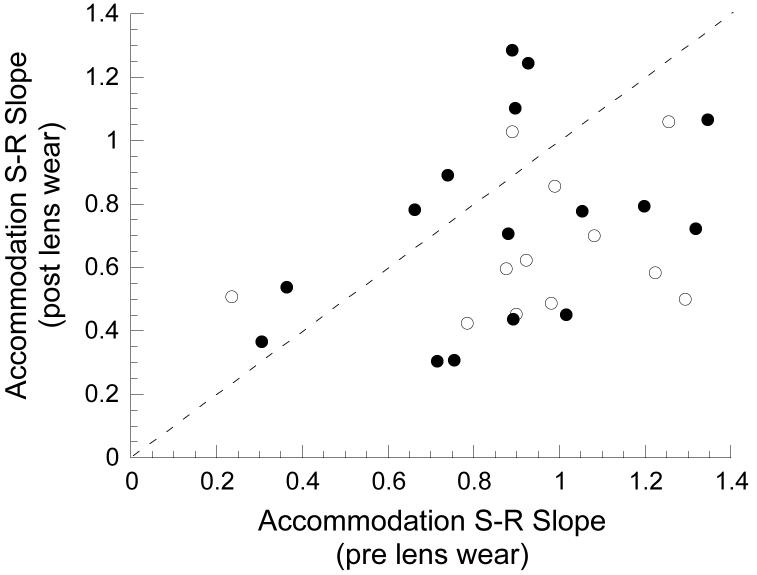
The older lens-treated marmosets had S-R functions measured both before and after lens treatment and showed a significant reduction in slope following lens wear (see Table 3 and Figures 7-9). Figures 7 and 8 show the S-R functions before and after lens treatment measured under monocular or binocular conditions respectively. For example, in Figure 7 the top right panel shows the data collected under monocular conditions from the right eye of marmoset E5. Differences in the slopes of the polynomial fits to the data collected before (solid line) and after (dashed line) lens treatment are clearly seen. Statistical comparisons were restricted to the slopes in the overlapping regions of the functions. On average, the accommodative slopes measured during monocular viewing were found to be significantly reduced after lens rearing compared to the slopes measured before whether using both eyes of each marmoset (mean±SE, change in slope: −0.30 ±0.09, one sample t-test, p<0.01) or just the right eye (mean±SE slope change: −0.34 ±0.12, one sample t-test, p<0.05). However, change in the S-R slopes measured under binocular conditions (see Figures 8 and 9) was not significant using either both eyes of each marmoset (mean±SE slope change: −0.14 ±0.08, one sample t-test, p<0.12) or just the right eye (mean±SE slope change: −0.16 ±0.11, one sample t-test, p<0.18).
Figure 9.
Changes in individual accommodation S-R slope measured under monocular (white circles) and binocular (black circles) conditions are represented in this scatter plot of slopes measured before (x-axis) and after (y-axis) lens-induced changes in eye size and refractive state. The diagonal dashed line has a slope of 1. Points below the line indicate reduced slopes following lens wear. Points above the line indicate increasing slopes.
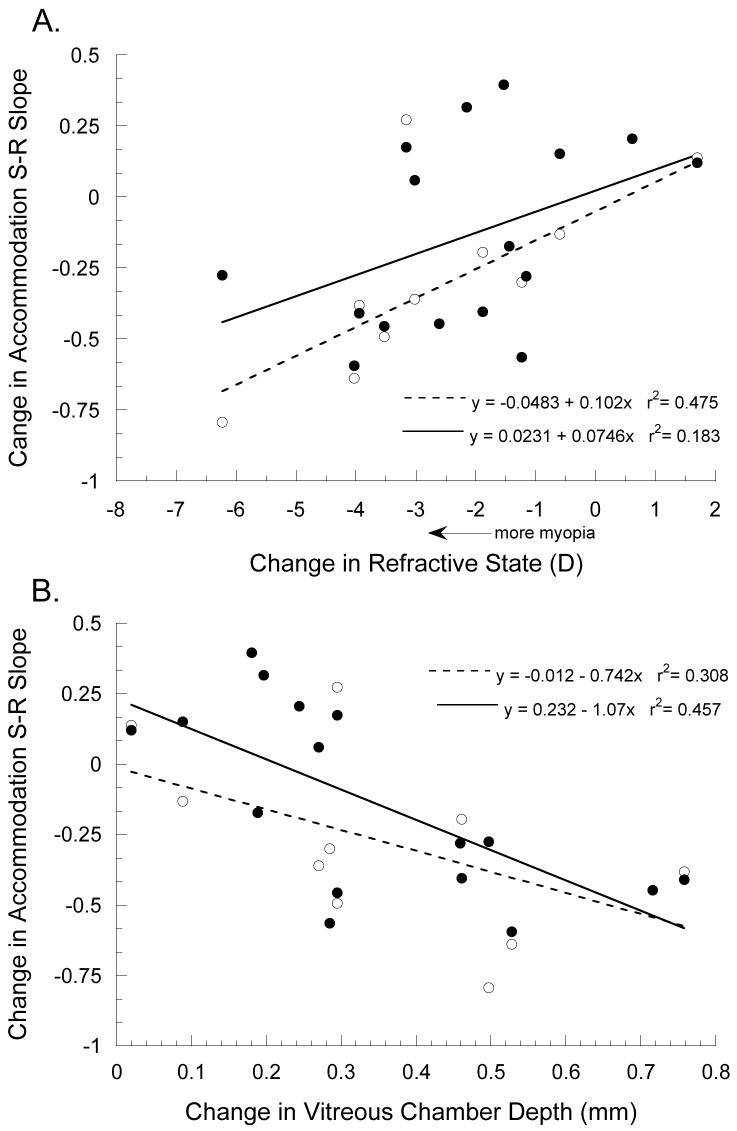
To examine the relationship of experimental myopia and accommodative function, the change in accommodative slope over the period of lens wear was compared to the induced changes in eye size and refractive state (Figure 10). Analysis of simple linear regressions showed that the change in accommodation S-R slope is proportional to the lens-induced change in vitreous chamber depth and myopia. Greater reductions in monocular accommodative slope are associated with increasing degrees of lens-induced myopia (Figure 10A: using both eyes, F=9.04, ANOVA, p<0.05; using right eyes only, F=99.13, ANOVA, p<0.01). Although the relationship of slope reduction to increasing vitreous chamber depth did not reach statistical significance (Figure 10B: using both eyes, F=4.46, ANOVA, p=0.061; using right eyes only, F=4.34, ANOVA, p=0.11), the direction of change was consistent with the associated refractive state data. For accommodation S-R slopes measured under binocular conditions, the change in slope was not significantly correlated with the lens-induced change in refractive state (Figure 10A: using both eyes, F=3.14, ANOVA, p=0.098; using right eyes only, F=0.28, ANOVA, p=0.615), but it was significantly reduced with induced increases in vitreous chamber depth (Figure 10B: using both eyes, F=11.78, ANOVA, p<0.01; using right eyes only, F=3.99, ANOVA, p=0.093).
Figure 10.
The change in accommodative slope (post lens wear – pre lens wear) plotted against the change in refractive state (A) and vitreous chamber depth (B) induced in experimental marmosets raised with binocular spectacle lenses. Black circles show slopes measured under binocular conditions and are fit with solid linear regression lines. White circles show slopes measured under monocular conditions and are fit with dashed regression lines.
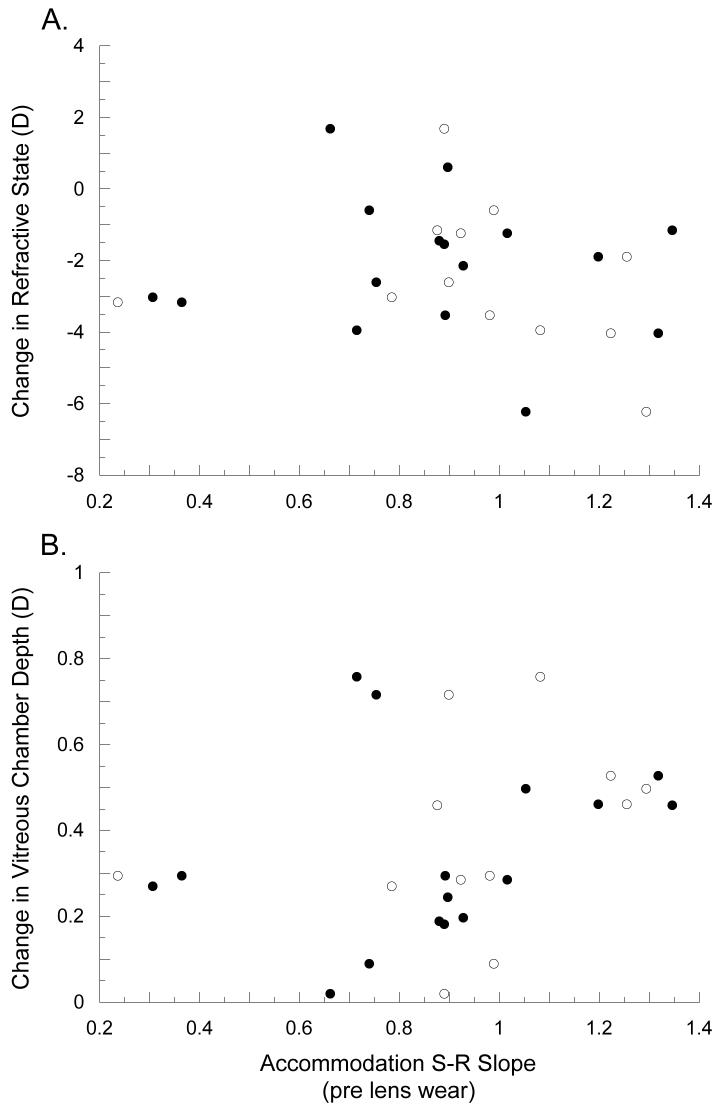
The pre-treatment accommodation S-R slope did not affect the response to lens induced defocus (Figure 11). There were no significant correlations between pre-treatment SR slopes measure during monocular viewing and the induced change in refractive state (F=0.61, ANOVA, p=0.453) or vitreous chamber depth (F=1.15, ANOVA, p=0.308), nor were there significant correlations between pre-lens slopes measure during binocular viewing and the induced change in refractive state (F=0.51, ANOVA, p=0.824) or vitreous chamber depth (F=1.01, ANOVA, p=0.331).
Figure 11.
Induced change in refractive state (A) and vitreous chamber depth (B) plotted against the accommodative slope measured in experimental marmosets before being treated with binocular spectacle lenses. Black circles show slopes measured under binocular conditions and white circles show slopes measured under monocular. There are no statistically significant correlations between S-R slopes measured before lens rearing and the induced change in either refractive state or vitreous chamber depth.
4. Discussion
This study sheds new light on the long-standing question of the relationship between accommodation and the development of myopia and provides a bridge between human clinical studies of the development of refractive state and animal models of emmetropization using a primate model. The aims of this study were to (1) develop a system for measuring accommodation S-R functions in free-viewing marmoset monkeys, (2) determine whether accommodation is altered by lens-induced changes in eye growth and refractive state, and (3) determine whether accommodation is a predictor of the response to lens-rearing. We successfully measured monocular and binocular accommodation S-R functions in marmosets using IR videorefraction. We found that untreated marmosets possess accommodation S-R functions very similar to those seen in humans, with slopes, on average, close to 1.0 under either monocular or binocular viewing conditions. There was, however, considerable inter-individual variability and under monocular viewing conditions the interocular S-R slopes were not correlated.
We found that overall both binocular and monocular measured S-R slopes were significantly correlated with refractive state. In a subset of marmosets that were measured both before and after lens treatment, there was also a significant reduction in the mean monocular S-R slope after the lens treatment, but the mean binocular S-R slope was not significantly changed. We speculate that this may be because of the contribution of vergence accommodation under binocular conditions. Accommodation is generally accepted to act as a negative-feedback proportional control system (Toates, 1970, Toates, 1972). In such a system there is a non-linearity between the S-R slope and the underlying gain of the accommodation controller, as gain = slope/(1−slope) (Flitcroft, 1991). This means that for a given change in gain there is a greater change in slope at lower slopes. So under binocular conditions, where accommodation gain is higher because of the contribution from convergence, changes in S-R slope would be expected to be less. We found further, that the amount of change in monocular S-R slope was significantly correlated with the amount of axial myopia induced and that, despite the lack of a mean reduction in binocular S-R slope, the amount of change in binocular S-R slope was correlated with the amount of lens induced axial growth as measured by vitreous chamber depth.
Accommodative performance before the lens treatment did not predict the amount of myopia or change in vitreous chamber depth induced. This suggests that the change in accommodation S-R slope observed with lens-induced axial myopia is more likely a consequence of developing myopia than a causal factor. Our results support a recent longitudinal study of accommodative lags in human subjects before and after the onset of myopia (Mutti et al., 2006), which concluded that increased accommodative lag do not generally precede the onset of myopia and is not a reliable predictor of myopia development. However, a reduction in accommodation S-R slope during negative lens wear may increase the hyperopic defocus being experienced and so may yet contribute to the development of myopia. Similarly, young human myopes with low accommodation S-R slopes may also experience increased hyperopic defocus during near work that may increase the development of myopia.
4.1. What may be responsible for low S-R slopes in myopes?
There are several factors that could contribute, either alone or in combinations, to a reduction in accommodative performance in myopes. In the discussion that follows we summarize the principal possibilities as (1) nearpoint oculomotor responses, (2) changes in accommodative error detection, and (3) accommodative plant changes.
4.1.1. Nearpoint Oculomotor Responses
The interaction of accommodation and vergence control systems during lens imposed hyperopic defocus or changing refractive state may affect accommodative lag, which has been considered a factor in emmetropization and development of myopia (Flitcroft, 1998, Flitcroft, 1999). It is commonly accepted that accommodation is driven mainly by image defocus related to object proximity, vergence is driven mainly by retinal disparity, and the two control systems are interconnected by cross-links (Fincham & Walton, 1957, Hung & Semmlow, 1980, Schor, 1985) that are known to be adaptively regulated (Judge & Miles, 1985, Miles, 1985, Miles, Judge & Optican, 1987, Schor, 1988, Schor, 1986, Schor & Kotulak, 1986). Changes in the performance of these cross-links could result in reduced accommodation gain as an oculomotor compromise between accurate accommodation and accurate convergence for near targets. So under conditions of imposed hyperopic blur, lower accommodation S-R slopes would be tolerated in order to avoid esotropia (excess tonic vergence), loss of binocular fusion, and diplopia. Elevated AC/A ratios would also be expected (Flitcroft, 1998, Schor, 1999) and have been found to be higher in human myopes (Gwiazda, Grice & Thorn, 1999, Jiang, 1995, Mutti, Jones, Moeschberger & Zadnik, 2000), and even before the onset of myopia (Gwiazda et al., 2005). Reduced CA/C ratios would also be expected, but have not been found (Allen & O'Leary, 2006, Jiang, 1995, Rosenfield & Gilmartin, 1988). Nevertheless, myopes with nearpoint esophoria have more rapid myopia progression (Goss, 1991), and are more responsive to therapy with progressive lenses (Gwiazda et al., 2004) supporting the view that nearpoint oculomotor responses are factors in the development of myopia.
4.1.2. Accommodative Error Detection
Visual acuity and contrast sensitivity are reduced with increasing myopia (Collins & Carney, 1990, Comerford, Thorn & Corwin, 1987, Fiorentini & Maffei, 1976, Strang, Winn & Bradley, 1998). Reduced sensitivity to defocus has also been reported in myopes (Rosenfield & Abraham-Cohen, 1999), and could result in greater accommodative lags and lower S-R slopes because of an associated increase in depth of focus (Flitcroft, 1998, Wang & Ciuffreda, 2006). The cause of such reduced sensitivity to defocus could be decreased optical image quality itself. For example, changes in monochromatic aberrations can affect accommodative demand and result in a reduced accommodative response. Increased monochromatic aberrations have been found in myopic eyes (Charman, 2005, Collins, Buehren & Iskander, 2006, Collins, Wildsoet & Atchison, 1995, He, Sun, Held, Thorn, Sun & Gwiazda, 2002, Llorente, Barbero, Cano, Dorronsoro & Marcos, 2004), and in preliminary studies of experimental myopia in marmosets we have also observed increased aberrations, particularly in negative spherical aberration (Coletta, Troilo, Moskowitz, Nickla & Marcos, 2004). During accommodation, spherical aberration has been observed to become more negative (Atchison, Collins, Wildsoet, Christensen & Waterworth, 1995, Hazel, Cox & Strang, 2003, He et al., 2002), or to actually change from positive to negative spherical aberration (Cheng, Barnett, Vilupuru, Marsack, Kasthurirangan, Applegate & Roorda, 2004, Plainis, Ginis & Pallikaris, 2005). In such eyes, the optimal image quality for near targets would be slightly behind the retina and so greater accommodative lags could result (Plainis et al., 2005). The effect of spherical aberration on the modulation transfer function of defocused eyes depend on the spatial frequency of the target (Jansonius & Kooijman, 1998), and a recent study showed that contrast sensitivity for low to middle spatial frequencies (1-8 c/deg) was actually improved in myopes when a slight (<1 D) hyperopic defocus was imposed (Radhakrishnan, Pardhan, Calver & O'Leary, 2004). Because middle spatial frequencies (3-5 c/deg) drive accommodation best (Mathews & Kruger, 1994), such a selective increase in sensitivity to these frequencies during hyperopic defocus could reduce accommodative demand and result in a reduced accommodative response.
Neural changes to the retina or central visual system of myopes may also affect the processing of the accommodative stimulus and result in a reduced accommodative response. Morphological changes to the retina associated with ocular growth and retinal stretching could contribute to the reduction in accommodative function by reducing visual acuity and sensitivity to changes in retinal blur, but the evidence for this possibility is mixed. Several studies in humans have considered whether axial myopia is associated with changes in visual resolution because of reduced optical quality, changes in retinal magnification, or the possibility of reduced sampling density due to retinal stretch. However, results have been equivocal because of the difficulties in controlling magnification changes from optical corrections (Strang et al., 1998). Optical techniques using interferometry (Atchison, Schmid & Pritchard, 2006, Coletta & Watson, 2006) or application of Knapp's law (Chui, Yap, Chan & Thibos, 2005) bypass these optical correction problems. Coletta and Watson (2006) and Atchison et al. (2006) reported reduced visual performance in myopes and Chui et al. (2005) found variable foveal acuity in myopes, but generally reduced acuity in higher myopes. Experimentally induced myopia has been reported to stretch the retina in chicks and affect retinal organization (Troilo, Xiong, Crowley & Finlay, 1996), but preliminary studies with marmosets indicate that the foveal photoreceptor density may actually increase during induced axial growth (Troilo, 1998, and see Hendrickson, Troilo, Possin & Springer, 2006, Springer & Hendrickson, 2004a, Springer & Hendrickson, 2004b, Springer & Hendrickson, 2005), suggesting that foveal changes are not a likely factor to explain the accommodation changes observed in this study.
Another possibility is that changes in the central visual system take place in marmosets with induced myopia. Experimental anisometropia in macaques produced with lenses (Chui et al., 2005) or atropine (Kiorpes & Wallman, 1995) is known to produce amblyopia, which may be associated with changes in accommodative function. This seems unlikely to be a factor here, however. The marmosets in this study were treated with binocular lenses of equal sign and power, and although five of them exhibited myopic anisometropia greater than 1 D (mean = 1.7 D), those animals did not show significantly different changes in accommodation S-R slope compared to the nine other lens-treated marmosets.
Adaptation to blur has been suggested to explain increased visual acuity following a period of imposed blur (Mon-Williams, Tresilian, Strang, Kochhar & Wann, 1998, Pesudovs & Brennan, 1993, Rosenfield, Hong & George, 2004), so it may be possible that a lack of blur adaptation might reduce accommodative function in lens-treated marmosets. While this was not specifically examined in the present study, evidence from studies in human myopes does not support this for an explanation for reduced accommodation. Blur adaptation has been reported to be greater in myopes than emmetropes (George & Rosenfield, 2004) and Vera-Diaz et al. (2004) reported that accommodative lags were reduced in myopes, but not emmetropes, following a period of exposure to blur.
4.1.3. Accommodative Plant Changes
There has been some speculation that morphological changes to the anterior segment of the eye associated with increased axial growth in myopia might affect the ciliary body and its control of lens shape, and could lead to a condition of “pseudocycloplegia” seen in myopic eyes that might be responsible for the observed reductions in accommodation S-R slope (Mutti et al., 2006). Accommodation has been shown to affect slightly the distance from cornea to retina (Drexler, Findl, Schmetterer, Hitzenberger & Fercher, 1998), and possibly eye shape as well (Walker & Mutti, 2002), so it is possible that by altering eye shape the accommodative plant and its function may also be affected. However, there is no direct evidence presently supporting this idea.
Finally, uncorrected myopes might have a lower accommodative response because accommodative demand would be generally diminished and disuse could result in low responses. This seems unlikely to be the case in this study because the marmosets actually experienced larger hyperopic demands while initially wearing the negative power spectacle lenses, and when they did develop myopia it at least partially compensated for the hyperopic defocus imposed by the lenses so accommodative demands would be comparable to those seen in untreated marmosets. It is conceivable that imposing hyperopic blur may have caused accommodative fatigue that reduced accommodative accuracy and S-R slope. The demands imposed, however, were considerably less than the maximal accommodative response in marmoset (estimated at up to at least 20 D (Troilo, Howland & Judge, 1993)), but we cannot say how accommodation responds to continuous sub-maximal accommodative demands over long periods of time. In fact, little is known about accommodative behavior through lens-imposed defocus in experimental animals, or for that matter during natural viewing conditions in animals or humans. Related to this, it remains unclear how accommodation interacts with the visual control of the development of refractive state (emmetropization).
4.2. What is the relationship between accommodation and emmetropization?
Because both accommodation and emmetropization use hyperopic defocus as a stimulus, the feedback loops controlling them must interact in some way so that accommodation does not eliminate the error signal for emmetropization. The nature of this interaction, however, is unclear. One possibility is that emmetropization uses residual hyperopic defocus from accommodative errors and is the basis for the hypothesis that large accommodative lag during near work, and their attendant hyperopic defocus, could drive the eye to elongate and become myopic. Alternative possibilities also exist however. For example, it is possible that the time constants for the accommodation and emmetropization controllers may differ sufficiently so that emmetropization is largely unaffected by normal levels of accommodation. It is conceivable, for example, that long periods of hyperopic defocus during near work do not result in myopia if they are interspersed with periods of distance vision or myopic defocus (Winawer, Zhu, Choi & Wallman, 2005).
We know very little about the temporal pattern of accommodation behavior under natural free viewing conditions. Two possibilities exist: (1) Accommodation may have a major effect on the defocus experienced. (2) Accommodation behavior may be infrequent enough that the changes in focus are too brief and inconsistent to affect the integration of retinal defocus for visually guided eye growth. In support of this latter view, our earlier studies of accommodation to near-targets in chicks and under free-viewing conditions in primates show that accommodative behavior is typically brief and variable (Troilo, Boisvert & Nau, 2000, Troilo, Harb, Totonelly, Merriwhether & Bradley, 2005). In humans during reading, the average accommodative response is steady but there is also considerable variability, the degree of which is, moreover, proportional to the subject's myopia (Harb, Thorn & Troilo, 2006). There are also significant individual differences in the lags and stability of accommodation that generally are greater in myopes. We speculate that variability in the accommodation response may be a factor in the development of refractive state, and some fluctuation in the steady state accommodative response may even be useful in detecting the sign of defocus for emmetropization. Microfluctuations of accommodation, measured from Fourier derived power spectra of the accommodative response, are also significantly increased in more myopic subjects (Day, Strang, Seidel, Gray & Mallen, 2006, Harb et al., 2006, Seidel, Gray & Heron, 2005). While the sources of accommodative microfluctuations are varied and debated (Judge & Flitcroft, 2000), and may be too small to stimulate eye growth, the higher frequency components may reflect instabilities in the accommodative controller or the accommodative plant of myopes that could have long-term effects on the visual control of eye growth.
How temporal fluctuations in accommodation interact with the temporal integration of the visual signal for eye growth is an important question and one that may be closely related to near work and the development of myopia. Experimental evidence with chicks suggests that myopic defocus is more heavily weighted than hyperopic defocus and their temporal integration for emmetropization is non-linear (Schmid & Wildsoet, 1996, Winawer & Wallman, 2002, Winawer et al., 2005).
Relatively little is known, however, about the temporal integration and weighting of different defocus stimuli in primates. The effect of brief periods of unrestricted vision without lenses as compared to brief periods of myopic defocus on the development of experimental myopia has not been extensively studied in primates, but what is known suggests that imposed myopic defocus is less effective than unrestricted vision, opposite to what is reported in chicks (Zhu, Winawer & Wallman, 2003). In macaques, one hour of clear vision each day resulted in approximately a 67% reduction in vitreous chamber elongation and axial myopia in the otherwise form deprived eyes (Smith III, Hung, Kee & Qiao, 2002). Similarly, in tree shrews wearing negative power lenses, removing the lenses for one hour per day effectively reduced the amount of myopia induced by approximately 50% (Shaikh, Siegwart & Norton, 1999). One abstract in macaques (Kee, Hung, Qiao, Ramamirtham, Winawer, Wallman & Smith, 2002), and a recent paper in tree shrews (Norton, Siegwart & Amedo, 2006), examined the effects on negative-lens-induced myopia of brief periods of positive lens defocus versus periods of clear vision for short periods per day. Both report a better protective response with unrestricted vision compared to positive lens defocus, and only about half of the tree shrews receiving the positive lenses did not become myopic. Interestingly, human myopes have been reported to have fewer fixation breaks then emmetropes during sustained reading, particularly at larger accommodative demands, and so possess a different pattern of interspersed distance vision that may contribute to the progression of myopia (Harb et al., 2006). These studies suggest that even short periods of clearing hyperopic defocus with accommodation or distance vision may help protect against myopia, and that inaccurate and variable accommodative responses may be a factor in myopia development.
5. Conclusion
In summary, in this study we found that, similar to reports in children with developing myopia, accommodation S-R slopes were reduced in marmosets with experimentally induced myopia. The changes in S-R slope observed are most likely a consequence of the induced myopia; we found no evidence that low S-R slopes make the marmoset eye more susceptible to the effects of experimental lens rearing. Because accommodation alters the hyperopic retinal defocus that drives the eye to increase its axial growth and become myopic, some interaction between the eye growth and accommodative controllers seems likely. The explanation may lie in a better understanding of the ethology of accommodation, and specifically the interaction of the temporal pattern of accommodation behavior under natural conditions and the temporal integration of the retinal defocus signals driving eye growth and the development of refractive state.
Acknowledgements
The authors are grateful to Heidi Denman and Kristen Totonelly for their assistance with animal care and to Dr. Debora Nickla for her assistance with some of the optometric measures. The authors thank Drs. Nancy Coletta, Elise Harb, Chea-su Kee, Frank Thorn, and Josh Wallman for their comments and suggestions.
Footnotes
Support: NIH R01-EY011228, T35-EY07149
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic and Physiological Optics. 1998;18(1):13–20. [PubMed] [Google Scholar]
- Allen PM, O'Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Research. 2006;46(4):491–505. doi: 10.1016/j.visres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Angle J, Wissmann DA. The epidemiology of myopia. America Journal of Epidemiology. 1980;111:220–228. doi: 10.1093/oxfordjournals.aje.a112889. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Collins MJ, Wildsoet CF, Christensen J, Waterworth MD. Measurement of monochromatic ocular aberrations of human eyes as a function of accommodation by the Howland aberroscope technique. Vision Research. 1995;35(3):313–323. doi: 10.1016/0042-6989(94)00139-d. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Schmid KL, Pritchard N. Neural and optical limits to visual performance in myopia. Vision Research. 2006;46(21):3707–3722. doi: 10.1016/j.visres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bedrossian RH. The effect of atropine on myopia. Annals of Ophthalmology. 1971;3:891–897. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- Brodstein RS, Brodstein DE, Olson RJ, Hunt SC, Williams RR. The treatment of myopia with atropine and bifocals; a long term prospective study. Ophthalmology. 1984;91:1373–1379. doi: 10.1016/s0161-6420(84)34138-0. [DOI] [PubMed] [Google Scholar]
- Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic and Physiological Optics. 1999;19(2):126–133. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Charman WN. Aberrations and myopia. Ophthalmic and Physiological Optics. 2005;25(4):285–301. doi: 10.1111/j.1475-1313.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, Barnett JK, Vilupuru AS, Marsack JD, Kasthurirangan S, Applegate RA, Roorda A. A population study on changes in wave aberrations with accommodation. Journal of Vision. 2004;4(4):272–280. doi: 10.1167/4.4.3. [DOI] [PubMed] [Google Scholar]
- Chou AC, Shih YF, Ho TC, Lin LLK. The effectiveness of 0.5% atropine in controlling high myopia in children. Journal of Ocular Pharmacological and Therapeutics. 1997;13(1):61–67. doi: 10.1089/jop.1997.13.61. [DOI] [PubMed] [Google Scholar]
- Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Chui TY, Yap MK, Chan HH, Thibos LN. Retinal stretching limits peripheral visual acuity in myopia. Vision Research. 2005;45(5):593–605. doi: 10.1016/j.visres.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Coletta NJ, Troilo D, Moskowitz A, Nickla DL, Marcos S. Ocular wavefront aberrations in the awake marmoset. Investigative Ophthalmology and Visual Science (ARVO Supplement) 2004;25(12) E-abstract 4298. [Google Scholar]
- Coletta NJ, Watson T. Effect of myopia on visual acuity measured with laser interference fringes. Vision Research. 2006;46(5):636–651. doi: 10.1016/j.visres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Collins JW, Carney LG. Visual performance in high myopia. Current Eye Research. 1990;9(3):217–223. doi: 10.3109/02713689009044516. [DOI] [PubMed] [Google Scholar]
- Collins MJ, Buehren T, Iskander DR. Retinal image quality, reading and myopia. Vision Research. 2006;46(12):196–215. doi: 10.1016/j.visres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Collins MJ, Wildsoet CF, Atchison DA. Monochromatic aberrations and myopia. Vision Research. 1995;35(9):1157–1163. doi: 10.1016/0042-6989(94)00236-f. [DOI] [PubMed] [Google Scholar]
- Comerford JP, Thorn F, Corwin TR. Effect of luminance level on contrast sensitivity in myopia. American Journal of Optometry and Physiological Optics. 1987;64(11):810–814. doi: 10.1097/00006324-198711000-00002. [DOI] [PubMed] [Google Scholar]
- Curtin BJ. The Myopias: Basic Science and Clinical Management. Harper and Row; Philadelphia: 1985. [Google Scholar]
- Day M, Strang NC, Seidel D, Gray LS, Mallen EA. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthalmic and Physiological Optics. 2006;26(1):88–96. doi: 10.1111/j.1475-1313.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Research. 1997;37(6):659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Drexler W, Findl O, Schmetterer L, Hitzenberger CK, Fercher AF. Eye elongation during accommodation in humans: Differences between emmetropes and myopes. Investigative Ophthalmology and Visual Science. 1998;39(11):2140–2147. [PubMed] [Google Scholar]
- Drobe B, de Saint-Andre R. The pre-myopic syndrome. Ophthalmic and Physiological Optics. 1995;15(5):375–378. [PubMed] [Google Scholar]
- Fincham EF, Walton J. The reciprocal actions of accommodation and convergence. Journal of Physiology (London) 1957;137:488–508. doi: 10.1113/jphysiol.1957.sp005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Maffei L. Spatial contrast sensitivity of myopic subjects. Vision Research. 1976;16:437–438. doi: 10.1016/0042-6989(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI. Accommodation and flicker: evidence of a role for temporal cues in accommodation control? Ophthalmic and Physiological Optics. 1991;11(1):81–90. [PubMed] [Google Scholar]
- Flitcroft DI. A model of the contribution of oculomotor and optical factors to emmetropization and myopia. Vision Research. 1998;38(19):2869–2879. doi: 10.1016/s0042-6989(98)00087-x. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI. The lens paradigm in experimental myopia: oculomotor, optical and neurophysiological considerations. Ophthalmic and Physiological Optics. 1999;19(2):103–111. doi: 10.1046/j.1475-1313.1999.00432.x. [DOI] [PubMed] [Google Scholar]
- George S, Rosenfield M. Blur adaptation and myopia. Optometry and Vision Science. 2004;81(7):543–547. doi: 10.1097/00006324-200407000-00016. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E. On the Etiology of Myopia: An Epidemiological Study. Munksgaard; Copenhagen: 1968. [PubMed] [Google Scholar]
- Goss DA. Clinical accommodation and heterophoria findings preceding juvenile onset of myopia. Optometry and Vision Science. 1991;68(2):110–116. doi: 10.1097/00006324-199102000-00005. [DOI] [PubMed] [Google Scholar]
- Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Research. 1999;39(2):189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Research. 1995;35(9):1299–1304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Grice K, Thorn F. Response AC/A ratios are elevated in myopic children. Ophthalmic and Physiological Optics. 1999;19(2):173–179. doi: 10.1046/j.1475-1313.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Marran L. Trends in Optics and Photonics. Vol. 35. Optical Society of America; Washington, D.C.: 2000. The many facets of the myopic eye: a review of genetic and environmental factors; pp. 393–406. [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Investigative Ophthalmology and Visual Science. 1993;34(3):690–694. [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optometry and Vision Science. 2005;82(4):273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, Manny R, Wang Y, Everett D. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Investigative Ophthalmology and Visual Science. 2004;45(7):2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Research. 2006;46(16):2581–2592. doi: 10.1016/j.visres.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel CA, Cox MJ, Strang NC. Wavefront aberration and its relationship to accommodative stimulus-response function in myopic subjects. Optometry and Vision Science. 2003;80(2):151–158. doi: 10.1097/00006324-200302000-00011. [DOI] [PubMed] [Google Scholar]
- He JC, Sun P, Held R, Thorn F, Sun XR, Gwiazda JE. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vision Research. 2002;42(8):1063–1070. doi: 10.1016/s0042-6989(02)00035-4. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Troilo D, Possin DE, Springer A. Development of neural retina and vasculature in the marmoset Callithrix jacchus. Journal of Comparative Neurology. 2006;497(2):270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- Hung GK, Semmlow JL. Static behavior of accommodation and vergence: Computer simulation of an interactive dual-feedback system. IEEE Transactions on Biomedical Engineering. 1980;27(8):439–447. doi: 10.1109/TBME.1980.326752. [DOI] [PubMed] [Google Scholar]
- Hung L-F, Crawford MLJ, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Jansonius NM, Kooijman AC. The effect of spherical and other aberrations upon the modulation transfer of the defocussed human eye. Ophthalmic and Physiological Optics. 1998;18(6):504–513. [PubMed] [Google Scholar]
- Jiang B. Parameters of accommodative and vergence systems and the development of late-onset myopia. Investigative Ophthalmology and Visual Science. 1995;36(8):1737–1742. [PubMed] [Google Scholar]
- Jiang B, Morse SE. Oculomotor functions and late-onset myopia. Ophthalmic and Physiological Optics. 1999;19(2):165–172. doi: 10.1046/j.1475-1313.1999.00431.x. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Flitcroft DI. Control of accommodation. In: Burnstock G, Sillito AM, editors. Nervous Control of the Eye. Vol. 13. Harwood Academic Publications; Australia: 2000. pp. 93–115. [Google Scholar]
- Judge SJ, Miles FA. Changes in the coupling between accommodation and vergence eye movements induced in human subjects by altering the effective interocular separation. Perception. 1985;14:617–629. doi: 10.1068/p140617. [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung L-F, Qiao Y, Ramamirtham R, Winawer JA, Wallman J, Smith EL. Temporal constraints on experimental emmetropization in infant monkeys. Investigative Ophthalmology and Visual Science (ARVO Supplement) 2002;43(12) doi: 10.1167/iovs.06-0743. E-Abstract 2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Wallman J. Does experimentally-induced amblyopia cause hyperopia in monkeys? Vision Research. 1995;35(9):1289–1297. doi: 10.1016/0042-6989(94)00239-i. [DOI] [PubMed] [Google Scholar]
- Llorente L, Barbero S, Cano D, Dorronsoro C, Marcos S. Myopic versus hyperopic eyes: axial length, corneal shape and optical aberrations. Journal of Vision. 2004;4(4):288–298. doi: 10.1167/4.4.5. [DOI] [PubMed] [Google Scholar]
- Mathews S, Kruger PB. Spatiotemporal transfer function of human accommodation. Vision Research. 1994;34(15):1965–1980. doi: 10.1016/0042-6989(94)90026-4. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Investigative Ophthalmology and Visual Science. 1993;34(1):205–215. [PubMed] [Google Scholar]
- Miles FA. Adaptive regulation in the vergence and accommodation control systems. In: Berthoz A, Melvill Jones G, editors. Adaptive Mechanisms in Gaze Control: Facts and Theories. Vol. 1. Elsevier; Amsterdam: 1985. pp. 81–94. [PubMed] [Google Scholar]
- Miles FA, Judge SJ, Optican LM. Optically induced changes in the couplings between vergence and accommodation. Journal of Neuroscience. 1987;7(8):2576–2589. [PMC free article] [PubMed] [Google Scholar]
- Mon-Williams M, Tresilian JR, Strang NC, Kochhar P, Wann JP. Improving vision: neural compensation for optical defocus. Proceedings of the Royal Society London B. 1998;265:71–77. doi: 10.1098/rspb.1998.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Investigative Ophthalmology and Visual Science. 2000;41(9):2469–2478. [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. Investigative Ophthalmology and Visual Science. 2006;47(3):837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Investigative Ophthalmology and Visual Science. 2002;43(12):3633–3640. [PubMed] [Google Scholar]
- Nakatsuka C, Hasebe S, Nonaka F, Ohtsuki H. Accommodative lag under habitual seeing conditions: comparison between myopic and emmetropic children. Japanese Journal of Ophthalmology. 2005;49(3):189–194. doi: 10.1007/s10384-004-0175-7. [DOI] [PubMed] [Google Scholar]
- Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR Journal. 1999;40(2):59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopia defocus in competition with a myopiagenic stimulus in tree shrew eyes. Investigative Ophthalmology and Visual Science. 2006;47(11):4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesudovs K, Brennan N. Decreased uncorrected vision after a period of distance fixation with spectacle wear. Optometry and Vision Science. 1993;70(7):528–531. doi: 10.1097/00006324-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Plainis S, Ginis HS, Pallikaris A. The effect of ocular aberrations on steady-state errors of accommodative response. Journal of Vision. 2005;5(5):466–477. doi: 10.1167/5.5.7. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan H, Pardhan S, Calver RI, O'Leary DJ. Effect of positive and negative defocus on contrast sensitivity in myopes and non-myopes. Vision Research. 2004;44(16):1869–1878. doi: 10.1016/j.visres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Richler A, Bear JC. Refraction, nearwork, and education. A population study in Newfoundland. Archives of Ophthalmology. 1980;58:468–478. doi: 10.1111/j.1755-3768.1980.tb05748.x. [DOI] [PubMed] [Google Scholar]
- Rosenfield M. Accommodation and myopia. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth Heinemann; Oxford: 1998. pp. 91–116. [Google Scholar]
- Rosenfield M, Abraham-Cohen JA. Blur sensitivity in myopes. Optometry and Vision Science. 1999;76(5):303–307. doi: 10.1097/00006324-199905000-00018. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Desai R, Portello JK. Do progressing myopes show reduced accommodative responses? Optometry and Vision Science. 2002;79(4):268–273. doi: 10.1097/00006324-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Gilmartin B. Disparity-induced accommodation in late-onset myopia. Ophthalmic and Physiological Optics. 1988;8:353–355. [PubMed] [Google Scholar]
- Rosenfield M, Hong SE, George S. Blur adaptation in myopes. Optometry and Vision Science. 2004;81(9):657–662. doi: 10.1097/01.opx.0000144743.34976.da. [DOI] [PubMed] [Google Scholar]
- Sato T. The cause and prevention of school myopia. Excerpta Medica; Amsterdam: 1993. [Google Scholar]
- Saw SM, Chua WH, Hong CY, Wu HM, Chan WY, Chia KS, Stone RA, Tan D. Nearwork in early-onset myopia. Investigative Ophthalmology and Visual Science. 2002;43(2):332–339. [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Eikermann J, Collett T. Lower-field myopia and astigmatism in amphibians and chickens. Journal of the Optical Society of America. 1994;11(2):487–495. doi: 10.1364/josaa.11.000487. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Visual Neuroscience. 1990;4:177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans - relation to age and refractive errors. Journal of Physiology (London) 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on compensatory reponses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research. 1996;36(7):1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Schor C. Imbalanced adaptation of accommodation and vergence produces opposite extremes of the AC/A and CA/C ratios. American Journal of Optometry and Physiological Optics. 1988;65(5):341–348. doi: 10.1097/00006324-198805000-00006. [DOI] [PubMed] [Google Scholar]
- Schor C. The influence of interactions between accommodation and convergence on the lag of accommodation. Ophthalmic and Physiological Optics. 1999;19(2):134–150. doi: 10.1046/j.1475-1313.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Schor CM. Models of mutual interactions between accommodation and convergence. American Journal of Optometry and Physiological Optics. 1985;62:369–374. doi: 10.1097/00006324-198506000-00003. [DOI] [PubMed] [Google Scholar]
- Schor CM. Adaptive regulation of accommodative vergence and vergence accommodation. American Journal of Optometry and Physiological Optics. 1986;63(8):587–609. [PubMed] [Google Scholar]
- Schor CM, Kotulak JC. Dynamic interactions between accommodation and convergence are velocity sensitive. Vision Research. 1986;26(6):927–942. doi: 10.1016/0042-6989(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Schwahn HN, Schaeffel F. Chick eyes under cycloplegia compensate for spectacle lenses despite 6-hydroxy dopamine treatment. Investigative Ophthalmology and Visual Science. 1994;35(9):3516–3524. [PubMed] [Google Scholar]
- Seidel D, Gray LS, Heron G. The effect of monocular and binocular viewing on the accommodation response to real targets in emmetropia and myopia. Optometry and Vision Science. 2005;82(4):279–285. doi: 10.1097/01.opx.0000159369.85285.21. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for minus lens in tree shrews. Optometry and Vision Science. 1999;76(5):308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Shih YF, Chen CH, Chou AC, Ho TC, Lin LLK, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. Journal of Ocular Pharmacology and Therapeutics. 1999;15(1):85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Laboratory Animal Science. 1994;44(3):292–294. [PubMed] [Google Scholar]
- Smith EL., III . Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth Heinemann; Oxford: 1998. pp. 57–90. [Google Scholar]
- Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology and Visual Science. 2002;43(2):291–299. [PubMed] [Google Scholar]
- Sokol RS, Rohlf FJ. Biometry: The Principals and Practice of Statistics in Biological Research. W.H. Freeman; San Francisco: 1981. [Google Scholar]
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Archives of Ophthalmology. 1983;101:405–407. doi: 10.1001/archopht.1983.01040010405011. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Visual Neuroscience. 2004a;21(1):53–62. doi: 10.1017/s0952523804041057. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retinal and pars plana growth. Visual Neuroscience. 2004b;21(5):775–790. doi: 10.1017/S0952523804215115. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Visual Neuroscience. 2005;22(2):171–185. doi: 10.1017/S095252380522206X. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Experimental Eye Research. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- Strang NC, Winn B, Bradley A. The role of neural and optical factors in limiting visual resolution in myopia. Vision Research. 1998;38(11):1713–1721. doi: 10.1016/s0042-6989(97)00303-9. [DOI] [PubMed] [Google Scholar]
- Toates FM. A model of accommodation. Vision Research. 1970;10:1069–1076. doi: 10.1016/0042-6989(70)90083-0. [DOI] [PubMed] [Google Scholar]
- Toates FM. Accommodation function of the human eye. Physiological Reviews. 1972;52(4):828–863. doi: 10.1152/physrev.1972.52.4.828. [DOI] [PubMed] [Google Scholar]
- Troilo D. Vision Science and Its Applications. Vol. 1. Optical Society of America; Santa Fe: 1998. Changes in retinal morphology following experimentally induced myopia; pp. 206–209. [Google Scholar]
- Troilo D, Boisvert N, Nau A. How is accommodation related to the development of refractive state? Evidence from experimental studies using animal models. In: Thorn F, Troilo D, Gwiazda J, editors. Myopia 2000: Proceedings of the VIII International Conference on Myopia; Boston, MA. Myopia 2000, Inc; 2000. pp. 254–258. [Google Scholar]
- Troilo D, Harb E, Totonelly K, Merriwhether L, Bradley D. Accommodation behavior in rhesus macaques during free viewing of an operant conditioned visual task. Investigative Ophthalmology and Visual Science (ARVO Supplement) 2005;46(12) E-Abstract 715. [Google Scholar]
- Troilo D, Howland HC, Judge SJ. Visual optics and retinal cone topography in the common marmoset (Callithrix jacchus) Vision Research. 1993;33(10):1301–1310. doi: 10.1016/0042-6989(93)90038-x. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Investigative Ophthalmology and Visual Science. 2005;46(6):1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Xiong M, Crowley JC, Finlay BL. Factors controlling the dendritic arborization of retinal ganglion cells. Visual Neuroscience. 1996;13(4):721–734. doi: 10.1017/s0952523800008609. [DOI] [PubMed] [Google Scholar]
- Vera-Diaz FA, Gwiazda J, Thorn F, Held R. Increased accommodation following adaptation to image blur in myopes. Journal of Vision. 2004;4(12):1111–1119. doi: 10.1167/4.12.10. [DOI] [PubMed] [Google Scholar]
- Walker TW, Mutti DO. The effect of accommodation on ocular shape. Optometry and Vision Science. 2002;79(7):424–430. doi: 10.1097/00006324-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wang B, Ciuffreda KJ. Depth-of-Focus of the Human Eye: Theory and Clinical Implications. Survey of Ophthalmology. 2006;51(1):75–85. doi: 10.1016/j.survophthal.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wildsoet CE. Neural pathways subserving negative lens-induced emmetropization in chicks - Insights from selective lesions of the optic nerve and ciliary nerve. Current Eye Research. 2003;27(6):371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthalmic and Physiological Optics. 1997;17(4):279–290. [PubMed] [Google Scholar]
- Wildsoet CF, Howland HC, Falconer S, Dick K. Chromatic aberration and accommodation: their role in emmetropization in the chick. Vision Research. 1993;33(12):1593–1604. doi: 10.1016/0042-6989(93)90026-s. [DOI] [PubMed] [Google Scholar]
- Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Research. 2002;42(24):2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Winawer J, Zhu X, Choi J, Wallman J. Ocular compensation for alternating myopic and hyperopic defocus. Vision Research. 2005;45(13):1667–1677. doi: 10.1016/j.visres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Investigative Ophthalmology and Visual Science. 2003;44(7):2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]
- Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. Journal of Pediatric Ophthalmology Strabismus. 1993;30(5):319–322. doi: 10.3928/0191-3913-19930901-12. [DOI] [PubMed] [Google Scholar]